Published online Oct 27, 2012. doi: 10.4240/wjgs.v4.i10.228
Revised: September 14, 2012
Accepted: September 21, 2012
Published online: October 27, 2012
AIM: To investigate the status of the lymphatic vessels in the small bowel affected by Crohn’s disease (CD) at the moment of surgery.
METHODS: During the period January 2011-June 2011, 25 consecutive patients affected by CD were operated on in our Institution. During surgery, Patent Blue V was injected subserosally and the way it spread along the subserosa of the intestinal wall, through the mesenterial layers towards the main lymphatic collectors and eventually to the lymph nodes was observed and recorded. Since some patients had been undergone strictureplasty at previous surgery, we also examined the status of intestinal lymph vessels after previous strictureplasties. The same procedure was performed in a control group of 5 patients affected by colorectal cancer. Length of lesions, caliber, maximal thickness of the diseased intestinal wall, thickness of the wall at injection site and thickness of the mesentery were evaluated at surgery.
RESULTS: We observed three features after the injection of Patent Blue V in the intestinal loops: (1) Macroscopically healthy terminal ileum of patients with CD or colon cancer showed thin lymphatic vessels linearly directed toward the mesentery; (2) In mild lesions in which the intestinal wall did not reach 8 mm of thickness, we observed short, wide and tortuous lymphatic vessels directed longitudinally along the intestinal axis toward disease-free areas and then transversally toward the mesentery; and (3) Injection in the severely affected lesions, that had a thickness of the intestinal wall over 10 mm, did not show any feature of lymphatic vessels at least on the subserosal surface. There was a correlation between the thickness of the parietal wall and the severity of the lymphatic alterations. Normal lymphatic vessels were observed at previous strictureplasties in the presence of complete regression of the inflammation.
CONCLUSION: Injection of Patent Blue V in the intestinal wall could help distinguish healthy tracts of the small bowel from those macroscopically borderline.
- Citation: Tonelli F, Giudici F, Liscia G. Is lymphatic status related to regression of inflammation in Crohn's disease? World J Gastrointest Surg 2012; 4(10): 228-233
- URL: https://www.wjgnet.com/1948-9366/full/v4/i10/228.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i10.228
Crohn’s disease (CD) is a chronic inflammation occurring mainly in the terminal ileum, but potentially affecting any part of the intestinal tract. Although in recent years several studies had clarified that CD occurs in genetically predisposed subjects and is associated with defective intestinal permeability and compromised immune response, a definitive etiology remains unknown. Pathologists who have studied surgical specimens of CD describe the microscopic appearance of the disease as a chronic lymphangitis[1]. These authors described focal collections of lymphocytes and histiocytes, dilation of the lymphatic channels, swelling and proliferation of the lymphatic endothelium with a consequent occlusion of the lymphatics[2-4].
In experimental animals CD was induced by obstruction of the mesenteric lymphatics either by injecting formalin or acrylic resin into the regional lymph nodes or by ligature[5-8]. It was then observed that lymphatic vessels may play an important role in inducing or maintaining bowel inflammation. The primary aim of this paper is to observe the status of the lymphatic vessels in the small bowel of CD patients at the time of primary surgery or of recurrence of the disease by injecting Patent Blue V (a dark bluish synthetic dye used in lymphangiography and for sentinel node biopsy as a dye to color lymph vessels) in the subserosa of the affected intestinal loops. The opportunity to perform recurring surgery in some CD patients allowed us to observe the status of intestinal lymph vessels after previous strictureplasties and to try to clarify whether a “restitutio ad integrum” of lymphatic channels is accompanied by regression of inflammatory lesions: it is the secondary aim of this paper.
During the period January 2011-June 2011, 25 consecutive patients (14 males and 11 females) affected by ileal CD were operated on in our Institution. The mean age was 38.7 years (range 17-58 years). Thirty-nine percent of the patients were at primary surgery while 61% were affected by recurrent CD. In this second group 5 patients had previously undergone a side-to-side isoperistaltic strictureplasty, 5 were treated with strictureplasty according to Finney technique and 6 with strictureplasty according to Heineke-Mikulicz (H-M) technique. Former intestinal resections of the terminal ileum were performed followed by an ileocolic resection in 9 patients of this group. Seventeen CD patients underwent surgery for intestinal obstruction 6 for multiple intestinal fistulas.
No patient had been subjected to biological therapy for at least one month before the surgical operation.
During laparotomy, once the affected intestinal loops were freed from adhesions, 0.2 mL of Patent Blue V were injected using a 25-gauge needle introduced tangentially for a length of 3-4 mm and a depth of 1-2 mm under the surface of the intestinal serosa in macroscopically disease-free intestine some centimeters from the macroscopic lesions, in diseased intestine close to the resection limit and in CD lesions. If skip lesions or those previously treated with strictureplasty were present, Patent Blue V injection was repeated at these sites.
Once the Patent Blue V was injected we observed if and how it spread along the subserosa of the intestinal wall and through the mesenterial layers towards the main lymphatic collectors and eventually the lymph nodes. The following parameters were evaluated at surgery or on the surgical specimen: length of lesions, caliber, maximal thickness of the diseased intestinal wall, thickness of the wall at injection site and thickness of the mesentery at that level. Furthermore, 5 patients (3 males and 2 females, mean age 64.4 years), operated on for colorectal cancer at our Institution during the same period, underwent the same procedure of Patent Blue V injection at the level of the terminal ileum, and they were evaluated as control sample.
Statistical analysis was performed on the data of patients affected by CD. These data collected in a database were checked and underwent a univariate statistical analysis using SPSS software version 15.0 (SPSS Inc. Chicago, IL). All data were studied through a log-rank test and a P value less than 0.05 was assumed as significant. Given the small number of patients, the P value was evaluated only for the parameters homogeneously distributed.
We observed the following three features after the injection of Patent Blue V in the intestinal loops.
Pattern I
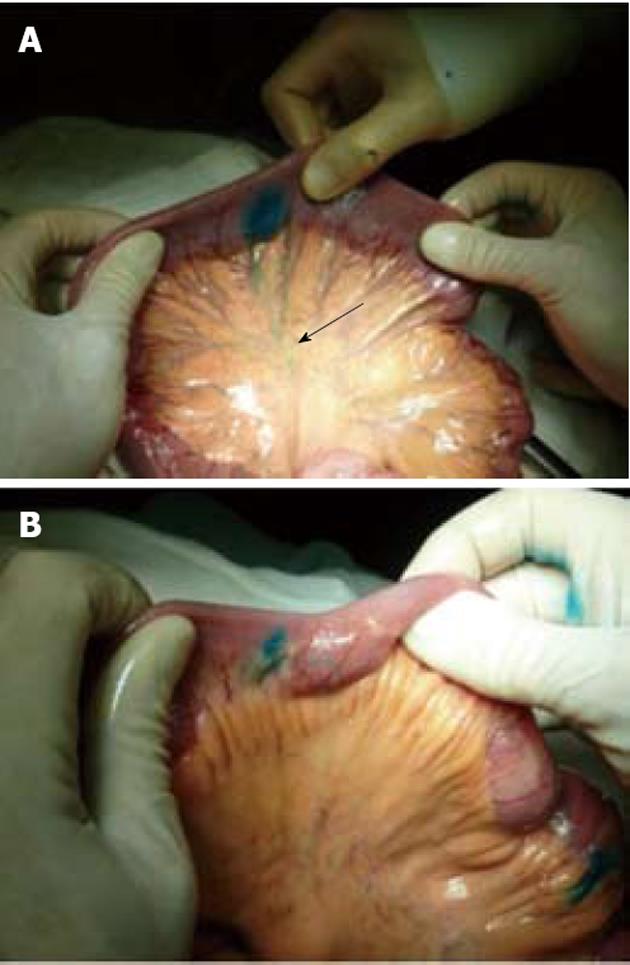
Macroscopically healthy terminal ileum of patients with CD or colon cancer showed thin lymphatic vessels linearly directed toward the mesentery. The dye quickly reaches and colors the node in the mesentery a few centimeters distally (Figure 1).
Pattern II
In mild lesions in which the intestinal wall did not reach 8 mm of thickness, we observed short, wide and tortuous lymphatic vessels directed longitudinally along the intestinal axis either orally or aborally toward disease-free areas and then transversally toward the mesentery. In skip lesions the features were comparable to those we observed in mild lesions (Figure 2).
Pattern III
Injection in the severely affected lesions, that had a thickness of the intestinal wall over 10 mm, did not show any feature of lymphatic vessels at least on the subserosal surface. The dye spread uniformly through the thick intestinal wall and reached the bowel lumen.
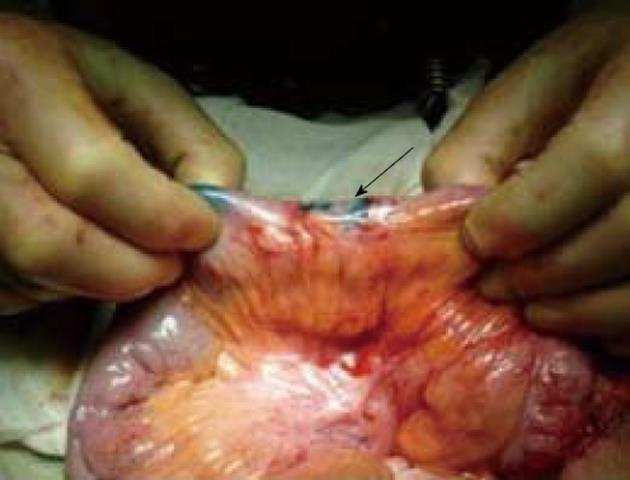
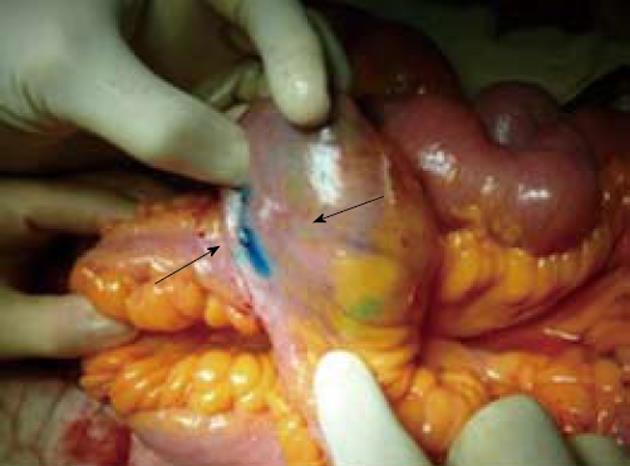
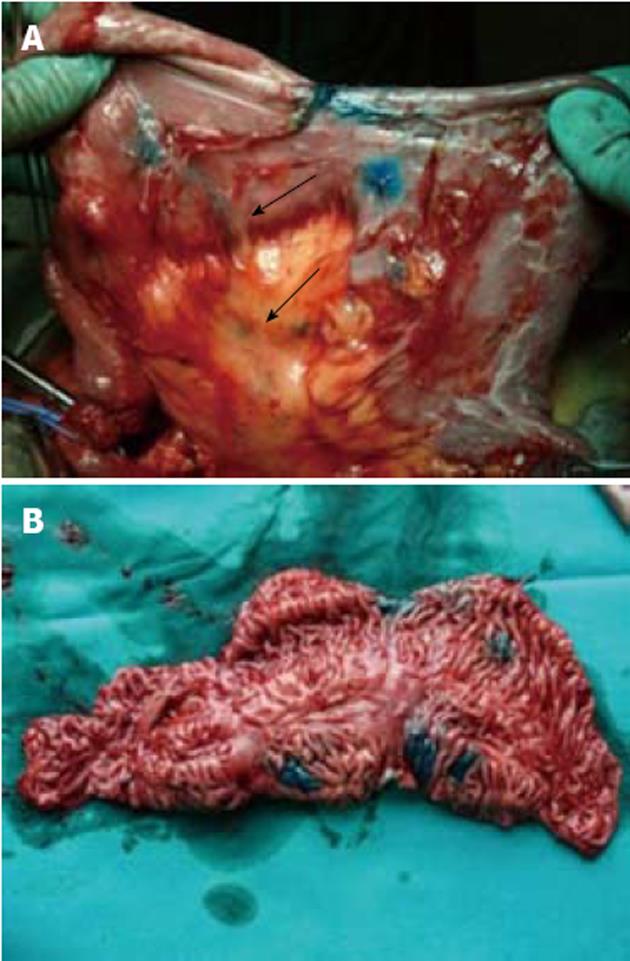
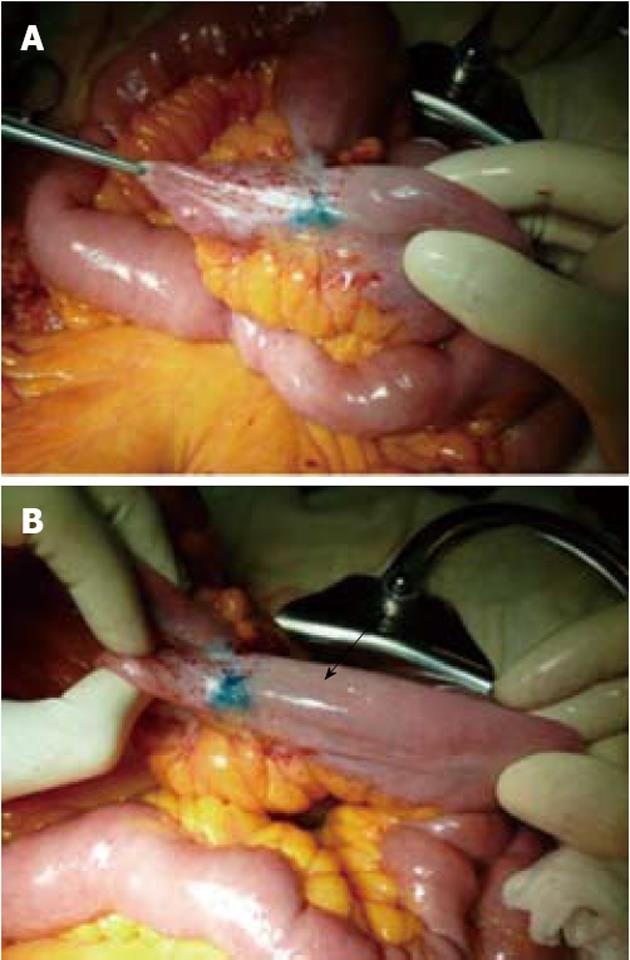
When we injected Patent Blue V in patients undergoing surgery at the site of previous strictureplasty or anastomosis, the dye had two different patterns: (1) If the area was disease-free, the lymphatics appeared linear and thin, like those we found in healthy intestinal loops. This was evident either in some cases of short stenosis previously treated with H-M or Finney strictureplasty, but also in a case of a 36-cm long SISS. During surgery the intestinal tract treated with strictureplasty appeared soft with a thin intestinal wall and thin mesentery, and the injected dye had the features of the healthy intestine (Figures 3 and 4A). Once we opened the specimen the mucosa appeared completely normal without any macroscopic signs of inflammation (Figure 4B); and (2) If the area was affected by persistent or recurrent CD, the lymphatic vessels appeared like those we found in mild or severe lesions depending on the severity of CD. Also some intestinal tracts previously treated by strictureplasty, that did not appear severely affected macroscopically, showed an altered lymphatic pattern with a few short, wide and tortuous lymphatic vessels. In these cases, once we performed an enterotomy at the site of the previous strictureplasty, we found signs of active disease such as ulcers at the mucosal site (Figure 5).
Length of lesions, caliber and maximal thickness of the diseased intestinal wall and thickness of the mesentery at that level have been evaluated. Statistical analysis showed a direct correlation between thickness of the diseased intestinal wall and thickness of its mesentery; furthermore, a significant (P < 0.05) correlation between the different lymphatic patterns and wall thickness was found (Table 1).
| Small bowel wall thickness | Lymphatic vessel pattern | |||
| I | II | III | P value | |
| < 5 mm (n = 17) | 14 (82) | 3 (18) | 0 (0) | < 0.05 |
| 5-8 mm (n = 22) | 2 (9) | 16 (73) | 4 (18) | < 0.05 |
| > 8 mm (n = 16) | 0 (0) | 3 (19) | 13 (81) | < 0.05 |
| P value | < 0.05 | < 0.05 | < 0.05 | |
Although many reports have been clarified the peculiar features of immunologic and inflammatory features of CD, its pathogenesis and the reason why its main localization is the terminal ileum remain unexplained[9]. The inflammatory process of CD is characterized by mucosal ulcers, submucosal edema and transmural diffusion. This process results in parietal thickness and bowel stricture[10]. The peculiar distribution of the inflammation, the segmental intestinal involvement and the prevalent localization in the terminal ileum suggested a lymphatic disorder. Dilation of the lymphatic vessels, occlusion of the lymphatics by endothelial proliferation and/or lymphocytic thrombi, and foci of lymphocytes are scattered throughout all layers of the bowel[11,12]. A specific lymphatic endothelial marker (D2-40), has allowed better characterization of the lymphatic channels in the context of the parietal inflammatory infiltrate[13]. A higher number of lymphatic vessels have been observed in the ileal and colonic wall in comparison to the healthy small and large bowel[14]. The diameter of the lymph vessels in the intestinal wall affected by CD was approximately ten times larger than normal controls and was significantly increased in comparison to other inflammatory bowel diseases, such as ulcerative colitis, ischemic ileitis or pseudomembranous colitis[15]. Interestingly, lymphangiogenesis and lymphangiectasia were present also in normal intestinal tract near the bowel affected by CD[15]. These last aspects suggest that alteration of the lymphatic system could be an inducer of CD[16].
Our study clearly shows an alteration of the course and the features of the lymphatic vessels in the presence of CD bowel. In the tracts markedly affected by CD, the injection of the dye does not color the lymph vessels but is diffused in the subserosal layer. This could be due to the obstruction of the lymphatic vessels or to inflammation and thickening of the intestinal wall that could mask the lymphatic vessels at the subserosal layer.
In the tracts where the disease is less severe there is a distorted, tortuous, increase in size and longitudinally directed lymphatic network. This different aspect is also seen in the areas where CD recurs, either at the site of the ileocolic anastomosis or the strictureplasted bowel.
There is a direct correlation between the different lymphatic patterns and wall thickness (Table 1). We have previously demonstrated a correlation between wall thickness and perforating disease[17]. One of the theories of the formation of fistulas in CD is that it results from the spread of bacteria through altered and dilated obstructed lymphatic vessels[10]. We must assume that when the wall thickness reaches a critical point this happens more easily due to the alteration of the lymphatic network.
The dysfunction of the lymphatic vessels could have an essential role in inducing and maintaining chronic inflammation in CD, similarly to other pathological clinical or experimental situations[18]. On the other hand, the lymphatic vessels play a fundamental part in the resolution of inflammation by draining the activated inflammatory cells into the mesenterial lymph nodes and transporting extravasated fluids from interstitial tissue into circulating blood.
It has been clearly shown that CD strictures can be treated by strictureplasty instead of resection[19-26]. The regression of inflammatory activity of CD at the strictureplasty site has been well documented by radiological, ultrasonographic, endoscopic or histological investigations[27-31]. During a reoperation, the surgeon can appreciate the softness and normal thickness of the healty strictured bowel and the normality of the mesenteric layers[17]. Yamamoto et al[32,33] reviewed the experience with strictureplasty in the literature and, using meta-regressive analysis, showed that strictureplasty is accompanied by a 5-year recurrence rate of 28 percent: in 90 percent of these patients, recurrence occurred at non-strictureplasty sites, and the site-specific recurrence rate is only 3 percent. The mechanism of this inflammatory regression has not been clarified. One hypothesis is that by releasing bowel obstruction the pressure inside the intestine is markedly decreased and consequently the passage of the enteric allogenic or bacterial content through the fissures or ulcers dramatically disappears. This complex mechanism could be triggered by perturbation of the intracellular redox state (glutathione decreases and oxidized glutathione increases)[34]. The myofibroblasts of CD patients exhibit an increased oxidative state due to a decrease in the glutathione/oxidized glutathione ratio and that this alteration is strictly related to upregulated production of interleukin-6[35]. The reduction of endoluminal intestinal pressure where a strictureplasty has been performed, could determine better oxidative status, the progressive reduction of activated myofibroblasts and production of pro-inflammatory cytokines. The decrease in intestinal wall inflammation may induce a progressive disappearance of the obstruction of the lymph vessels. By restoring normal function of the lymphatic vessels, the tissutal edema of the mesentery and of the intestinal wall progressively disappear and finally return to normal.
Furthermore, the injection of Patent Blue V in the intestinal wall could clinically help distinguish healthy tracts of the small bowel from those who have macroscopical borderline inflammatory lesions, making it possible to perform a more appropriate surgical resection during the primary operation or at reoperation for CD.
In conclusion, the presence of lymphatic alterations in CD strictures and their disappearence after strictureplasty, both confirmed in our experience, demonstrates the pathogenetic role of the lymphatic damage in the progression and maintenance of CD and the possibility that strictureplasty restores a normal configuration of the lymphatic vessels and consequently a normal lymphatic function in the intestinal wall.
Crohn’s disease (CD) is a chronic inflammation occurring mainly in the terminal ileum, but potentially affecting any part of the intestinal tract. Although in recent years several studies had clarified that CD occurs in genetically predisposed subjects and is associated with defective intestinal permeability and compromised immune response, a definitive etiology remains unknown. Pathologists who have studied surgical specimens of CD describe the microscopic appearance of the disease as a chronic lymphangitis
The primary aim of this paper is to observe the status of the lymphatic vessels in the small bowel of CD patients at the time of primary surgery or of recurrence of the disease by injecting Patent Blue V (a dark bluish synthetic dye used in lymphangiography and for sentinel node biopsy as a dye to color lymph vessels) in the subserosa of the affected intestinal loops. The opportunity to perform recurring surgery in some CD patients allowed us to observe the status of intestinal lymph vessels after previous strictureplasties and to try to clarify whether a “restitutio ad integrum” of lymphatic channels is accompanied by regression of inflammatory lesions: it is the secondary aim of this paper.
During the period January 2011-June 2011 25 consecutive patients affected by CD were operated on in the authors’ Institution. During surgery, Patent Blue V was injected subserosally and the way it spread along the subserosa of the intestinal wall, through the mesenterial layers towards the main lymphatic collectors and eventually to the lymph nodes was observed and recorded. Since some patients had been undergone strictureplasty at previous surgery, the authors also examined the status of intestinal lymph vessels after previous strictureplasties. The same procedure was performed in a control group of 5 patients affected by colorectal cancer. Length of lesions, caliber, maximal thickness of the diseased intestinal wall, thickness of the wall at injection site and thickness of the mesentery were evaluated at surgery.
The authors found that there was a correlation between the thickness of the parietal wall and the severity of the lymphatic alterations. Normal lymphatic vessels were observed at previous strictureplasties in the presence of complete regression of the inflammation. Injection of Patent Blue V in the intestinal wall could help distinguish healthy tracts of the small bowel from those macroscopically borderline.
The article revealed a correlation between small bowel wall thickness in CD and alteration of the lymphatic vessels, using Ptent Blue infection method. The authors discussed that such alteration in lymphatic drainage may contribute to the pathogenesis of chronic inflammation in CD.
Peer reviewer: Tsuyoshi Konishi, MD, PhD, Department of Gastroenterological Surgery, Cancer Institute Hospital, 3-10-6 Ariake, Koto-ku, Tokyo 135-8550, Japan
S- Editor Wen LL L- Editor A E- Editor Xiong L
| 1. | Van Kruiningen HJ, Colombel JF. The forgotten role of lymphangitis in Crohn's disease. Gut. 2008;57:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Blackburn G, Hadfield G, Hunt AH. Regional ileitis. St Bart's Hosp Rep. 1939;72:181–224. [Cited in This Article: ] |
| 3. | Warren S, Sommers SC. Cicatrizing enteritis as a pathologic entity; analysis of 120 cases. Am J Pathol. 1948;24:475-501. [PubMed] [Cited in This Article: ] |
| 4. | Van Patter WN. Pathology and pathogenesis of regional enteritis (Dissertation). Minneapolis, Minn. : University of Minnesota 1952; . [Cited in This Article: ] |
| 5. | Kalima TV. Experimental lymphatic obstruction in the ileum. Ann Chir Gynaecol Fenn. 1970;59:187-201. [PubMed] [Cited in This Article: ] |
| 6. | Kalima TV, Saloniemi H, Rahko T. Experimental regional enteritis in pigs. Scand J Gastroenterol. 1976;11:353-362. [PubMed] [Cited in This Article: ] |
| 7. | Pace M, Cardona G, Cataliotti L, Brugnola D, Pirillo M, Bandettini L, Nannelli A. Experimental induction, by a personal method, of chronic lymphatic block of the small intestine: reproduction of the histogenesis of Crohn’s enteritis (Preliminary report). Surgery in Italy. 1973;3:4. [Cited in This Article: ] |
| 8. | Danese CA, Georgalas-Penesis M, Kark AE, Dreiling DA. Studies of the effects of blockage of intestinal lymphatics. I. Experimental procedure and structural alterations. Am J Gastroenterol. 1972;57:541-546. [PubMed] [Cited in This Article: ] |
| 9. | Caprilli R. Why does Crohn's disease usually occur in terminal ileum? J Crohns Colitis. 2008;2:352-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Tonelli P. il linfedema intestinale come causa della malattia di Crohn. Il Gastroenterologo. 2008;30:4. [Cited in This Article: ] |
| 11. | Baumgart DC. What's new in inflammatory bowel disease in 2008? World J Gastroenterol. 2008;14:329-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650-5659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Geleff S, Schoppmann SF, Oberhuber G. Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch. 2003;442:231-237. [PubMed] [Cited in This Article: ] |
| 15. | Pedica F, Ligorio C, Tonelli P, Bartolini S, Baccarini P. Lymphangiogenesis in Crohn's disease: an immunohistochemical study using monoclonal antibody D2-40. Virchows Arch. 2008;452:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1399] [Cited by in F6Publishing: 1425] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 17. | Tonelli F, Ficari F. Strictureplasty in Crohn's disease: surgical option. Dis Colon Rectum. 2000;43:920-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Yamamoto T, Umegae S, Kitagawa T, Matsumoto K. Postoperative change of mucosal inflammation at strictureplasty segment in Crohn's disease: cytokine production and endoscopic and histologic findings. Dis Colon Rectum. 2005;48:749-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Futami K, Arima S. Role of strictureplasty in surgical treatment of Crohn's disease. J Gastroenterol. 2005;40 Suppl 16:35-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Fearnhead NS, Chowdhury R, Box B, George BD, Jewell DP, Mortensen NJ. Long-term follow-up of strictureplasty for Crohn's disease. Br J Surg. 2006;93:475-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, Imbesi V, Molteni M, Danelli PG, Taschieri AM. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology. 2004;127:730-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Michelassi F, Upadhyay GA. Side-to-side isoperistaltic strictureplasty in the treatment of extensive Crohn's disease. J Surg Res. 2004;117:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Shatari T, Clark MA, Yamamoto T, Menon A, Keh C, Alexander-Williams J, Keighley M. Long strictureplasty is as safe and effective as short strictureplasty in small-bowel Crohn's disease. Colorectal Dis. 2004;6:438-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Dietz DW, Laureti S, Strong SA, Hull TL, Church J, Remzi FH, Lavery IC, Fazio VW. Safety and longterm efficacy of strictureplasty in 314 patients with obstructing small bowel Crohn's disease. J Am Coll Surg. 2001;192:330-337; discussion 337-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Roy P, Kumar D. Strictureplasty for active Crohn's disease. Int J Colorectal Dis. 2006;21:427-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Sampietro GM, Cristaldi M, Maconi G, Parente F, Sartani A, Ardizzone S, Danelli P, Bianchi Porro G, Taschieri AM. A prospective, longitudinal study of nonconventional strictureplasty in Crohn's disease. J Am Coll Surg. 2004;199:8-20; discussion 20-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lawrance IC, Welman CJ, Shipman P, Murray K. Correlation of MRI-determined small bowel Crohn's disease categories with medical response and surgical pathology. World J Gastroenterol. 2009;15:3367-3375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 61] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Vogel J, da Luz Moreira A, Baker M, Hammel J, Einstein D, Stocchi L, Fazio V. CT enterography for Crohn's disease: accurate preoperative diagnostic imaging. Dis Colon Rectum. 2007;50:1761-1769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn's disease. Am J Gastroenterol. 2007;102:2541-2550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Paulsen SR, Huprich JE, Fletcher JG, Booya F, Young BM, Fidler JL, Johnson CD, Barlow JM, Earnest F. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics. 2006;26:641-657; discussion 657-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 345] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 31. | Yamamoto T. Factors affecting recurrence after surgery for Crohn's disease. World J Gastroenterol. 2005;11:3971-3979. [PubMed] [Cited in This Article: ] |
| 32. | Yamamoto T. The current status of strictureplasty for Crohn’s disease. CML Gastroenterology. 2007;26:57. [Cited in This Article: ] |
| 33. | Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn's disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50:1968-1986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Catarzi S, Favilli F, Romagnoli C, Marcucci T, Picariello L, Tonelli F, Vincenzini MT, Iantomasi T. Oxidative state and IL-6 production in intestinal myofibroblasts of Crohn's disease patients. Inflamm Bowel Dis. 2011;17:1674-1684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Wittig BM, Zeitz M. Critical comment: analyzing the effect of novel therapies on cytokine expression in inflammatory bowel disease: do cytokine levels reflect clinical response? Int J Colorectal Dis. 2006;21:505-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |