Published online Jul 27, 2017. doi: 10.4240/wjgs.v9.i7.167
Peer-review started: February 6, 2017
First decision: March 13, 2017
Revised: April 25, 2017
Accepted: May 22, 2017
Article in press: May 24, 2017
Published online: July 27, 2017
To evaluate the presence of submucosal and myenteric plexitis and its role in predicting postoperative recurrence.
Data from all patients who underwent Crohn’s disease (CD)-related resection at the University of Szeged, Hungary between 2004 and 2014 were analyzed retrospectively. Demographic data, smoking habits, previous resection, treatment before and after surgery, resection margins, neural fiber hyperplasia, submucosal and myenteric plexitis were evaluated as possible predictors of postoperative recurrence. Histological samples were analyzed blinded to the postoperative outcome and the clinical history of the patient. Plexitis was evaluated based on the appearance of the most severely inflamed ganglion or nerve bundle. Patients underwent regular follow-up with colonoscopy after surgery. Postoperative recurrence was defined on the basis of endoscopic and clinical findings, and/or the need for additional surgical resection.
One hundred and four patients were enrolled in the study. Ileocecal, colonic, and small bowel resection were performed in 73.1%, 22.1% and 4.8% of the cases, respectively. Mean disease duration at the time of surgery was 6.25 years. Twenty-six patients underwent previous CD-related surgery. Forty-three point two percent of the patients were on 5-aminosalicylate, 20% on corticosteroid, 68.3% on immunomodulant, and 4% on anti-tumor necrosis factor-alpha postoperative treatment. Postoperative recurrence occurred in 61.5% of the patients; of them 39.1% had surgical recurrence. 92.2% of the recurrences developed within the first five years after the index surgery. Mean disease duration for endoscopic relapse was 2.19 years. The severity of submucosal plexitis was a predictor of the need for second surgery (OR = 1.267, 95%CI: 1.000-1.606, P = 0.050). Female gender (OR = 2.21, 95%CI: 0.98-5.00, P = 0.056), stricturing disease behavior (OR = 3.584, 95%CI: 1.344-9.559, P = 0.011), and isolated ileal localization (OR = 2.671, 95%CI: 1.033-6.910, P = 0.043) were also predictors of postoperative recurrence. No association was revealed between postoperative recurrence and smoking status, postoperative prophylactic treatment and the presence of myenteric plexitis and relapse.
The presence of severe submucosal plexitis with lymphocytes in the proximal resection margin is more likely to result in postoperative relapse in CD.
Core tip: This is a retrospective study to evaluate the presence of submucosal and myenteric plexitis and its role in predicting postoperative recurrence (POR) in Crohn’s disease. Demographic data, smoking habits, previous resection, treatment before and after surgery, and histological findings were evaluated as possible predictors of POR. We found that the severity of submucosal plexitis was a predictor of the need for second surgery. Other predictors of POR were female gender, stricturing disease behavior, and isolated ileal localization. Our results did not confirm the hypothesis that myenteric plexitis can be predictive of postoperative relapse.
- Citation: Milassin Á, Sejben A, Tiszlavicz L, Reisz Z, Lázár G, Szűcs M, Bor R, Bálint A, Rutka M, Szepes Z, Nagy F, Farkas K, Molnár T. Analysis of risk factors - especially different types of plexitis - for postoperative relapse in Crohn’s disease. World J Gastrointest Surg 2017; 9(7): 167-173
- URL: https://www.wjgnet.com/1948-9366/full/v9/i7/167.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i7.167
Surgery is not curative in Crohn’s disease (CD), hence postoperative recurrence still remains a significant problem in the treatment of CD. More than 70% of all patients with CD require surgery in the course of their disease. A second surgery is required in 34%-53% of the cases; the highest recurrence rate has been observed in the ileocolic disease location[1]. Farmer et al[2] also demonstrated that operative incidence was the highest (91.5%) among patients with ileocolic disease. Therefore it is important to identify the predictors of postoperative recurrence in order to optimize treatment and surveillance after surgery. Currently conflicting data are available on the different risk factors. The IBSEN study group found that the probability of surgery was 37.9% in a 10-year follow-up. Terminal ileal location, stricturing, penetrating behavior, and age younger than 40 years at diagnosis were independent risk factors of subsequent surgery[3]. A large meta-analysis of 2962 patients with CD revealed that smoking significantly increases the risk of clinical and surgical recurrence. This high risk of postoperative relapse and reoperation is significantly reduced if a patient quits smoking[4]. A recently published study only identified preoperative steroid use as a risk factor for early postoperative endoscopic recurrence[5], while another study found a higher risk for postoperative endoscopic recurrence in case of previous use of two or more anti-tumor necrosis factor (TNF)-α agents[6].
Histological changes in the enteric nervous system are common in CD. The major structural abnormalities are irregular hypertrophy and hyperplasia of nerve fibers and alterations of neuronal cell bodies and enteric glial cells in the ganglia of the submucosal and myenteric plexus[7]. Ferrante et al[8] showed that the presence of myenteric plexitis in proximal resection margins of ileocolonic resection specimens is highly associated with postoperative CD recurrence, and the severity of myenteric plexitis in the proximal resection margin correlated with the severity of endoscopic recurrence[8-10]. Sokol et al[11] revealed an association between submucosal plexitis and early clinical recurrence, moreover lymphocytic plexitis in the proximal surgical margin was related to a higher risk of endoscopic or surgical recurrence after ileocolonic resection[12,13].
Our aim was to evaluate the frequency and predictors of postoperative recurrence and the role of submucosal and myenteric plexitis in predicting postoperative recurrence on the basis of endoscopic findings and/or the need for additional surgical resection.
Patients were selected retrospectively from the database of the Department of Pathology, University of Szeged (Hungary). All patients who underwent CD-related surgery between 2004 and 2014 were included in the study.
Diagnosis of CD was based on clinical, endoscopic and histological findings. The following data were extracted retrospectively from the medical chart of each patient: Age, sex, year of the diagnosis of CD, phenotype of CD according to the Montréal classification[14], smoking habits, date of the CD-related surgery, type of the anastomosis, CD-related therapy before and after surgery, and the presence of postoperative relapse. Postoperative relapse was defined on the basis of endoscopic and clinical findings, and/or the need for additional surgical resection. Patients were regularly followed up with colonoscopy after the surgery. Postoperative endoscopy findings were classified on the basis of the Rutgeerts score in case of ileocolonic resection[15]; remission was defined as Rutgeerts endoscopic score i0-i1, and recurrence as a score of i2-i4[15].
Postoperative recurrences were defined on the basis of the work of Ng et al[10] Clinical recurrence was defined as the presence of CD-related symptoms associated with radiologic or endoscopic findings, considered severe enough to change the current therapy (requires steroid treatment or an increase in existing treatment). Surgical recurrence was defined as a need for further operation (refractory to medical treatment or new complications developed)[10].
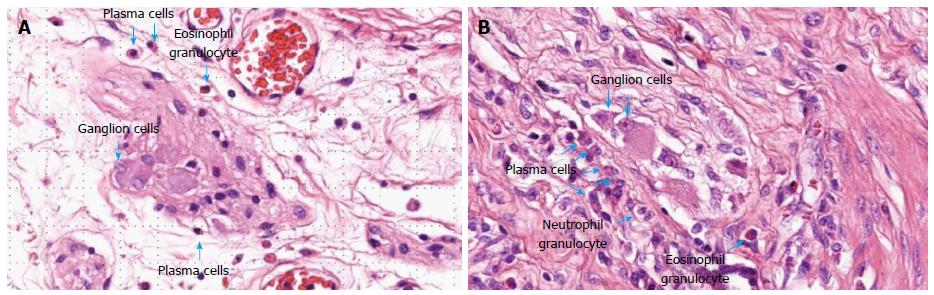
Histological samples were analyzed retrospectively by two expert pathologists, blinded to the postoperative outcome and the clinical history of the patient. Both resection margins (ileal and colonic margins) were investigated for typical CD lesions (inflammatory infiltrates, granuloma, etc). Further investigations focused on the proximal resection margin. Special attention was given to the enteric nervous system, namely to the myenteric and submucosal plexuses. Plexitis was evaluated based on the appearance of the most severely inflamed ganglion or nerve bundle[12]. The severity of plexitis was graded according to the classification proposed by Ferrante et al[8]: Mild plexitis if the ganglion or nerve bundle contained 0-4 inflammatory cells (G1), moderate plexitis if it contained 4 to 9 cells (G2), or severe if containing ≥ 10 cells (G3). Evaluation was performed independently for each cellular type: Mast cell, plasmocyte, lymphocyte, eosinophil and neutrophil cell counts were also evaluated[12]. Each sample was fixed in buffered formalin and analyzed using hematoxylin-eosin staining. Some examples are demonstrated in Figure 1.
The statistical analysis of the data was performed by a biomedical statistician using SPSS. To identify predictors of postoperative recurrence (clinical recurrence or surgical recurrence) among patients’ baseline characteristics, histological findings such as severity of myenteric and submucosal plexitis univariable logistic regression analysis was used. P values < 0.05 were considered statistically significant. Survival was examined with the Kaplan-Meier method.
One hundred and four patients with CD were enrolled in the study. The baseline characteristics of the patients are reported in Table 1. Mean age at index CD-related surgery was 34.8 ± 13.24 years, mean disease duration at the time of the index surgery was 6.25 ± 6.12 years. 86.5% of the patients were on specific CD-related treatment at the time of the index surgery; 37.5% of patients were on aminosalicylates, 13.5% on anti-TNF-α therapy, 51% on corticosteroid, 12.5% on budesonide, 43.3% on azathioprine, 6.7% on methotrexate, and 35.6% on antibiotics. Operations were performed for specific reasons: abscess (20.2%), fistulas (13.5%), perforation (4.8%), stenosis (67.3%) and other (1%). Ileocecal, colonic and small bowel resection were performed in 73.1%, 22.1% and 4.8% of the cases, respectively. Twenty-six patients had undergone previous CD-related surgery. Forty-three point two percent of the patients were on 5-aminosalicylate, 20% on corticosteroid, 68.3% on immunomodulant, and 4% on anti-TNF-α postoperative treatment. Postoperative recurrence occurred in 61.5% of the patients; of them 39.1% had surgical recurrence. 92.2% of the recurrences developed within the first five years after the index surgery. Mean disease duration for postoperative relapse was 2.70 ± 2.11 years.
| Baseline characteristics of patients | n = 104 |
| Mean age at diagnosis (yr) | 41.3 ± 14.047 |
| Mean disease duration at the time of the operation (yr) | 6.25 ± 6.12 |
| Sex | |
| Female | 50 (48) |
| Male | 54 (52) |
| Age at index resection (yr) | |
| Younger than 40 | 74 (71.2) |
| 40 and older | 30 (28.8) |
| Smoking history at index surgery | |
| Current smoker | 32 (30.8) |
| Never smoked | 68 (65.4) |
| Ex-smoker | 4 (3.8) |
| Montréal classification | |
| A1 (< 16 yr) | 15 (14.4) |
| A2 (between 17 and 40 yr) | 71 (68.3) |
| A3 (> 40 yr) | 18 (17.3) |
| B1 (nonstricturing, nonpenetrating) | 12 (11.5) |
| B2 (stricturing) | 52 (50) |
| B3 (penetrating) | 40 (38.5) |
| L1 (isolated ileal disease) | 51 (49) |
| L2 (isolated colonic disease) | 22 (21.2) |
| L3 (ileocolonic disease) | 31 (29.8) |
| L4 (isolated upper disease) | 0 (0) |
| p (perianal disease modifier) | 14 (13.5) |
| Type of index resection | |
| Ileocolonic resection | 76 (73.1) |
| Colonic resection | 23 (22.1) |
| Small bowel resection | 5 (4.8) |
| Previous resection before index surgery | 26 (25) |
Typical Crohn’s lesions, such as inflammatory cell infiltration, architectural alterations, crypt abscesses, ulcers, and granulomas were detected in both resection margins. Typical CD lesions were found in proximal resection margins (5.8%), distal resection margins (5.8%), and in both resection margins (16.3%). Neural fiber hyperplasia was present in 37.5% of proximal resection margins. The pathological examination focused on proximal resection margins with quantitative evaluation of myenteric and submucosal plexitis. Inflammatory cell count (mastocyte, plasmocyte, lymphocyte, eosinophil and neutrophil granulocyte) for myenteric and submucosal plexuses are summarized in Table 2. Median severity of submucosal plexitis was 1 and median severity of myenteric plexitis was 2. Submucosal plexitis was mainly constituted by lymphocytes (median: 2), while myenteric plexitis was mainly constituted by lymphocytes (median: 2) and plasmocytes (median: 2). Other cell types, such as mastocytes, eosinophils and neutrophil granulocytes were less frequently observed.
| Median | IQR, 25th to 75th | |
| Myenteric plexus | ||
| Eosinophils | 0 | 0-1 |
| Lymphocytes | 2 | 1-4 |
| Neutrophils | 0 | 0-0 |
| Plasmocytes | 2 | 1-3 |
| Mastocytes | 0 | 0-0 |
| Submucosal plexus | ||
| Eosinophils | 0 | 0-0 |
| Lymphocytes | 2 | 1-3 |
| Neutrophils | 0 | 0-0 |
| Plasmocytes | 1 | 0-3 |
| Mastocytes | 0 | 0-0 |
We found that perianal disease [odds ratio (OR) = 3.78, 95%CI: 1.164-12.312, P = 0.027] and female gender (OR = 2.21, 95%CI: 0.98-5.00, P = 0.056) are risk factors for postoperative relapse. Stricturing disease behavior (OR = 3.584, 95%CI: 1.344-9.559, P = 0.011) and isolated ileal disease localization (OR = 2.671, 95%CI: 1.033-6.910, P = 0.043) increased the risk of second surgery. Stricturing disease behavior (OR = 6.417, 95%CI: 0.999-41.212, P = 0.050) and ileocecal disease (OR = 6.00, 95%CI: 0.832-43.293, P = 0.076) also increased the risk of relapse in previously operated CD patients.
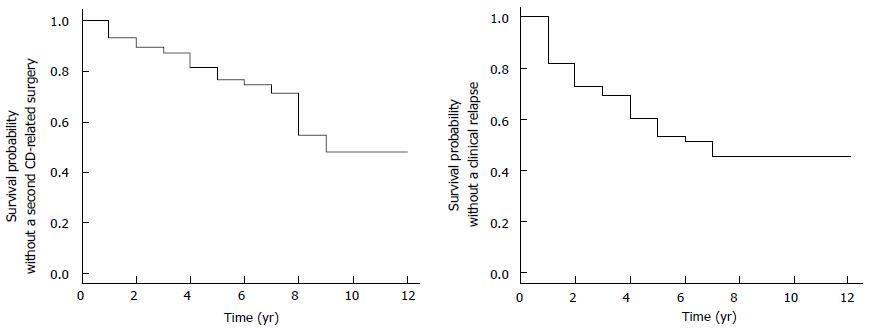
Higher lymphocyte cell count in the submucosal plexus was a risk factor for surgical or clinical relapse (OR = 1.267, 95%CI: 1.000-1.606, P = 0.050). Moderate submucosal plexitis reduced the risk of second surgery by 85.4% compared to severe submucosal plexitis (OR = 0.146, 95%CI: 0.029-0.738, P = 0.020). No association was revealed between postoperative recurrence and smoking status, postoperative prophylactic treatment and the presence of myenteric plexitis and relapse. Figure 2 shows the survival probability without a second CD-related surgery and the probability without clinical recurrence.
We have demonstrated that severity of submucosal plexitis in proximal resection margins, perianal manifestation and stricturing disease behavior, as well as isolated ileal disease were all associated with postoperative recurrence. Over the last few years, several studies focused on plexitis and its role in the postoperative recurrence of CD. Ferrante et al[8] demonstrated that inflammation of the myenteric plexus was significantly associated with postoperative CD endoscopic recurrence; moreover they found a positive correlation between the severity of the inflammatory infiltration of the plexus and the severity of endoscopic recurrence. These data are in concordance with the findings of recent studies: Misteli et al[9] revealed that severe myenteric plexitis at the proximal resection margin is associated with surgical resection; Ng et al[10] demonstrated that myenteric plexitis can be present in otherwise uninvolved proximal resection margins. Sokol et al[11] demonstrated an association between submucosal plexitis and early clinical recurrence; they found that mast cell-associated submucosal plexitis in proximal resection margins is a predictor of early postoperative clinical recurrence. Aude et al[12] revealed that submucosal plexitis of > 0 eosinophils and/or > 6 lymphocytes in proximal resection margins and early surgical revision after the first ileocecal resection are predictive of a second surgery in CD; Lemmens et al[13] found that submucosal lymphocytic plexitis in the proximal surgical margin was significantly associated with a higher risk of endoscopic recurrence after ileocolonic resection. All these studies found that plexitis is more frequent in the proximal resection margin, but data on the prognostic value of histological factors in postoperative CD recurrence are conflicting. This is the reason why we used a comprehensive approach by analyzing all inflammatory cell types in both submucosal and myenteric plexuses in proximal resection margins. Data of the most severely inflamed plexus were involved in the study.
Studies found myenteric plexitis in 42.5%-69.7%-88% of proximal surgical margins[8-10]. We could evaluate myenteric and submucosal plexitis of different severity in every sample, in accordance with Aude et al[12], while the rate of typical CD-lesions was low (5.7%) in proximal resection margins. A higher lymphocyte cell count in the submucosal plexus was a risk factor for surgical or clinical relapse (P = 0.050), while moderate submucosal plexitis reduced the risk of a second surgery by 85.4% compared to severe submucosal plexitis (P = 0.020), which is in accordance with other studies. No association was revealed between postoperative recurrence and the presence of myenteric plexitis.
We found no relationship between the presence of granulomas and clinical or surgical recurrence; however, we could find granulomas only in approximately half of the samples. A few studies found a positive association between the presence of granulomas and the likelihood of recurrence or a more aggressive disease process[16-18], while other studies suggested the opposite[19,20]. It has also been reported that the need for immunosuppressive therapy and surgical interventions were significantly higher in patients with granulomas.
We found no association between postoperative recurrence and neural hypertrophy. Ferrante et al[8] found that patients who had both neural hypertrophy in the terminal ileum and myenteric plexitis in the proximal resection margin had a tendency to develop a higher endoscopic recurrence rate compared with patients who only had myenteric plexitis.
Postoperative recurrence occurred in 61.5% of patients with a median duration of 2 years between the index surgery and relapse; of them 39.1% had surgical recurrence. Ninety-two point two percent of the recurrences occurred within five years. Our data are similar to previously published data: Surgical recurrence was reported in 11%-32% of patients at 5 years[21]. Mean disease duration for endoscopic relapse on the basis of the Rutgeerts score was 2.70 years. Postoperative recurrence was divided into two groups on the basis of the paper of Ng et al[10] Clinical recurrence was defined as the presence of CD-related symptoms associated with radiologic or endoscopic findings considered severe enough to change the current therapy (requires steroid treatment or an increase in existing treatment). Surgical recurrence was defined as a need for further operation if the disease was refractory to medical treatment or new complications developed.
No association was revealed between postoperative recurrence and preoperative or postoperative prophylactic treatment. Forty-three point two percent of patients were on 5-aminosalicylate, 20% on corticosteroid, 68.3% on immunomodulant, and 4% on anti-TNF-α postoperative treatment. 5-aminosalicylic acid (5-ASA) has been extensively studied in the postoperative management of CD. Studies showed that the early administration of oral mesalazine following surgery is effective in preventing postoperative endoscopic recurrence in CD over a 2-year period[22] and it can also decrease the rate and severity of endoscopic recurrences[23]. In a meta-analysis, 5-ASA significantly reduced the risk of symptomatic relapse[24]. In a prospective, open-label randomized study, azathioprine was more effective than mesalazine in preventing clinical relapse in patients with previous intestinal resections[25]. These studies suggest that 5-ASA is safe in postoperative CD prophylaxis, even if it seems to provide only a small reduction in clinical and endoscopic recurrence[26].
Our study has certain limitations including its retrospective nature, although it is one of the largest series looking at myenteric and submucosal plexitis. As the course of CD may differ from one patient to another, many studies have looked for potential predictors of CD recurrence as these can modify the intensity of surveillance and the type of medical therapy.
In conclusion, the presence of severe submucosal plexitis with lymphocytes in the proximal resection margin is more likely to result in postoperative relapse. Postoperative assessment of plexitis could be performed routinely by every pathologist in every center as proximal resection margins are systematically analyzed. This requires no special immunostaining. Histological analysis of the proximal resection margin may be useful when making a decision on early postoperative treatment without a postoperative follow-up colonoscopy, thus possibly modifying the natural course of CD. However, further studies with a prospective design and a longer follow-up period are needed.
Crohn’s disease (CD) can affect the entire gastrointestinal tract, but the most commonly affected sites are the ileum and the ascending colon. More than 70% of all patients with CD require surgery in the course of their disease, which is not curative; the disease recurs in most cases. Currently, there are no reliable tools to predict when and in whom the disease will recur.
In the last decade, particular attention was paid to histological features to assess the risk of postoperative relapse (POR). Inflammatory changes in the enteric nervous system (myenteric and submucosal plexus) of the resection margins are probably the most promising factors.
The authors confirmed the significant value of investigating the presence of submucosal plexitis in the proximal resection margin of ileo-colonic resection specimens; the severity of submucosal plexitis (higher lymphocyte cell count in the submucosal plexus) was a risk factor for surgical and clinical POR of CD. These investigations can be performed by analyzing proximal resection margins with routine staining.
All available data, including ours, suggest that lymphocyte cell count plays the most important role in predicting the POR of CD. Routine histological analysis of the proximal resection margin for submucosal plexitis can be useful to stratify patients according to their risk to decide the need for early postoperative treatment.
POR was defined as the reappearance of lesions after complete surgical resection. Clinical recurrence was defined as the presence of CD-related symptoms associated with radiologic or endoscopic findings, considered severe enough to change the therapy (requires steroid treatment or an increase in existing treatment). Surgical recurrence was defined as a need for further operation.
In the article, the clinical and pathological data of 140 postoperative patients with CD were analyzed, so as to study the risk factors of postoperative recurrence of CD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Zhong YQ S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Whelan G, Farmer RG, Fazio VW, Goormastic M. Recurrence after surgery in Crohn’s disease. Relationship to location of disease (clinical pattern) and surgical indication. Gastroenterology. 1985;88:1826-1833. [PubMed] [Cited in This Article: ] |
| 2. | Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818-1825. [PubMed] [Cited in This Article: ] |
| 3. | Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 488] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 4. | Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | de Barcelos IF, Kotze PG, Spinelli A, Suzuki Y, Teixeira FV, de Albuquerque IC, Saad-Hossne R, da Silva Kotze LM, Yamamoto T. Factors affecting the incidence of early endoscopic recurrence after ileocolonic resection for Crohn’s disease: a multicentre observational study. Colorectal Dis. 2017;19:O39-O45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Collins M, Sarter H, Gower-Rousseau C, Koriche D, Libier L, Nachury M, Cortot A, Zerbib P, Blanc P, Desreumaux P. Previous Exposure to Multiple Anti-TNF Is Associated with Decreased Efficiency in Preventing Postoperative Crohn’s Disease Recurrence. J Crohns Colitis. 2017;11:281-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Geboes K. What histologic features best differentiate Crohn’s disease from ulcerative colitis? Inflamm Bowel Dis. 2009;15:1438-47. [PubMed] [Cited in This Article: ] |
| 8. | Ferrante M, Gert de H, Hlavaty T, D’Haens G, Penninckx F, D’Hoore A, Vermeire S, Rutgeerts P, Geboes K, Van Assche G. The Value of Myenteric Plexitis to Predict Early Postoperative Crohn’s Disease Recurrence The Value of Myenteric Plexitis to Predict Early Postoperative. Gastroenterology. 2006;130:1595-1606. [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Misteli H, Koh CE, Wang LM, Mortensen NJ, George B, Guy R. Myenteric plexitis at the proximal resection margin is a predictive marker for surgical recurrence of ileocaecal Crohn’s disease. Colorectal Dis. 2015;17:304-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Ng SC, Lied GA, Kamm MA, Sandhu F, Guenther T, Arebi N. Predictive Value and Clinical Significance of Myenteric Plexitis in Crohn’s Disease. Inflamm Bowel Dis. 2009;15:1499-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Sokol H, Polin V, Lavergne-Slove A, Panis Y, Treton X, Dray X, Bouhnik Y, Valleur P, Marteau P. Plexitis as a predictive factor of early postoperative clinical recurrence in Crohn’s disease. Gut. 2009;58:1218-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Aude B, Chevaux JB, Williet N, Oussalah A, Germain A, Gauchotte G, Wissler MP, Vignaud JM, Bresler L, Bigard MA. Submucosal Plexitis as a Predictor of Postoperative Surgical Recurrence in Crohn ’ s Disease. Inflamm Bowel Dis. 2013;19:1654-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Lemmens B, de Buck van Overstraeten A, Arijs I, Sagaert X, Van Assche G, Vermeire S, Tertychnyy A, Geboes K, Wolthuis A, D’Hoore A. Submucosal Plexitis as a Predictive Factor for Postoperative Endoscopic Recurrence in Patients with Crohn’s Disease Undergoing a Resection with Ileocolonic Anastomosis: Results from a Prospective Single-centre Study. J Crohns Colitis. 2017;11:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] [Cited in This Article: ] |
| 15. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. [PubMed] [Cited in This Article: ] |
| 16. | Trnka YM, Glotzer DJ, Kasdon EJ, Goldman H, Steer ML, Goldman LD. The long-term outcome of restorative operation in Crohn’s disease: influence of location, prognostic factors and surgical guidelines. Ann Surg. 1982;196:345-355. [PubMed] [Cited in This Article: ] |
| 17. | Anseline PF, Wlodarczyk J, Murugasu R. Presence of granulomas is associated with recurrence after surgery for Crohn’s disease: experience of a surgical unit. Br J Surg. 1997;84:78-82. [PubMed] [Cited in This Article: ] |
| 18. | Molnár T, Tiszlavicz L, Gyulai C, Nagy F, Lonovics J. Clinical significance of granuloma in Crohn’s disease. World J Gastroenterol. 2005;11:3118-3121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (3)] |
| 19. | Wolfson DM, Sachar DB, Cohen A, Goldberg J, Styczynski R, Greenstein AJ, Gelernt IM, Janowitz HD. Granulomas Do Not Affect Postoperative Recurrence Rates in Crohn’s Disease. Gastroenterology. 1972;83:405-409. [DOI] [Cited in This Article: ] |
| 20. | Glass RE, Baker WN. Role of the granuloma in recurrent Crohn’s disease. Gut. 1976;17:75-77. [PubMed] [Cited in This Article: ] |
| 21. | Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 654] [Cited by in F6Publishing: 686] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 22. | Caprilli R, Andreoli A, Capurso L, Corrao G, D’Albasio G, Gioieni A, Assuero Lanfranchi G, Paladini I, Pallone F, Ponti V. Oral mesalazine (5-aminosalicylic acid; Asacol) for the prevention of post-operative recurrence of Crohn’s disease. Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Aliment Pharmacol Ther. 1994;8:35-43. [PubMed] [Cited in This Article: ] |
| 23. | Brignola C, Cottone M, Pera A, Ardizzone S, Scribano ML, De Franchis R, D’Arienzo A, D’Albasio G, Pennestri D. Mesalamine in the prevention of endoscopic recurrence after intestinal resection for Crohn’s disease. Italian Cooperative Study Group. Gastroenterology. 1995;108:345-349. [PubMed] [Cited in This Article: ] |
| 24. | Cammà C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohn’s disease: a meta-analysis adjusted for confounding variables. Gastroenterology. 1997;113:1465-1473. [PubMed] [Cited in This Article: ] |
| 25. | Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, Imbesi V, Molteni M, Danelli PG, Taschieri AM. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn’s disease. Gastroenterology. 2004;127:730-740. [PubMed] [Cited in This Article: ] |
| 26. | Spinelli A, Sacchi M, Fiorino G, Danese S, Montorsi M, Goll R, Heise P. Risk of postoperative recurrence and postoperative management of Crohn’s disease. World J Gastroenterol. 2011;17:3213-3219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |