Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. Apr 26, 2012; 3(4): 61-72
Published online Apr 26, 2012. doi: 10.4331/wjbc.v3.i4.61
Published online Apr 26, 2012. doi: 10.4331/wjbc.v3.i4.61
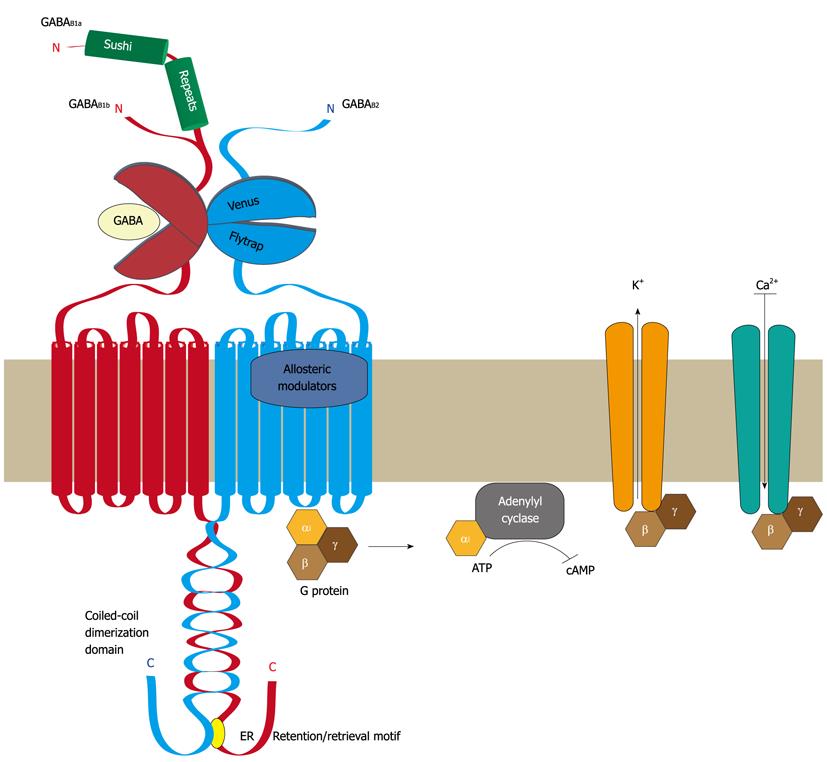
Figure 1 Structural organization of GABAB receptors.
Functional GABAB receptors are heterodimers composed of the two subunits GABAB1 and GABAB2. Both subunits are heptahelical membrane proteins with a large extracellular located N-terminal domain containing a “Venus flytrap” module and a large intracellular C-terminal domain containing a coiled-coil protein-protein interaction module. GABAB1 and GABAB2 heterodimerize via their “Venus flytrap” and coiled-coiled domains. An endoplasmic reticulum (ER) retention/retrieval signal is present distal to the coiled-coil domain in GABAB1 and prevents ER exit of GABAB1 unless it is masked by heterodimerization with GABAB2. The “Venus flytrap” module of GABAB1 constitutes the GABA binding site, whereas that of GABAB2 is inactive and not involved in ligand binding. Instead, the heptahelical domain of GABAB2 contains a binding site for allosteric modulators, which affects the affinity of ligands binding to the GABA site. Binding of GABA results in the recruitment and activation of Gαi/o proteins via GABAB2. The activated Gαi/o subunit inhibits the adenylyl cyclase, resulting in lowered cAMP levels, while the Gβγ dimer activates K+ channels and inhibits Ca2+ channels, leading in either case to neuronal inhibition. There exist two isoforms of GABAB1, named GABAB1a and GABAB1b, which are generated by alternative promoter usage. They only differ by the additional presence of two so-called “sushi repeats” (protein-protein interaction modules) in the N-terminal domain of GABAB1a. GABA: γ-Aminobutyric acid; ATP: Adenosine-5'-triphosphate; cAMP: 3'-5'-cyclic adenosine monophosphate.
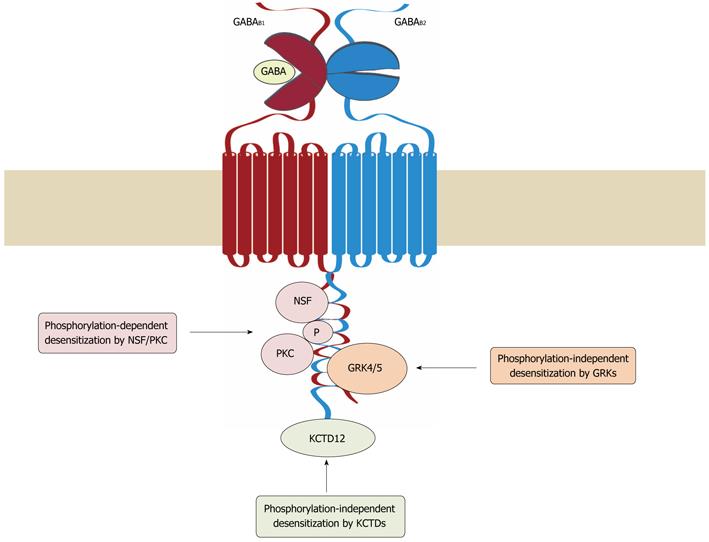
Figure 2 Mechanisms of GABAB receptor desensitization.
Three distinct mechanisms have been so far implicated in the desensitization of GABAB receptors. In cerebellar granule cells, G protein receptor kinase (GRK) 4 and 5 associate with GABAB receptors and induce desensitization of the receptors in a phosphorylation-independent manner. In cortical and hippocampal neurons, desensitization of the receptors involves the interaction of NEM-sensitive fusion protein (NSF) with GABAB1 and GABAB2, which is thought to prime the receptor for phosphorylation by protein kinase C (PKC). Association of potassium channel tetramerization domain-containing (KCTD) proteins 12 and 12b with the C-terminus of GABAB2 appears to render the receptor complex competent for desensitization. GABA: γ-Aminobutyric acid.
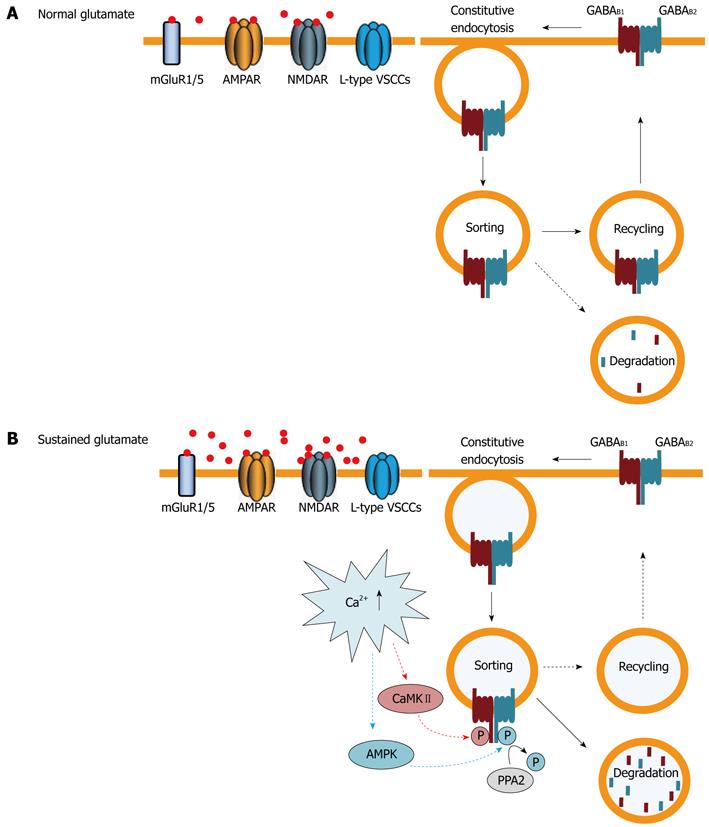
Figure 3 Regulation of cell surface GABAB receptors by trafficking.
A: Under normal conditions GABAB receptors are constitutively internalized and recycled back to the plasma membrane. Only a small fraction of receptors are sorted to lysosomes for degradation; B: Sustained activation of glutamate receptors [primarily 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl) propanoic acid (AMPA) and N-methyl-D-aspartic acid (NMDA) receptors] and L-type voltage-gated Ca2+ channels raises intracellular Ca2+ levels. This induces phosphorylation of GABAB1 at serine 867 by calmodulin-dependent protein kinaseII (CaMKII) and of GABAB2 at serine 783 by adenosine monophosphate (AMP) kinase, followed by slow dephosphorylation, by protein phosphatase 2 (PPA2). These events shift the recycling/degradation equilibrium towards degradation so that the majority of GABAB receptors are no longer recycled, but instead degraded in lysosomes. Since constitutive endocytosis of the receptors remains unaffected, this mechanism results in a rapid down-regulation of GABAB receptors. AMPK: 5’AMP-dependent protein kinase; GABA: γ-Aminobutyric acid; VSCCs: Voltage-sensitive calcium channels.
- Citation: Benke D, Zemoura K, Maier PJ. Modulation of cell surface GABAB receptors by desensitization, trafficking and regulated degradation. World J Biol Chem 2012; 3(4): 61-72
- URL: https://www.wjgnet.com/1949-8454/full/v3/i4/61.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i4.61