INTRODUCTION
Worldwide, colorectal cancer is the third most prevalent malignancy accounting for over 1 million new cases per year with more than 500000 deaths[1]. Incidence in Europe exceeds 400000 per year[2].
More than 25%-35% of patients with either early or advanced colorectal cancer will develop peritoneal recurrence alone after a first line treatment; peritoneal carcinomatosis is present in up to 44% of patients with recurrent colorectal cancer; the presence of synchronous or metachronous peritoneal metastasis is associated with poor prognosis[3,4] accounting for more than one third of all deaths.
Despite recent advances in gaining a thorough knowledge of clinical, biological and pathological behavior of colorectal cancer, the most commonly used staging systems for colorectal cancer are the Tumor-Node-Metastasis (TNM) and the modified Duke’s staging systems[5-7]. Expected prognosis, treatment choice and adjuvant chemotherapy are based on results provided by these staging systems which consider the local extension of the disease, the lymph nodes involvement and the eventual presence of distant metastases[8].
Both these staging systems are highly heterogeneous within each stage, sometimes resulting in very different prognosis for patients that share the same stage. This is particularly true in patients in stage II and III[9-11] and it may explain why also patients with early stage cancers could develop local or distant recurrence. Furthermore, according to this staging procedure, early stage patients are not appropriate candidates for adjuvant chemotherapy even though the probability of peritoneal recurrence in these patients seems to be equal to those with more advanced cancers.
Recent studies identified only a limited subset of patients suitable for the extensive surgical treatment and the intraperitoneal heated chemotherapy (HIPEC) with promising results in terms of both recovery from disease and overall survival[12-16]. Nevertheless, ten years survival in this limited cohort of patients appears to be a disappointing accomplishment in most qualified studies, in 10% of patients[17]. More recently extensive surgery and HIPEC has been proposed to prevent peritoneal recurrence in selected cases found to have advanced mucinous cancers with positive peritoneal lavage: Although the first results seem promising, such an approach is still merely investigational[18-20].
Peritoneal cytology from peritoneal lavage is routinely performed in in staging esophageal - gastric and pancreatic malignancies and it has a definitive prognostic role in ovarian cancer[21-26]. Free intra-peritoneal cancer cells (IFCC) dissemination can occur either spontaneously or because of surgical manipulation, and follows a complex mechanism of circulation, adhesion and invasion of peritoneal surfaces.
MECHANISM OF PERITONEAL SHEDDING, CIRCULATION AND SEEDING OF CANCER CELLS
IFCC are found in peritoneal washing of as much as 25% of colorectal cancer patients[27]. Mechanisms of seeding and the cascade of events, which may lead to their adhesion to peritoneal surface and subsequent peritoneal metastasis development, consist in several well-defined steps. Detachment of cells from primary tumor is the first and it can occur spontaneously. Down regulation of cell adhesion molecules CAMs, such as E-cadherin, associated with high interstitial pressure due to the lack of a well organized lymphatic drainage inside the tumor explains this mechanism, which is effective just when the tumor involves the colon serosal surface (T3) or when spontaneous bowel perforation occurs[28,29].
Surgery itself represents a highly effective mechanism to (that favors) peritoneal cancer spread. Theoretically, even when tumor’s manipulation is limited, tumor spill is possible from blood or lymphatic vessels section[30]. This cells show proliferation and invasive potentials and are capable of developing metastasis. Once detached, cells follow well known peritoneal routes which are the same of peritoneal fluid drainage and reabsorption, driven by gravity force, diaphragmatic excursion and mesenteric reflections, towards and from the pelvis, along the right para-colic gutter and the sub-diaphragmatic space. Moreover, tumor cells showed inherent motility[31]. Another possible iatrogenic mechanism of free cancer cells spilling and diffusion could be associated with the laparoscopic technique[32]. However, large clinical trials found no differences in peritoneal recurrence risk between open and laparoscopic surgery for colorectal cancer, possibly because the carbon dioxide potential effect is minimized by the reduced peritoneal trauma of the laparoscopic access[33]. A preferred location for free tumor cells seeding is represented by the omentum because of its discontinuous mesothelial lining and the presence of milky spots.
Surgery contributes to tumor cells spilling and adhesion even by other post surgical physiological effects: Post-operative tissue inflammation and wound healing is mediated by macrophages which produce an array of mediators able to enhance tumor growth, while pro-inflammatory cytokines enhance mesothelial adhesion molecules expression. Even fibrin can entrap tumor cells during the wound healing process. After mesothelial adhesion, tumor cells become able to pass through the peritoneal discontinuity areas or even to promote mesothelial cells apoptosis as well[34]. Matrix metalloproteinases inhibition seems also to be associated with extracellular matrix degradation, thus allowing tumor cells invasion of layers.
IFCC DETECTION METHODS
Several studies tried to detect IFCC immediately before and after curative surgery for colorectal cancer[35-37], using different methods and arrays with different sensitivity. A recent large review on this issue by Mohan et al[38] revised 18 studies out of 3805 found, on 3197 colorectal cancer patients; large heterogenicity was found in peritoneal washing methods in terms of volume and solutions, timing of washing, and laboratory techniques. Most used techniques include conventional cytology, immunological or radio-immunoassays methods, molecular techniques as real time or endpoint polymerase chain reaction (PCR). Heterogeneity of peritoneal washing techniques, timing and samples analysis accounts for the main issue in clarifying the impact of intraperitoneal free cancer cells on prognosis and risk to develop peritoneal recurrence in colorectal cancer patients[39].
Disseminated intraperitoneal cancer cells in colorectal cancer patients may be detected using a range of techniques including examination of peritoneal fluid using conventional cytology, cytology following immune-marker staining, PCR or immunocytochemistry.
The timing of the detection may vary and can occur either pre or post-tumor resection[40].
CONVENTIONAL CYTOLOGY AND CYTOLOGY FOLLOWING IMMUNE-MARKERS STAINING
Peritoneal cytology can be performed without lavage when free peritoneal fluid is present. In the absence of peritoneal fluid, a lavage with saline serum (NaCl 0.9%) is needed. The volume of fluid used is extremely variable, ranging from 50 to 1000 mL, but most authors use a small amount of liquid (100-200 mL) delivered around the tumor, where most cells are usually found. Wet fixed direct smears are prepared from the aspirated material after centrifugation and discarding the supernatant. Two or three slides are fixed immediately to prevent cell degeneration. Papanicolaou is a highly suitable staining method also to sediment preparations from fluids. It offers a great advantage with regard to comparative cell studies in histological sections. However Hematoxylin-Eosin, May-Grünwald-Giemsa, Diff-Quik, and other staining methods are also used.
Cyto-centrifuge preparation is recommended for small amounts of fluid with sparse cellular content. Thin layer preparation (cytospin, ThinPrep, and others) is becoming more and more popular. The method may be preferred for adjacent analyses. The remaining cellular material should be retained and stored at 4 °C, mixed up with a certain amount of the supernatant.
Adenocarcinoma of the large bowel may be suggested by those cases that display small and large compact irregular papillary clusters. The epithelial glandular cells are large and cylindrical, and show a palisade arrangement.
Immuno-stains for CK7 and CK20 expression yield a negative and a positive staining result, respectively[41,42].
IMMUNO-CYTOCHEMISTRY
Immuno-cytochemical methods are based on the staining of tumor cells using specific antibodies against tissue (tumor) specific antigens. Target antigens include EpCAM members of the cytokeratin family or other antigens, which are overexpressed on tumor cells (HER2-neu or MUC-1) but not on other normal cells. These antibodies are either directly labeled with horseradish peroxidase, alkaline phosphatase or fluorescent proteins, or otherwise the antigen-antibody complex is visualized by a labeled second antibody, as in the alkaline phosphatase anti-alkaline phosphatase method. To discriminate between malignant cells and non-specifically stained non-malignant cells, an additional evaluation step that includes morphological criteria and/or an additional counterstaining is necessary. This step employs a second tumor cell-specific antigen or an antibody against a CD marker as CD45. During the last years several computeraided search systems have been developed which are used for scanning of microscope slide analysis. Enriched fractions are commonly centrifuged onto microscope slides (cytospins) for immune-cytochemical detections.
PCR-BASED METHODS
PCR-based methods are widely used for the detection of IFCC, targeting both DNA and RNA markers. DNA is generally stable and independent of the transcription mechanism of the cell. DNA markers are used based on specific genetic abnormalities that occur in certain types of cancers, although it has been reported that, at least in some cases, disseminated tumor cells are not necessarily clonal to the primary tumor. In general, few chromosomal alterations specifically characterize certain types of cancer, or even are frequent enough to serve as molecular markers. The most frequently encountered genomic alterations in colorectal cancer, commonly used for the detection of free peritoneal cancer cells, include mutations in k-Ras and p53 genes, sometimes investigated together with mRNA markers. More specifically, the detection of occult tumor cells engages targeting of tumor specific mRNA, meaning mRNA that encodes for antigens that are specific either for the malignant phenotype or for the normal tissue. The use of mRNA markers is based on the notion that tumor cells continue to display the same pattern of antigen expression as their normal tissue of origin. Once released from malignant cells, mRNA is relatively unstable; therefore, once detected, mRNA markers are indicative of the presence of viable tumor cells in the examined sample[43].
In a recent meta-analysis, positive peritoneal washing was seen as an independent prognostic factor for poor survival and was associated with a greater risk of both local and systemic recurrence in colorectal cancer patients[44]. Yield rates of intraperitoneal neoplastic cells ranged from 5% to 40% depending on the methods and on the time of detection. In general, immunocytochemistry appears to result in a far greater yield of intraperitoneal neoplastic cells than either PCR or cytopathology. Furthermore it must be considered that immunocytochemistry (along with other histological staining techniques) is subjective and depends on the strength of cellular staining, while PCR-based methods have inherent problems as they detect DNA, not viable cells, and cannot delineate cancerous cells from nonmalignant cells or cellular debris.
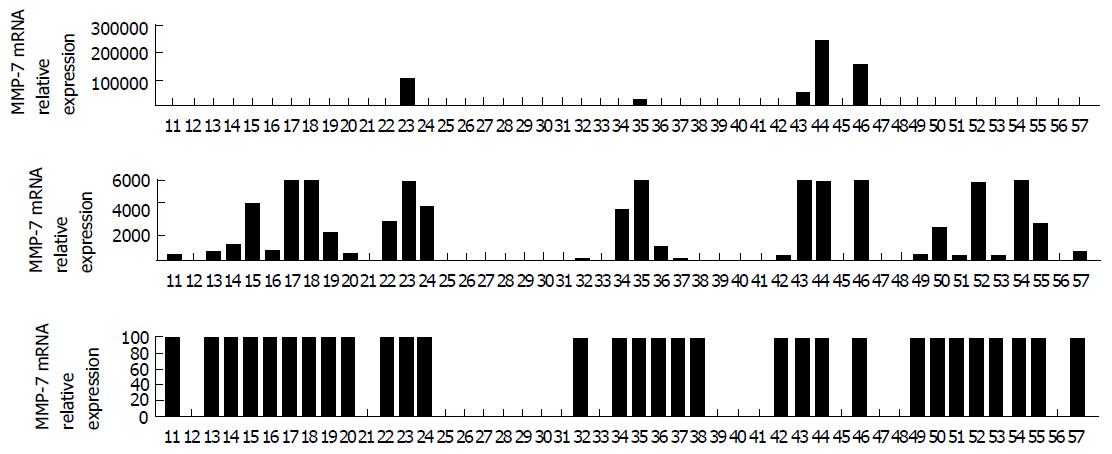
However, several cancer cell proteins may be identified by mean of PCR based methods, such as the matrix metallo-proteinase (MMP) class and specifically the MMP-7 (Figure 1) which has been recently proved a highly sensible predictive factor involved in colorectal cancer recurrence after curative treatment. In a recent article by Sica et al[45] expression of MMP-7 on IFCC correlated with higher recurrence rate after curative surgery for colorectal cancer and worse prognosis[45]. Patterns of expression of MMP-7 RNA transcripts in a sample of 47 patients who underwent surgery for colorectal cancer are shown in Figure 1.
Figure 1 Patterns of expression of matrix metallo-proteinase-7 RNA transcripts in 47 peritoneal washing samples taken from 47 patients who had undergone surgery for colorectal cancer[45].
MMP: Matrix metallo-proteinase.
CLINICAL AND PROGNOSTIC SIGNIFICANCE
In the last ten years, several studies attempted to state the prognostic and clinical meaning of free peritoneal cancer cells found during colorectal cancer surgery, investigating either their presence and prognostic impact[26,38-40,42,43,46].
If their clinical importance in gastric cancer has been clearly identified[47-51], results from this large series of studies on colorectal cancer are misleading. The first concern has to be moved to the large heterogeneity of detection techniques used: If conventional cytology appears to be very sensitive, easily applicable and low costing, its specificity is low, yielding positive results in 4% to 35.5% of series, also providing for a 2% of inconclusive examinations[42]. Immunoassays and PCR seem to be more specific as well as more expensive and subject to laboratory availability[37].
This variability partially explains the differences in results from the studies. A recently closed large trial by French authors, based on 1364 patients, found no relationship between positive cytology and incidence of recurrence and no predictive value regarding the development of peritoneal carcinomatosis. In this study positive cytology correlated with depth of invasion of colorectal wall, synchronous presence of minimal peritoneal carcinomatosis, lymph nodes metastasis, presence of ascites or not radical surgery; this reflected on survival analysis which led to worse survival in patients with positive cytology (P < 0.001) in univariate analysis although it didn’t reach statistical significance as independent prognostic factor[40]. Otherwise, other studies found higher risk of overall and loco-regional recurrence when peritoneal free cancer cells are found[39] as well as predictive of poorer outcome[38].
Two studies showed that poorer outcomes are associated with positive post resection washing compared to positive pre-resection one, in terms of recurrence[52] and survival[36].
In most studies, increasing disease staging correspond to higher rates of positive cytology[53] although they can be detected also in early stage patients[54], mostly in stage 2, where Lloyd et al[36] found worse survival among stage 2 patients with positive cytology rather than negative ones.
Some authors found correlation between positive cytology and poorly differentiated cancers[55] while the correlation with mucinous or signet ring cells histology remains unclear, even because these histological types are mostly found in advanced stage diseases.
When positive cytology is found, a prophylactic intraperitoneal chemotherapy may be considered in selected cases within clinical trials.
This proactive treatment is proposed in order to prevent peritoneal diffusion in colorectal cancer patients at high risk of peritoneal metastasis. The risk factors which were identified are as follow: Mucinous or signet ring cell hystologies, T3/T4 or perforated tumors and positive peritoneal cytology.
Two recent comparative studies by Sammartino et al[18,19] showed that on a sample of 25 patients affected by colorectal cancer at high risk of peritoneal metastasis, a more aggressive surgical treatment including omentectomy, appendectomy, hepatic round ligament resection and oophorectomy in non-fertile women, associated with prophylactic intraperitoneal hyperthermic chemotherapy led to better disease free survival and lower peritoneal recurrence rates[18-20]. It is clear, at this point, that peritoneal washing should become a standardized procedure and that the clinical implications of IFCC are potentially enormous. Effort should be spent on obtaining reliable results in terms of sensibility and specificity of the methods of analysis.
CONCLUSION
Positive peritoneal washing for IFCC is associated with worse outcome in colorectal cancer patients, however it is not clear whether it is associated with an increased risk of local recurrence or not. IFCC can be found in advanced stage or in the acute setting (occlusion or perforation). However, positive cytology can occur also in colorectal cancer at an earlier stage (especially TNM stage II patients) and it could affect the strategic plan of treatment. Nevertheless, available data still do not allow to include peritoneal washing and cytology as routine procedures procedures in staging colorectal cancer.
Potentially, peritoneal washing could improve the outcome of those selected patients with apparent early stage colorectal cancer, to receive adjuvant chemotherapy.
Patients with positive cytology may also become candidates to receive proactive intraperitoneal chemotherapy.
Peritoneal washing examination techniques must be improved in order to achieve a better sensitivity. It is the authors’ belief that until a proper reliable tumor marker for RT-PCR will be identified, probably the most suitable procedure remains conventional cytology. However, giving the recent studies in this direction it is desirable that highly sensible proteins such as the MMP class and specifically the MMP-7 are employed to increase the specificity of conventional cytology[45].
Further studies are needed to standardize detection and examination procedures, to determine if there are and which are the stages more likely to benefit from routine search for IFCC in the view of offering a proactive management, keeping in mind what Benjamin Franklin once stated: “an ounce of prevention is worth a pound of cure”.
P- Reviewer: He S, Hironaka S, Noguera J, Wang YH
S- Editor: Ji FF L- Editor: A E- Editor: Li D