Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Jan 26, 2010; 1(1): 13-20
Published online Jan 26, 2010. doi: 10.4331/wjbc.v1.i1.13
Published online Jan 26, 2010. doi: 10.4331/wjbc.v1.i1.13
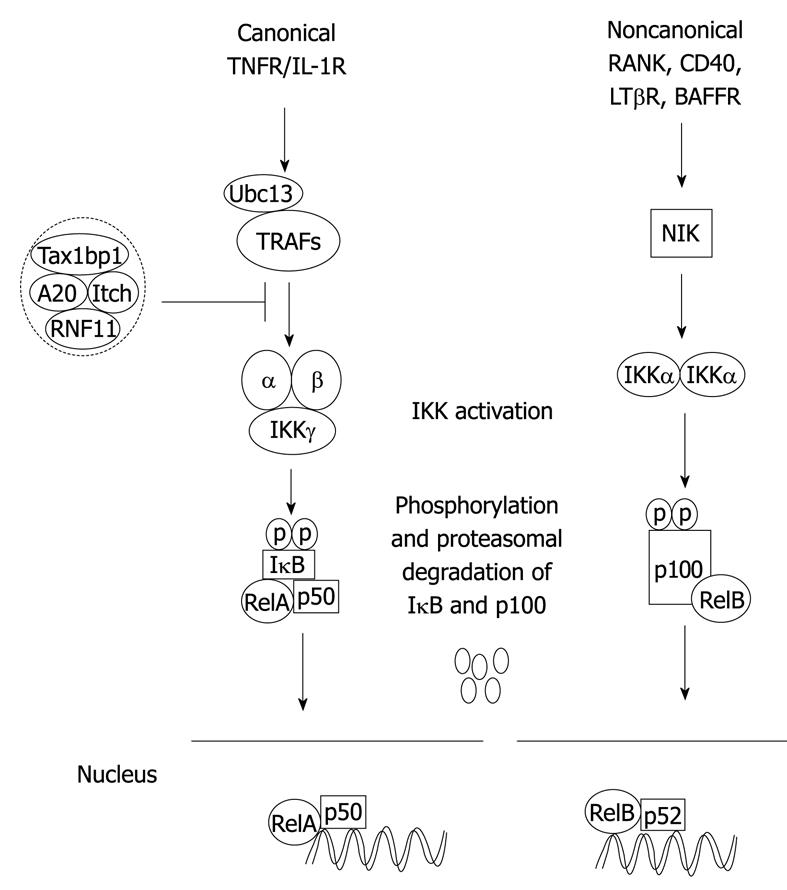
Figure 1 Canonical and noncanonical nuclear factor-κB (NF-κB) activation pathways.
The binding of a specific ligand to a receptor (i.e. tumor necrosis factor-α (TNF-α) binding to TNFR1) leads to the recruitment and activation of an IKK complex comprising IKKα, IKKβ catalytic subunits and the regulatory subunit IKKγ/NEMO. The IKK complex then phosphorylates IκBα leading to degradation by the proteasome and concomitant translocation of NF-κB to the nucleus where it activates target genes. The NF-κB negative regulators, A20, TAX1BP1, Itch and RNF11, form a complex and inhibit activation of NF-κB upstream of IKK in the canonical pathway. In the noncanonical pathway, NIK is activated downstream of select TNFR superfamily members, and phosphorylates IKKα that in turn phosphorylates p100 resulting in its ubiquitination, limited degradation by the proteasome and nuclear mobilization of RelB/p52 dimers. Ubc13: Ubiquitin-conjugating enzyme 13.
- Citation: Shembade N, Harhaj EW. Role of post-translational modifications of HTLV-1 Tax in NF-κB activation. World J Biol Chem 2010; 1(1): 13-20
- URL: https://www.wjgnet.com/1949-8454/full/v1/i1/13.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i1.13