Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2010; 1(5): 181-187
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.181
Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.181
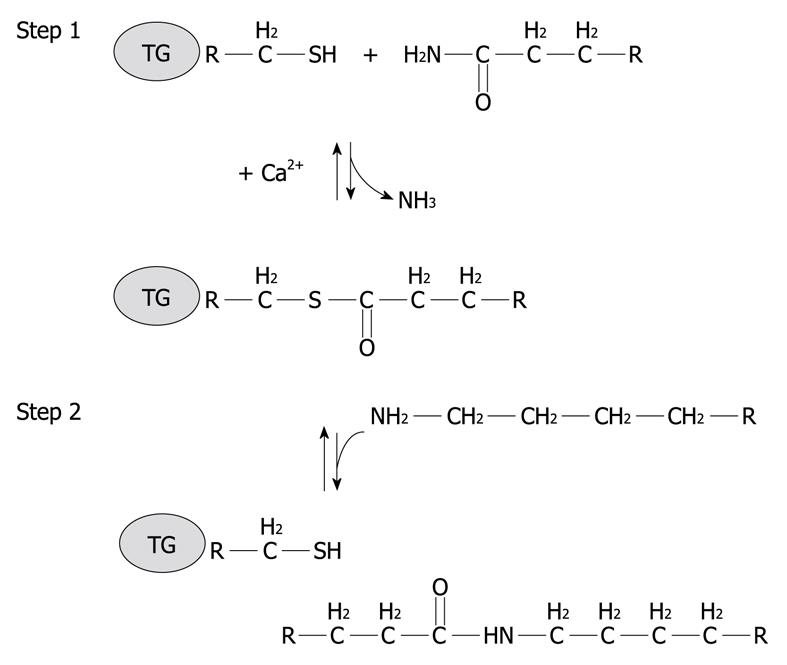
Figure 2 Schematic representation of a two step transglutaminase reaction.
Step 1: In the presence of Ca2+, the active-site cysteine residue reacts with the γ-carboxamide group of an incoming glutaminyl residue of a protein/peptide substrate to yield a thioacyl-enzyme intermediate and ammonia; Step 2: The thioacyl-enzyme intermediate reacts with a nucleophilic primary amine substrate, resulting in the covalent attachment of the amine-containing donor to the substrate glutaminyl acceptor and regeneration of the cysteinyl residue at the active site. If the primary amine is donated by the epsilon-amino group of a lysyl residue in a protein/polypeptide, a Nε-(γ-L-glutamyl)-L-lysine (GGEL) isopeptide bond is formed.
- Citation: Ricotta M, Iannuzzi M, Vivo GD, Gentile V. Physio-pathological roles of transglutaminase-catalyzed reactions. World J Biol Chem 2010; 1(5): 181-187
- URL: https://www.wjgnet.com/1949-8454/full/v1/i5/181.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i5.181