Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1097
Peer-review started: December 30, 2023
First decision: January 16, 2024
Revised: February 7, 2024
Accepted: March 5, 2024
Article in press: March 5, 2024
Published online: April 27, 2024
Roux-en-Y gastric bypass (RYGB) is a widely recognized bariatric procedure that is particularly beneficial for patients with class III obesity. It aids in significant weight loss and improves obesity-related medical conditions. Despite its effectiveness, postoperative care still has challenges. Clinical evidence shows that venous thromboembolism (VTE) is a leading cause of 30-d morbidity and mortality after RYGB. Therefore, a clear unmet need exists for a tailored risk assessment tool for VTE in RYGB candidates.
To develop and internally validate a scoring system determining the individualized risk of 30-d VTE in patients undergoing RYGB.
Using the 2016–2021 Metabolic and Bariatric Surgery Accreditation Quality Improvement Program, data from 6526 patients (body mass index ≥ 40 kg/m2) who underwent RYGB were analyzed. A backward elimination multivariate analysis identified predictors of VTE characterized by pulmonary embolism and/or deep venous thrombosis wi
Of the 26 initial variables, six predictors were identified. These included a history of chronic obstructive pulmonary disease with a regression coefficient (Coef) of 2.54 (P < 0.001), length of stay (Coef 0.08, P < 0.001), prior deep venous thrombosis (Coef 1.61, P < 0.001), hemoglobin A1c > 7% (Coef 1.19, P < 0.001), venous stasis history (Coef 1.43, P < 0.001), and preoperative anticoagulation use (Coef 1.24, P < 0.001). These variables were weighted according to their regression coefficients in an algorithm that was generated for the model predicting 30-d VTE risk post-RYGB. The risk model's area under the curve (AUC) was 0.79 [95% confidence interval (CI): 0.63-0.81], show
This simple risk model uses only six variables to assist clinicians in the preoperative risk stratification of RYGB patients, offering insights into factors that heighten the risk of VTE events.
Core Tip: Venous thromboembolism (VTE) is an uncommon but important cause of morbidity and mortality following Roux-en-Y gastric bypass (RYGB). Clinical evidence regarding VTE risk stratification after RYGB remains limited. Using a multicenter database, this is the first retrospective cross-sectional study that used supervised machine learning to develop and internally validate a scoring system to assess the 30-d individualized risk of VTE post-RYGB. Our model uses only six preoperative variables, including a history of chronic obstructive pulmonary disease, length of stay, previous deep venous thrombosis, hemoglobin A1c > 7%, prior venous stasis, and preoperative anticoagulation use. Our findings may help to improve clinical outcomes and procedural safety for patients undergoing RYGB.
- Citation: Ali H, Inayat F, Moond V, Chaudhry A, Afzal A, Anjum Z, Tahir H, Anwar MS, Dahiya DS, Afzal MS, Nawaz G, Sohail AH, Aziz M. Predicting short-term thromboembolic risk following Roux-en-Y gastric bypass using supervised machine learning. World J Gastrointest Surg 2024; 16(4): 1097-1108
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1097.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1097
Obesity is a chronic, relapsing disease that has numerous physical, psychological, and metabolic ramifications[1]. The World Obesity Atlas 2023 report states that 38% of the global population is currently overweight or obese, and the prevalence is projected to rise to 51% by 2035[2]. In the United States, the rates of severe obesity have increased from 1.5% in 1971/74 to 9% in 2017/20[3]. Recent advances in drug therapy have revolutionized obesity treatment, but anti-obesity medications may have questionable safety and inadequate efficacy for sustained weight loss[4,5]. Therefore, bariatric surgery procedures such as Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy have gained preeminence over the past decade[6,7]. A systematic review of 26 studies showed that bariatric surgery may be a clinically and financially advantageous choice for patients with moderate and severe obesity when compared to non-surgical therapy[8]. Con
Venous thromboembolism (VTE), such as pulmonary embolism (PE) and deep vein thrombosis (DVT), is an uncommon surgical sequela of bariatric surgery. However, it is a major cause of postoperative morbidity and mortality. The rate of VTE in bariatric patients ranges from 0.3% to 2.4%[21-24]. In a meta-analysis of 71 studies based on 107874 patients, the < 30-d PE rate following bariatric surgery was 1.17%[25]. Although PE has an incidence of around 1%, it accounts for 40% of all 30-d postoperative deaths[26-28]. Therefore, it is one of the major causes of mortality after bariatric surgery. In an analysis of the Nationwide Readmission Database, Mabeza et al[29] showed that bariatric surgery patients with VTE had a higher risk of mortality, an increased length of stay, and higher in-patient charges in their index hospital admission. Obesity is a hypercoagulability state, and it also leads to several systemic complications that may increase the risk of VTE[30]. Bariatric surgery involves several complex steps. Factors like long procedure durations, challenging surgery, the necessity for surgical reinterventions, and the need for blood transfusions may also increase VTE risk[31]. It is a preventable cause of morbidity and mortality. Therefore, effective prognostication may help to avoid postoperative VTE in patients undergoing bariatric surgery.
A number of assessment tools have previously been developed to predict the risk of VTE following bariatric surgery[32-37]. However, these models differ significantly in terms of high-risk criteria, predictive performance, and inclusion characteristics[38]. Furthermore, VTE risk factors may also vary based on bariatric surgery procedures, such as sleeve gastrectomy or RYGB[39]. Recent developments in artificial intelligence offer a chance to improve the accuracy of risk stratification through effective analysis of large volumes of patient data. Recently, Hsu et al[40] developed a machine learning model that outperformed logistic regression in predicting postoperative gastrointestinal bleeding in bariatric surgery patients. However, machine learning has not been used in VTE risk prediction in bariatric patients. Therefore, to our knowledge, this is the first study to develop and internally validate a 30-d RYGB-specific VTE risk scoring system using supervised machine learning. Our model can help mitigate the risk of VTE by identifying modifiable precipitating factors, setting reasonable expectations, and promoting communication between patients and healthcare providers. Moreover, our findings could contribute to the available clinical evidence, thereby further refining the recommendations for perioperative VTE prophylaxis[41].
This retrospective cross-sectional study is based on the Metabolic and Bariatric Surgery Accreditation Quality Impro
This research used data from the MBSAQIP database from 2016 to 2021. Patients with a baseline body mass index (BMI) ≥ 40 kg/m2 who had undergone RYGB were identified. The event of interest was the occurrence of postoperative VTE, such as PE and/or DVT, within 30 d after the procedure. Consistent with prior research, cases including individuals under the age of 18, those with a BMI below the cutoff point, missing or miscoded data, and those who had undergone revision or conversion procedures were excluded[43]. Preselected clinical factors (only preoperative variables) were considered based on existing scientific insights and recent scholarly works[24]. The variables with incomplete data were omitted. A correlation assessment was conducted to screen all potential explanatory variables for collinearity (Supplementary Figure 1). Factors that demonstrated a correlation score above 0.7 were removed. The short list of risk variables included patient demographics, clinical factors, and laboratory results.
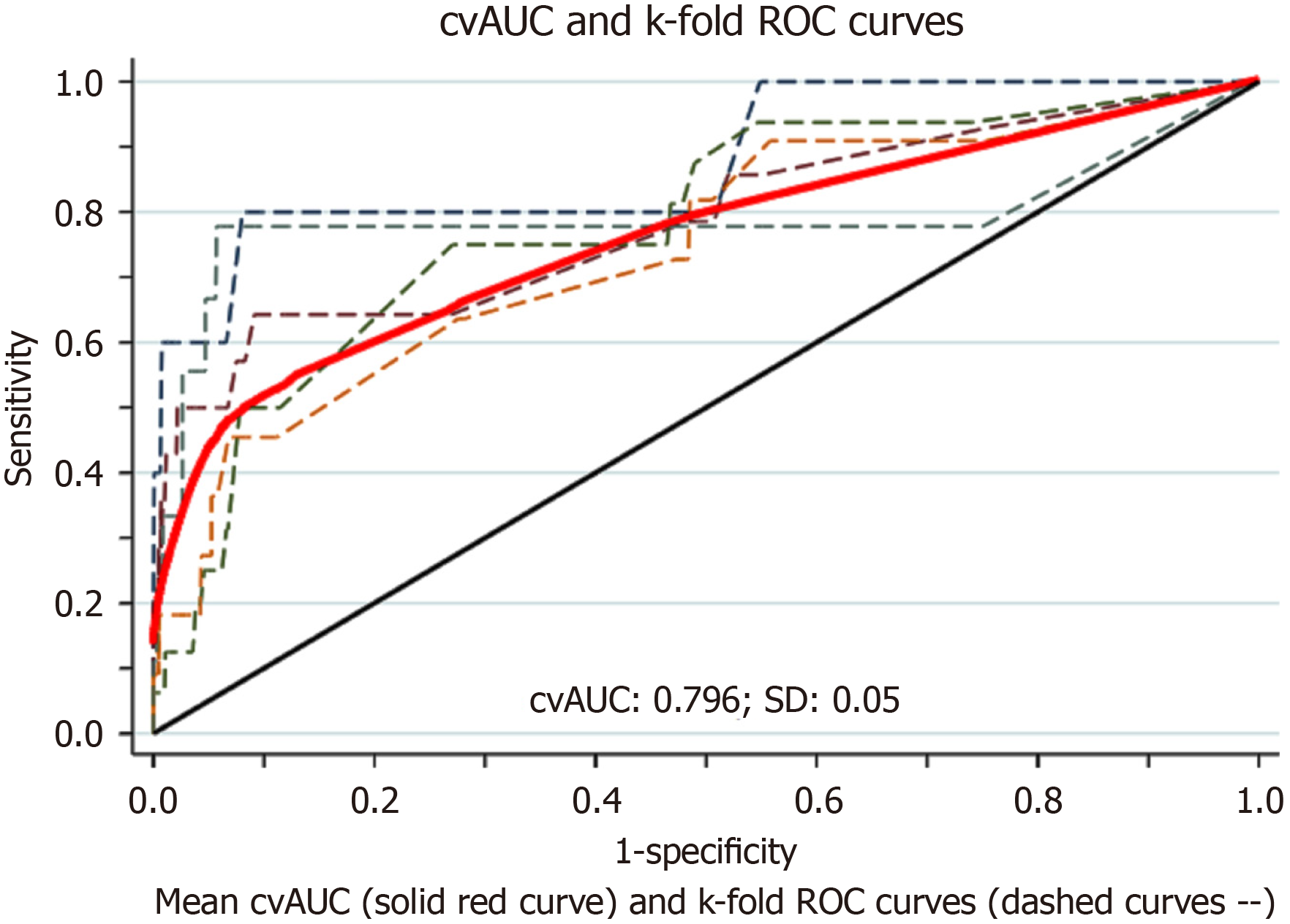
The "pmsampsize" command in the Statistical Software for Data Science (StataCorp LLC, College Station, TX) was used to determine the smallest sample size needed to generate a novel multivariable predictive model with a binary result; the anticipated C-statistic was 0.80, and the possible inclusions for this new predictive model were numbered at 26. The required minimum sample size for the risk assessment was specified as 2108. A backward elimination multivariate regression model was employed to isolate the significant predictors of the 30-d VTE risk with a cutoff P value of < 0.05. This method zeroed out the coefficients of less important variables, effectively eliminating them. To estimate the model's predictive quality, a 5-fold cross-validation method was applied. It helps avoid overly optimistic performance evaluations in neglected cases[44]. The area under the curve (AUC) was used to judge the model's discriminatory power for 30-d VTE risk, with values ranging as described[45].
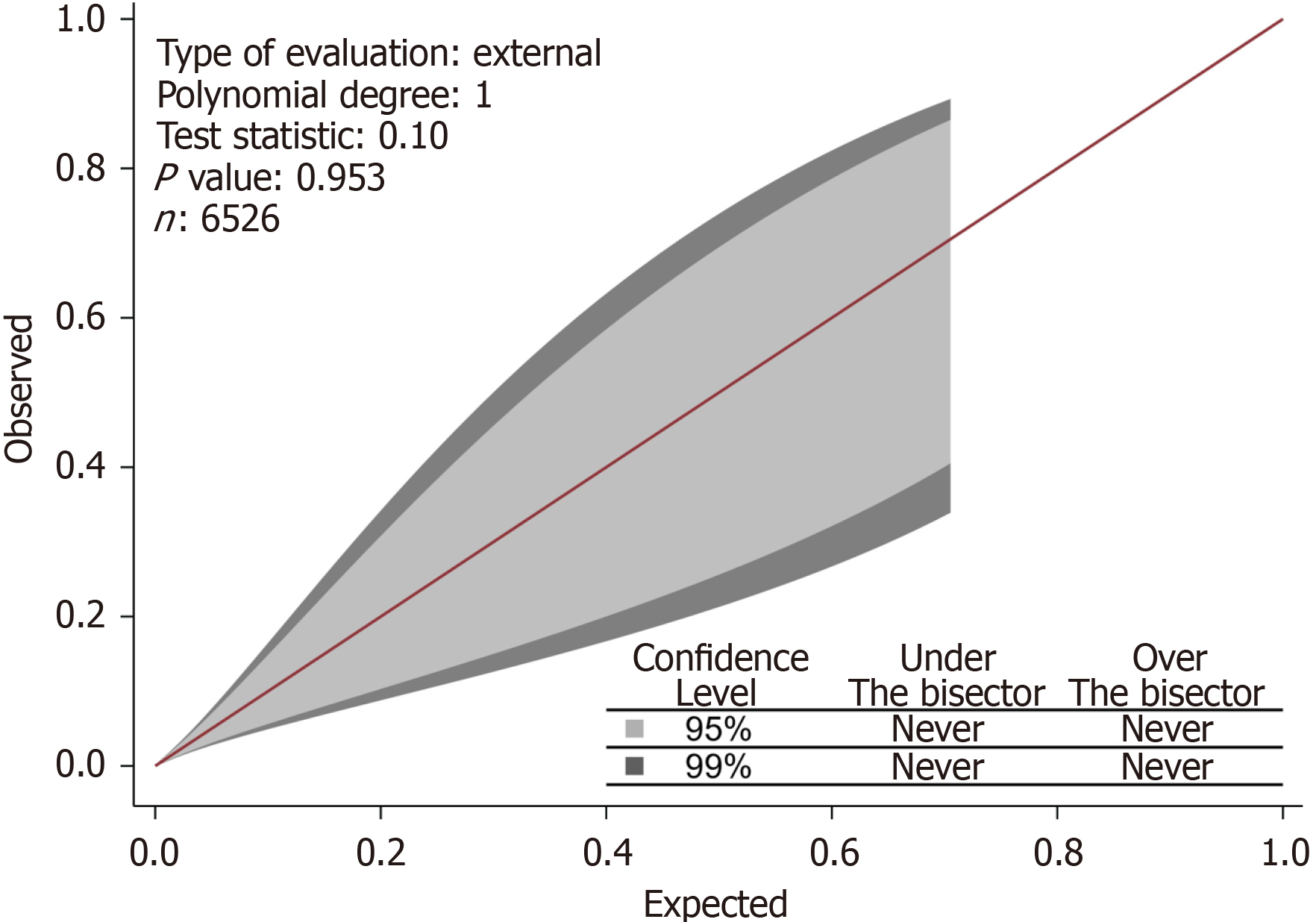
The match between the model-predicted and actual 30-d VTE risk was examined via calibration belt plots, enabling a visual comparison between expected and observed frequencies and the orientation of any miscalibration[46]. The plot included a calibration test to determine if any deviations from the line of perfect fit (bisector at 45 degrees) were sig
The MBSAQIP is based on de-identified aggregated data with accepted privacy standards in accordance with the guidelines for human subject research. In order to comply with privacy regulations, the third party acquired and de-identified the data, ensuring that no individual could be recognized either directly or indirectly. We adhered to all ethical standards in utilizing this dataset for research purposes. Therefore, this study did not require institutional review board approval. Moreover, informed consent did not apply as the patient data were anonymized.
A total of 6526 patients were included in the present analysis. The demographic and clinical characteristics are outlined (Table 1). The 30-d VTE rate for RYGB cases was 0.84%. Six out of the 26 predictors were retained by the backward regression for predicting 30-d VTE, including a history of preoperative chronic obstructive pulmonary disease (COPD) with a regression coefficient (Coef) of 2.54 [95% confidence interval (CI): 1.57-3.51, P < 0.001], length of stay (Coef 0.08, 95%CI: 0.06-0.11, P < 0.001), a history of DVT (Coef 1.62, 95%CI: 0.31-2.92, P = 0.015), hemoglobin A1c (HbA1c) > 7% (Coef 1.19, 95%CI: 0.52-1.86, P = 0.001), venous stasis history (Coef 1.44, 95%CI: 0.40-2.48, P = 0.007), and preoperative anticoagulation (Coef 1.24, 95%CI: 0.01-2.48, P = 0.049) (Table 2).
| Factor | Value |
| N | 6526 |
| Postoperative VTE within 30 d (%) | 49 (0.8) |
| Age, median (IQR) | 44 (36.1, 52.81) |
| Age > 50 yr (%) | 2083 (31.9) |
| Female gender (%) | 5282 (80.9) |
| Preop GERD requiring medication (%) | 2477 (38.0) |
| Preop history of MI (%) | 67 (1.0) |
| Previous PCI (%) | 140 (2.1) |
| Previous cardiac surgery (%) | 48 (0.7) |
| Preop hypertensive requiring medication (%) | 3340 (51.2) |
| Preop hyperlipidemia (%) | 1691 (25.9) |
| Preop weight closest to bariatric surgery, median (IQR) | 287 (256, 329) |
| Preop BMI closest to bariatric surgery, median (IQR) | 47.61 (44.26, 51.75) |
| Preop vein thrombosis (%) | 128 (2.0) |
| Preop venous stasis (%) | 195 (3.0) |
| Preop requiring or on dialysis (%) | 7 (0.1) |
| Preop renal insufficiency/CKD (%) | 56 (0.9) |
| Preop therapeutic anticoagulation (%) | 201 (3.1) |
| Previous foregut surgery (%) | 710 (10.9) |
| Current smoker within 1 yr (%) | 546 (8.4) |
| Preop history of COPD (%) | 126 (1.9) |
| Preop obstructive sleep apnea | 3196 (49.0) |
| Preop steroid/immunosuppressant use for chronic condition (%) | 113 (1.7) |
| Preop history of IVC filter placement (%) | 25 (0.4) |
| Preop history of DM (%) | 2009 (30.8) |
| Preop albumin (g/dL), median (IQR) | 3.9 (3.4, 4.2) |
| Preop serum creatinine (mg/dL), median (IQR) | 0.77 (0.69, 0.85) |
| Preop hematocrit (%), median (IQR) | 40.3 (37.7, 42.8) |
| Preop hemoglobin A1c > 7% | 741 (11.4) |
| Variables | Regression coefficient | Standard error | t value | P value | 95% confidence interval (lower) | 95% confidence interval (upper) |
| Preoperative COPD | 2.54 | 0.49 | 5.15 | 0 | 1.57 | 3.51 |
| Length of stay | 0.08 | 0.01 | 6.24 | 0 | 0.06 | 0.11 |
| Deep vein thrombosis history | 1.62 | 0.67 | 2.42 | 0.02 | 0.31 | 2.92 |
| Hemoglobin A1c level | 1.19 | 0.34 | 3.47 | 0 | 0.52 | 1.86 |
| History of venous stasis | 1.44 | 0.53 | 2.7 | 0.01 | 0.4 | 2.48 |
| Preoperative anticoagulation use | 1.24 | 0.63 | 1.97 | 0.05 | 0.01 | 2.48 |
| Model constant | -5.77 | 0.28 | -20.61 | 0 | -6.32 | -5.22 |
Using this multivariable model, a risk model was inferred based on the six factors weighted by their regression coefficients in the multivariable logistic regression model. It is represented by the following equation: VTE risk score = (2.54 × preoperative COPD) + (0.08 × length of stay) + (1.62 × previous DVT) + (1.19 × HbA1c > 7%) + (1.44 × venous stasis history) + (1.24 × preoperative anticoagulation use) - 5.77. The optimal cutoff point for the risk score was determined using the Youden index. This index maximizes the sum of sensitivity and specificity for each available cutoff and selects the maximum sum. For our risk score model, the optimal cutoff point was approximately -4.2 (high risk vs low risk).
With this cutoff, the model yielded a sensitivity of 60% and a specificity of 91%. The area under the receiver operating characteristic curve was 0.79 (95%CI: 0.63-0.80) (Figure 1). Without training, the same model performed satisfactorily in patients with laparoscopic sleeve gastrectomy with an AUC of 0.63 (95%CI: 0.62-0.64) and endoscopic sleeve gastroplasty with an AUC of 0.76 (95%CI: 0.75-0.78). The 95% and 99% confidence levels of the calibration belt plot indicated that there was no discernible miscalibration. At 95% confidence levels (the inner belt or light gray region) and 99% confidence levels (the outer belt or dark gray area), the predicted model probability matched the observed result rate (Figure 2).
This is the first population-based study that used supervised machine learning to develop and internally validate a scoring system based on only six preoperative variables to assess the individual risk of VTE within the first 30 d post-RYGB. The variables included a history of COPD, length of stay, previous DVT, HbA1c > 7%, venous stasis history, and prior anticoagulation use. Our simple model could aid clinicians in the preoperative risk stratification of RYGB patients for VTE. It may constitute a crucial step to improve clinical outcomes and procedural safety for bariatric patients.
VTE, including PE and DVT, continues to be a significant concern for postoperative morbidity and mortality in patients undergoing bariatric surgery[49]. Existing data suggest that PE affects 0.3%-2% of bariatric patients, and DVT develops in 1%-3% of cases[50,51]. The majority of VTE events following bariatric surgery have been reported after hospital discharge but within the 30-d postoperative period[52]. A number of risk factors have been recognized, including a higher BMI, increased age, male sex, past instances of VTE, obesity-related hypoventilation issues, limited mobility, pulmonary hyper
Our study suggested that preoperative COPD is an independent risk factor for VTE after RYGB. A national study revealed that preoperative COPD may double the risk of pulmonary complications after surgery[56]. Lawrence et al[57] suggested that symptoms such as diminished breath sounds, extended expiration, decreased oxygen levels, and whee
Previous VTE may serve as one of the predictors of thromboembolic complications in bariatric surgery patients. A case-cohort study revealed that patients who had surgery and were subsequently hospitalized following their initial VTE episode were at an increased risk of developing recurrent in-hospital VTE in comparison to those patients who had a VTE history but did not undergo surgery[60]. Furthermore, in a retrospective cohort study, Bahl et al[32] demonstrated that 4.2% of patients with a history of VTE developed recurrent VTE within 30 d of surgery. Nemeth et al[61] revealed that patients with a history of VTE undergoing gastrointestinal surgeries had a high risk of VTE recurrence at six months (hazard ratio, 8.4; 95%CI: 4.0-17.8). Similarly, Chao et al[62] also showed in their retrospective study that a history of VTE was the greatest driver of VTE post-bariatric surgery. Our results also validated these findings, as patients with a history of VTE were found to have a significantly increased risk of developing a recurrent VTE post-RYGB. Considering that VTE is a preventable cause of inpatient death and 60% of VTE incidents occur during or post-hospitalization, it is crucial to recognize the elevated risk of recurrence related to surgical procedures in patients with a prior history of VTE[63]. Pre
Studies evaluating the impact of glycemic control on the risk of VTE in patients with diabetes mellitus (DM) showed marked heterogeneity. Some studies have identified a positive correlation between increased glucose levels and the risk of developing VTE[68,69]. However, Lerstad et al[70] found no such association in their multivariable analysis of a Norwegian population-based cohort. Notably, their results demonstrated that the risk of VTE climbed by 5% per one standard deviation (0.7%) elevation of HbA1c level[70]. In a case-control analysis, Charlier et al[71] suggested that women with type 2 DM with HbA1c levels above 7% might have a marginally elevated risk of unprovoked VTE compared to those with HbA1c levels between 6.5% and 7%. However, no studies have specifically delved into the occurrence of VTE and its related risk factors post-bariatric surgery in diabetic patients. Our findings indicate a statistically significant increase in VTE risk among patients with an HbA1c level exceeding 7%.
The use of stepwise backward regression in this study represents a departure from traditional logistic regression approaches, adding a layer of complexity and predictive accuracy. This method allowed for efficient variable selection, pinpointing the most relevant risk factors from a broad set of potential predictors. This streamlining is particularly va
Sheikhtaheri et al[74] formulated a model using an artificial neural network (ANN) algorithm to forecast complications after one-anastomosis gastric bypass surgery within a 90-d timeframe. This model accounted for complications like bleeding, leakage at the anastomotic site, obstruction, an abscess within the abdomen, and PE[74]. The prediction model incorporated 32 factors, including age, BMI, smoking habits, and laboratory test outcomes[74]. Remarkably, the model showcased its peak accuracy (AUC score: 0.98) during the first 10 d post-surgery[74]. Similarly, Cao et al[75] utilized various machine learning methods to identify significant complications occurring within a month of bariatric surgery. Their study incorporated algorithms like decision trees, random forests, gradient boosting, support vector machine (SVM), and ANN[75]. The outcomes demonstrated model performances as follows: the decision tree achieved an accuracy of 92% with an AUC of 0.5, the random forest had 95% accuracy with 0.51 AUC, gradient boosting presented 96% accuracy with 0.58 AUC, SVM recorded 96% accuracy with an AUC of 0.5, and ANN reported 96% accuracy with a 0.54 AUC[75].
Cao et al[76] utilized ANN and convolutional neural network (CNN) models to forecast significant complications within 30 d post-bariatric surgery. They categorized serious complications using the Clavien-Dindo classification of grade 3b or above, which includes issues like anastomotic leakage, organ failure, or even death[76]. The effectiveness of each model was measured using accuracy and AUC metrics. Specifically, the ANN model achieved 84% accuracy with an AUC of 0.54, while the CNN model reached 95% accuracy with an AUC of 0.57 in predicting post-surgery complications[76]. Based on a cohort of 101721 laparoscopic sleeve gastrectomy patients, Wise et al[77] predicted a readmission rate of 3.1%, a reoperation and reintervention rate of 8.7%, and a mortality rate of 0.07% within 30 d following the procedure. Utilizing an ANN model, they identified an AUC of 0.59. Additionally, the following seven factors appeared to be necessary for the prediction of 30-d morbidity and mortality: Age, race, BMI, hypertension, DM, functional capacity, and a history of prior surgery[77].
The published research indicates that despite an increase in comprehensive studies on RYGB surgery, prediction of short- and long-term complications remains difficult. The American Society of Metabolic and Bariatric Surgery and the International Federation for the Surgery of Obesity and Metabolic Disorders have updated indications for bariatric surgery[78]. As a result, it is expected that the bariatric surgery procedure numbers will further increase. It underscores the clinical importance of a meticulous evaluation of all probable risk factors and a tailored algorithm to prevent complications. This is one area where supervised machine learning techniques might be particularly beneficial to reduce the risk of VTE after bariatric surgery. Our study shows that crafting an algorithm to evaluate the 30-d VTE risk following RYGB would enhance the understanding of related factors and equip the surgeon and the patient with the insights needed for informed decision-making.
There are certain limitations to our study. The primary factor was the lack of exact predictors that could improve the accuracy of the risk model, such as hospital-level data. Moreover, gathering detailed data on specific procedures and center volumes posed additional difficulties. The applicability of our model to a broader global population might be restricted as the sample population only involved patients from North America. Nonetheless, it is important to highlight that the study used robust 5-fold cross-validation, reinforcing the reliability of the predictive model despite these limi
This study utilized supervised machine learning to develop a preoperative risk stratification model for VTE in patients undergoing RYGB. Our simple risk model predicting 30-d VTE risk after the RYGB procedure incorporated only six variables, including a history of COPD, length of stay, prior DVT, HbA1c, a history of venous stasis, and preoperative anticoagulation use. This model has pertinent clinical implications as a preliminary VTE risk assessment method for RYGB candidates. Therefore, our results may help to avoid VTE-related morbidity and mortality in patients undergoing bariatric surgery. Future population-based studies are warranted to externally validate our findings.
The escalating global prevalence of obesity has prompted the advancement of various therapeutic interventions. Roux-en-Y gastric bypass (RYGB) has established efficacy, particularly for class III obesity. However, despite its benefits, postoperative complications like venous thromboembolism (VTE) remain a significant concern due to their contribution to morbidity and mortality within 30 d post-surgery. This study addresses the critical gap in clinical risk stratification and predictive modeling for VTE post-RYGB.
This research is driven by the need to develop a simple and reliable RYBG-specific predictive model for VTE. The goal is to mitigate the 30-d morbidity and mortality associated with VTE by enabling clinicians to identify high-risk individuals through a validated scoring system, thereby guiding preventive strategies and optimizing patient management post-RYGB.
The primary objective of this study was to construct and internally validate a scoring system for the prediction of individualized VTE risk within 30 d after RYGB. By focusing on preoperative variables, the study aimed to deliver a practical tool for clinicians to enhance preoperative risk stratification and improve overall patient outcomes.
Utilizing data from the Metabolic and Bariatric Surgery Accreditation Quality Improvement Program database, this re
Our study based on multivariate analysis identified six significant predictors: A history of chronic obstructive pulmonary disease, length of stay, prior deep venous thrombosis, hemoglobin A1c, a history of venous stasis, and preoperative anticoagulation use, each quantified by robust regression coefficients. The derived risk model exhibited commendable predictive performance with an area under the curve of 0.79, sensitivity of 0.60, and specificity of 0.91. This model also demonstrated satisfactory predictive capability in laparoscopic sleeve gastrectomy and endoscopic sleeve gastroplasty populations.
Our study concludes that the devised risk model, underpinned by supervised machine learning, constitutes a significant step forward in preoperative risk stratification for VTE. It provides a clinically relevant, evidence-based tool that sim
Our model stands out for its simplicity and clinical applicability, potentially aiding in the preoperative assessment of VTE risk and the tailoring of prophylactic measures. Future research should focus on external validation of the scoring system across diverse populations and healthcare settings. Moreover, incorporating additional variables, such as perioperative data, may further refine the predictive capability of the model. Expansion to include other surgical procedures may also be considered, broadening the scope and impact of the research findings.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soldera J, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | Allison DB, Downey M, Atkinson RL, Billington CJ, Bray GA, Eckel RH, Finkelstein EA, Jensen MD, Tremblay A. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity (Silver Spring). 2008;16:1161-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Lobstein T, Jackson-Leach R, Powis J, Brinsden H, Gray M. World Obesity Atlas 2023. [cited 27 December 2023] Available from: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023. [Cited in This Article: ] |
| 3. | Kranjac AW, Kranjac D. Explaining adult obesity, severe obesity, and BMI: Five decades of change. Heliyon. 2023;9:e16210. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 4. | Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine. 2023;58:101882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 49] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21:201-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 285] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 6. | Kamiński M, Miętkiewska-Dolecka M, Kręgielska-Narożna M, Bogdański P. Popularity of Surgical and Pharmacological Obesity Treatment Methods Searched by Google Users: the Retrospective Analysis of Google Trends Statistics in 2004-2022. Obes Surg. 2024;34:882-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Gulinac M, Miteva DG, Peshevska-Sekulovska M, Novakov IP, Antovic S, Peruhova M, Snegarova V, Kabakchieva P, Assyov Y, Vasilev G, Sekulovski M, Lazova S, Tomov L, Velikova T. Long-term effectiveness, outcomes and complications of bariatric surgery. World J Clin Cases. 2023;11:4504-4512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1-190, 215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 614] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 9. | Lazzati A. Epidemiology of the surgical management of obesity. J Visc Surg. 2023;160:S3-S6. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 10. | American Society for Bariatric and Metabolic Surgery. Estimate of Bariatric Surgery Numbers, 2011-2021. [Cited 27 December 2023] Available from: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. [Cited in This Article: ] |
| 11. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 811] [Cited by in F6Publishing: 849] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 12. | Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS Jr, Weidenbacher HJ, Livingston EH, Olsen MK. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016;151:1046-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 390] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 13. | Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, Hopkins PN, McKinlay R, Simper SC, Hunt SC. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017;377:1143-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 534] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 14. | Olbers T, Beamish AJ, Gronowitz E, Flodmark CE, Dahlgren J, Bruze G, Ekbom K, Friberg P, Göthberg G, Järvholm K, Karlsson J, Mårild S, Neovius M, Peltonen M, Marcus C. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5:174-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 15. | Wu T, Wong CKH, Lui DTW, Wong SKH, Lam CLK, Chung MSH, McAllister DA, Welbourn R, Dixon JB. Bariatric surgery, novel glucose-lowering agents, and insulin for type 2 diabetes and obesity: Bayesian network meta-analysis of randomized controlled trials. BJS Open. 2023;7. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 16. | Madani S, Shahsavan M, Pazouki A, Setarehdan SA, Yarigholi F, Eghbali F, Shahmiri SS, Kermansaravi M. Five-Year BAROS Score Outcomes for Roux-en-Y Gastric Bypass, One Anastomosis Gastric Bypass, and Sleeve Gastrectomy: a Comparative Study. Obes Surg. 2024;34:487-493. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Ontario Health (Quality). Bariatric Surgery for Adults With Class I Obesity and Difficult-to-Manage Type 2 Diabetes: A Health Technology Assessment. Ont Health Technol Assess Ser. 2023;23:1-151. [PubMed] [Cited in This Article: ] |
| 18. | Kassir R, Debs T, Blanc P, Gugenheim J, Ben Amor I, Boutet C, Tiffet O. Complications of bariatric surgery: Presentation and emergency management. Int J Surg. 2016;27:77-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Lim R, Beekley A, Johnson DC, Davis KA. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018;3:e000219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Goel R, Nasta AM, Goel M, Prasad A, Jammu G, Fobi M, Ismail M, Raj P, Palaniappan R, Aggarwal S, Bindal V, Katakwar A, Vennapusa A, Bhasker AG, Peters A, Goel D, Bedi D, Palep J, Kona L, Mehrotra M, Baijal M, Bhandari M, Dukkipati N, Wadhawan R, Baig S, Pattanshetti S, Ugale S. Complications after bariatric surgery: A multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group. J Minim Access Surg. 2021;17:213-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Winegar DA, Sherif B, Pate V, DeMaria EJ. Venous thromboembolism after bariatric surgery performed by Bariatric Surgery Center of Excellence Participants: analysis of the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2011;7:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Carvalho L, Almeida RF, Nora M, Guimarães M. Thromboembolic Complications After Bariatric Surgery: Is the High Risk Real? Cureus. 2023;15:e33444. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 23. | Hamad GG, Choban PS. Enoxaparin for thromboprophylaxis in morbidly obese patients undergoing bariatric surgery: findings of the prophylaxis against VTE outcomes in bariatric surgery patients receiving enoxaparin (PROBE) study. Obes Surg. 2005;15:1368-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Froehling DA, Daniels PR, Mauck KF, Collazo-Clavell ML, Ashrani AA, Sarr MG, Petterson TM, Heit JA. Incidence of venous thromboembolism after bariatric surgery: a population-based cohort study. Obes Surg. 2013;23:1874-1879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Chang SH, Freeman NLB, Lee JA, Stoll CRT, Calhoun AJ, Eagon JC, Colditz GA. Early major complications after bariatric surgery in the USA, 2003-2014: a systematic review and meta-analysis. Obes Rev. 2018;19:529-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Stein PD, Matta F. Pulmonary embolism and deep venous thrombosis following bariatric surgery. Obes Surg. 2013;23:663-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Jamal MH, Corcelles R, Shimizu H, Kroh M, Safdie FM, Rosenthal R, Brethauer SA, Schauer PR. Thromboembolic events in bariatric surgery: a large multi-institutional referral center experience. Surg Endosc. 2015;29:376-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | El Ansari W, El-Ansari K. Missing something? A scoping review of venous thromboembolic events and their associations with bariatric surgery. Refining the evidence base. Ann Med Surg (Lond). 2020;59:264-273. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 29. | Mabeza RM, Lee C, Verma A, Park MG, Darbinian K, Darbinian S, Yetasook A, Benharash P. Factors and Outcomes Associated With Venous Thromboembolism Following Bariatric Surgery. Am Surg. 2022;88:2525-2530. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 30. | Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: A review. Open J Prev Med. 2012;2:499-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Hamad GG, Bergqvist D. Venous thromboembolism in bariatric surgery patients: an update of risk and prevention. Surg Obes Relat Dis. 2007;3:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251:344-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 33. | Finks JF, English WJ, Carlin AM, Krause KR, Share DA, Banerjee M, Birkmeyer JD, Birkmeyer NJ; Michigan Bariatric Surgery Collaborative; Center for Healthcare Outcomes and Policy. Predicting risk for venous thromboembolism with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann Surg. 2012;255:1100-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Dang JT, Switzer N, Delisle M, Laffin M, Gill R, Birch DW, Karmali S. Predicting venous thromboembolism following laparoscopic bariatric surgery: development of the BariClot tool using the MBSAQIP database. Surg Endosc. 2019;33:821-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Aminian A, Andalib A, Khorgami Z, Cetin D, Burguera B, Bartholomew J, Brethauer SA, Schauer PR. Who Should Get Extended Thromboprophylaxis After Bariatric Surgery?: A Risk Assessment Tool to Guide Indications for Post-discharge Pharmacoprophylaxis. Ann Surg. 2017;265:143-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 36. | Cronin M, Dengler N, Krauss ES, Segal A, Wei N, Daly M, Mota F, Caprini JA. Completion of the Updated Caprini Risk Assessment Model (2013 Version). Clin Appl Thromb Hemost. 2019;25:1076029619838052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 37. | Edwards MA, Spaulding A, Brennan E, Elli EF. Risk stratified venous thromboembolism prophylaxis in bariatric patients using a Caprini assessment: practice patterns and opportunities for improvement. Surg Obes Relat Dis. 2024;20:221-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 38. | Imbus JR, Jung AD, Davis S Jr, Oyefule OO, Patel AD, Serrot FJ, Stetler JL, Majumdar MC, Papandria D, Diller ML, Srinivasan JK, Lin E, Hechenbleikner EM. Extended postoperative venous thromboembolism prophylaxis after bariatric surgery: a comparison of existing risk-stratification tools and 5-year MBSAQIP analysis. Surg Obes Relat Dis. 2023;19:808-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Cornejo J, Gunturu NS, Castillo-Larios R, Elli EF. Do sleeve gastrectomy and Roux-en-Y gastric bypass have different venous thromboembolism risk factors? Creation of 30-day Bariatric Hypercoagulation Score. Surg Obes Relat Dis. 2023;19:1246-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 40. | Hsu JL, Chen KA, Butler LR, Bahraini A, Kapadia MR, Gomez SM, Farrell TM. Application of machine learning to predict postoperative gastrointestinal bleed in bariatric surgery. Surg Endosc. 2023;37:7121-7127. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 41. | Aminian A, Vosburg RW, Altieri MS, Hinojosa MW, Khorgami Z; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. The American Society for Metabolic and Bariatric Surgery (ASMBS) updated position statement on perioperative venous thromboembolism prophylaxis in bariatric surgery. Surg Obes Relat Dis. 2022;18:165-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 42. | American College of Surgeons. Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. [cited 26 December 2023]. Available from: https://www.facs.org/quality-programs/mbsaqip. [Cited in This Article: ] |
| 43. | Ali H, Inayat F, Malik TF, Patel P, Nawaz G, Taj S, Rehman AU, Afzal A, Ishtiaq R, Afzal MS, Advani R, Watson RR. Operator-specific outcomes in endoscopic sleeve gastroplasty: a propensity-matched analysis of the US population using a multicenter database. Proc (Bayl Univ Med Cent). 2023;36:592-599. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 44. | Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 1062] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 45. | Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2564] [Cited by in F6Publishing: 2957] [Article Influence: 211.2] [Reference Citation Analysis (0)] |
| 46. | Fenlon C, O'Grady L, Doherty ML, Dunnion J. A discussion of calibration techniques for evaluating binary and categorical predictive models. Prev Vet Med. 2018;149:107-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Nattino G, Finazzi S, Bertolini G. A new calibration test and a reappraisal of the calibration belt for the assessment of prediction models based on dichotomous outcomes. Stat Med. 2014;33:2390-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1652] [Cited by in F6Publishing: 1582] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 49. | Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007;246:1002-7; discussion 1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Sapala JA, Wood MH, Schuhknecht MP, Sapala MA. Fatal pulmonary embolism after bariatric operations for morbid obesity: a 24-year retrospective analysis. Obes Surg. 2003;13:819-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Prystowsky JB, Morasch MD, Eskandari MK, Hungness ES, Nagle AP. Prospective analysis of the incidence of deep venous thrombosis in bariatric surgery patients. Surgery. 2005;138:759-63; discussion 763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Helm MC, Simon K, Higgins R, Kindel TL, Gould JC. Perioperative complications increase the risk of venous thromboembolism following bariatric surgery. Am J Surg. 2017;214:1135-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | O'Connor K, Garcia Whitlock AE, Tewksbury C, Williams NN, Dumon KR. Risk factors for postdischarge venous thromboembolism among bariatric surgery patients and the evolving approach to extended thromboprophylaxis with enoxaparin. Surg Obes Relat Dis. 2021;17:1218-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Wesley Vosburg R, Druar NM, Kim JJ. Factors Associated with Increased Risk for Pulmonary Embolism After Metabolic and Bariatric Surgery: Analysis of Nearly One Million Patients. Obes Surg. 2022;32:2433-2437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 55. | Becattini C, Agnelli G, Manina G, Noya G, Rondelli F. Venous thromboembolism after laparoscopic bariatric surgery for morbid obesity: clinical burden and prevention. Surg Obes Relat Dis. 2012;8:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Gupta H, Ramanan B, Gupta PK, Fang X, Polich A, Modrykamien A, Schuller D, Morrow LE. Impact of COPD on postoperative outcomes: results from a national database. Chest. 2013;143:1599-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 57. | Lawrence VA, Cornell JE, Smetana GW; American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:596-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 58. | Børvik T, Brækkan SK, Enga K, Schirmer H, Brodin EE, Melbye H, Hansen JB. COPD and risk of venous thromboembolism and mortality in a general population. Eur Respir J. 2016;47:473-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 59. | Salomon B, Dasa V, Krause PC, Hall L, Chapple AG. Hospital Length of Stay Is Associated With Increased Likelihood for Venous Thromboembolism After Total Joint Arthroplasty. Arthroplast Today. 2021;8:254-257.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Heit JA, Lahr BD, Ashrani AA, Petterson TM, Bailey KR. Predictors of venous thromboembolism recurrence, adjusted for treatments and interim exposures: a population-based case-cohort study. Thromb Res. 2015;136:298-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Nemeth B, Lijfering WM, Nelissen RGHH, Schipper IB, Rosendaal FR, le Cessie S, Cannegieter SC. Risk and Risk Factors Associated With Recurrent Venous Thromboembolism Following Surgery in Patients With History of Venous Thromboembolism. JAMA Netw Open. 2019;2:e193690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 62. | Chao GF, Montgomery JR, Abou Azar S, Telem DA. Venous thromboembolism: risk factors in the sleeve gastrectomy era. Surg Obes Relat Dis. 2021;17:1905-1911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Jha AK, Larizgoitia I, Audera-Lopez C, Prasopa-Plaizier N, Waters H, Bates DW. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf. 2013;22:809-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 64. | Carmody BJ, Sugerman HJ, Kellum JM, Jamal MK, Johnson JM, Carbonell AM, Maher JW, Wolfe LG, DeMaria EJ. Pulmonary embolism complicating bariatric surgery: detailed analysis of a single institution's 24-year experience. J Am Coll Surg. 2006;203:831-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Sugerman HJ, Sugerman EL, Wolfe L, Kellum JM Jr, Schweitzer MA, DeMaria EJ. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | Altieri MS, Yang J, Zhu C, Konstantinos Spaniolas L, Talamini MA, Pryor AD. Preoperative anticoagulation in patients undergoing bariatric surgery is associated with worse outcomes. Surg Endosc. 2020;34:4177-4184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Modasi A, Dang JT, Afraz S, Hefler J, Switzer N, Birch DW, Karmali S. Bariatric Surgery Outcomes in Patients on Preoperative Therapeutic Anticoagulation: an Analysis of the 2015 to 2017 MBSAQIP. Obes Surg. 2019;29:3432-3442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Bell EJ, Selvin E, Lutsey PL, Nambi V, Cushman M, Folsom AR. Glycemia (hemoglobin A1c) and incident venous thromboembolism in the Atherosclerosis Risk in Communities cohort study. Vasc Med. 2013;18:245-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Hermanides J, Cohn DM, Devries JH, Kamphuisen PW, Huijgen R, Meijers JC, Hoekstra JB, Büller HR. Venous thrombosis is associated with hyperglycemia at diagnosis: a case-control study. J Thromb Haemost. 2009;7:945-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Lerstad G, Brodin EE, Enga KF, Jorde R, Schirmer H, Njølstad I, Svartberg J, Braekkan SK, Hansen JB. Hyperglycemia, assessed according to HbA1c , and future risk of venous thromboembolism: the Tromsø study. J Thromb Haemost. 2014;12:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | R Charlier SH, Meier C, Jick SS, Meier CR, Becker C. Association between glycemic control and risk of venous thromboembolism in diabetic patients: a nested case-control study. Cardiovasc Diabetol. 2022;21:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Deng RX, Zhu XL, Zhang AB, He Y, Fu HX, Wang FR, Mo XD, Wang Y, Zhao XY, Zhang YY, Han W, Chen H, Chen Y, Yan CH, Wang JZ, Han TT, Chen YH, Chang YJ, Xu LP, Huang XJ, Zhang XH. Machine learning algorithm as a prognostic tool for venous thromboembolism in allogeneic transplant patients. Transplant Cell Ther. 2023;29:57.e1-57.e10. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 73. | Lee Y, Jehangir Q, Lin CH, Li P, Sule AA, Poisson L, Balijepally V, Halabi AR, Patel K, Krishnamoorthy G, Nair GB. 3D-PAST: Risk Assessment Model for Predicting Venous Thromboembolism in COVID-19. J Clin Med. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Sheikhtaheri A, Orooji A, Pazouki A, Beitollahi M. A Clinical Decision Support System for Predicting the Early Complications of One-Anastomosis Gastric Bypass Surgery. Obes Surg. 2019;29:2276-2286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Cao Y, Fang X, Ottosson J, Näslund E, Stenberg E. A Comparative Study of Machine Learning Algorithms in Predicting Severe Complications after Bariatric Surgery. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 76. | Cao Y, Montgomery S, Ottosson J, Näslund E, Stenberg E. Deep Learning Neural Networks to Predict Serious Complications After Bariatric Surgery: Analysis of Scandinavian Obesity Surgery Registry Data. JMIR Med Inform. 2020;8:e15992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Wise ES, Amateau SK, Ikramuddin S, Leslie DB. Prediction of thirty-day morbidity and mortality after laparoscopic sleeve gastrectomy: data from an artificial neural network. Surg Endosc. 2020;34:3590-3596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, de Luca M, Faria SL, Goodpaster KPS, Haddad A, Himpens JM, Kow L, Kurian M, Loi K, Mahawar K, Nimeri A, O'Kane M, Papasavas PK, Ponce J, Pratt JSA, Rogers AM, Steele KE, Suter M, Kothari SN. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes Surg. 2023;33:3-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 92] [Article Influence: 92.0] [Reference Citation Analysis (0)] |