Copyright
©2013 Baishideng Publishing Group Co.
World J Biol Chem. Aug 26, 2013; 4(3): 64-70
Published online Aug 26, 2013. doi: 10.4331/wjbc.v4.i3.64
Published online Aug 26, 2013. doi: 10.4331/wjbc.v4.i3.64
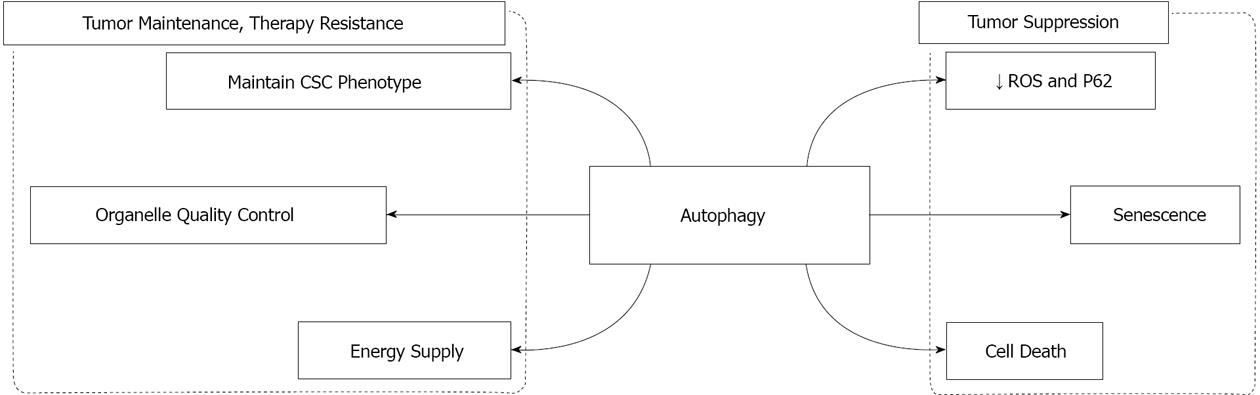
Figure 1 Schematic summary of the role of autophagy in cancer.
Autophagy contributes to tumor suppression as well as tumor maintenance and therapy resistance. The mechanisms by which autophagy is involved in tumor suppression include limiting the accumulation of ROS and P62, and inducing senescence and cell death. On the other hand, autophagy facilities tumor maintenance and therapy resistance by providing the tumor with metabolic substrates and maintaining intracellular homeostasis (organelle quality control), and by possibly contributing to the maintenance of CSC phenotype. CSC: Cancer stem cell; ROS: Reactive oxygen species.
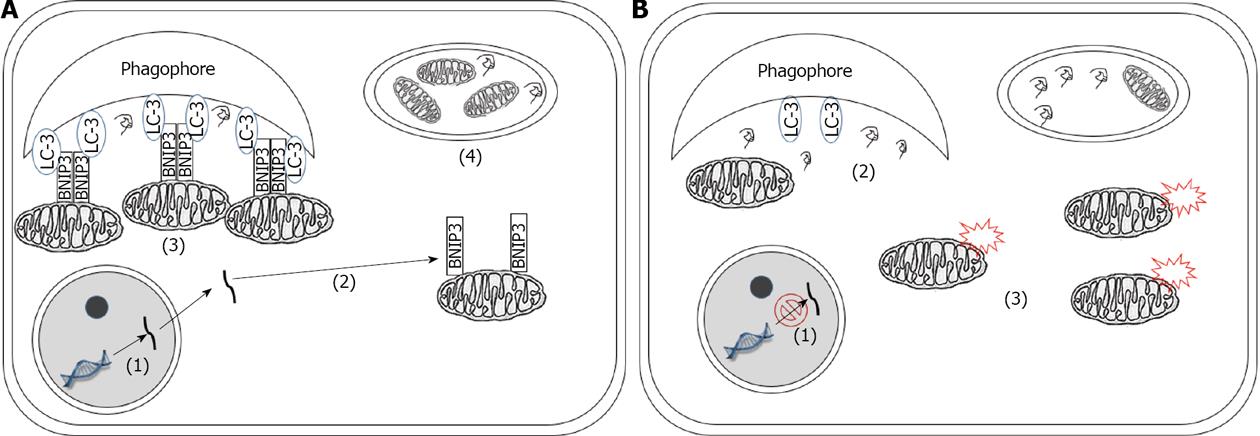
Figure 2 Schematic illustration of differential regulation of mitophagy participating in the progression of pancreatic cancer.
Numbers in parentheses indicate successive mitophagy processes that are interrelated. A: During the early stages of pancreatic cancer: (1) BNIP3 is transcribed; (2) translated and inserted into mitochondria membrane; (3) Active BNIP3 tethers mitochondria to the phagophore through its interaction with LC-3;(4) Mitochondria are therefore selectively engulfed in the autophagosome and degraded by the lysosome. In this way, mitochondria-induced ROS production is limited and genome stability is preserved; B: In the later stages of pancreatic cancer: (1) BNIP3 gene is silenced; (2) The absence of BNIP3 on the mitochondrial outer membrane will prevent the process of selective targeting of mitochondria to the autophagy machinery; (3) Accumulation of damaged mitochondria will result in elevated production of ROS and increased genome instability, which further contributes to the progression of cancer.
- Citation: Lu SZ, Harrison-Findik DD. Autophagy and cancer. World J Biol Chem 2013; 4(3): 64-70
- URL: https://www.wjgnet.com/1949-8454/full/v4/i3/64.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v4.i3.64