Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.307
Peer-review started: October 5, 2023
First decision: December 8, 2023
Revised: December 20, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 27, 2024
Gallstones are common lesions that often require surgical intervention. Laparoscopic cholecystectomy is the treatment of choice for symptomatic gallstones. Preoperatively, the anatomical morphology of the cystic duct (CD), needs to be accurately recognized, especially when anatomical variations occur in the CD, which is otherwise prone to bile duct injury. However, at present, there is no optimal classification system for CD morphology applicable in clinical practice, and the relationship between anatomical variations in CDs and gallstones remains to be explored.
To create a more comprehensive clinically applicable classification of the morphology of CD and to explore the correlations between anatomic variants of CD and gallstones.
A total of 300 patients were retrospectively enrolled from October 2021 to January 2022. The patients were divided into two groups: The gallstone group and the nongallstone group. Relevant clinical data and anatomical data of the CD based on magnetic resonance cholangiopancreatography (MRCP) were collected and analyzed to propose a morphological classification system of the CD and to explore its relationship with gallstones. Multivariate analysis was performed using logistic regression analyses to identify the independent risk factors using variables that were significant in the univariate analysis.
Of the 300 patients enrolled in this study, 200 (66.7%) had gallstones. The mean age was 48.10 ± 13.30 years, 142 (47.3%) were male, and 158 (52.7%) were female. A total of 55.7% of the patients had a body mass index (BMI) ≥ 24 kg/m2. Based on the MRCP, the CD anatomical typology is divided into four types: Type I: Linear, type II: n-shaped, type III: S-shaped, and type IV: W-shaped. Univariate analysis revealed differences between the gallstone and nongallstone groups in relation to sex, BMI, cholesterol, triglycerides, morphology of CD, site of CD insertion into the extrahepatic bile duct, length of CD, and angle between the common hepatic duct and CD. According to the multivariate analysis, female, BMI (≥ 24 kg/m2), and CD morphology [n-shaped: Odds ratio (OR) = 10.97, 95% confidence interval (95%CI): 5.22-23.07, P < 0.001; S-shaped: OR = 4.43, 95%CI: 1.64-11.95, P = 0.003; W-shaped: OR = 7.74, 95%CI: 1.88-31.78, P = 0.005] were significantly associated with gallstones.
The present study details the morphological variation in the CD and confirms that CD tortuosity is an independent risk factor for gallstones.
Core Tip: We propose a novel classification system for the morphology of cystic duct (CD) based on magnetic resonance cholangiopancreatography to guide clinical practice. We also found that CD tortuosity is an independent risk factor for gallstones, which provides a theoretical basis for the construction of predictive models and prevention in high-risk patients.
- Citation: Zhu JH, Zhao SL, Kang Q, Zhu Y, Liu LX, Zou H. Classification of anatomical morphology of cystic duct and its association with gallstone. World J Gastrointest Surg 2024; 16(2): 307-317
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/307.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.307
Gallstone disease is the most common inpatient digestive diagnosis and carries substantial costs for health care services[1,2]. There are three types of gallstones depending on the major constituents: Pure cholesterol, pure pigment, and mixed gallstones, of which cholesterol gallstones account for 80%-90% of all gallstones[3]. The formation of gallstones is a complex process involving interactions between the environment and genes. Major risk factors include age, sex, race, parity, obesity, and family history of gallstones[4,5]. Some scholars believe that the formation of gallstones is related to anatomical variation in the cystic duct (CD)[6,7]. The angle between the CD and common bile duct (CBD) junction (sistocholedochal angle: SCA) affects gallstone formation, and as the SCA increases, the incidence of gallstone formation increases[6]. The number of gallstones, the angle between the long axis of the gallbladder and the CD, and the diameter of the CD are significantly related to gallstone-related biliary events[7]. However, few scholars have focused on the relationship between the morphological variation in CDs and gallstones. The relationship between the two needs to be further explored and refined.
Currently, laparoscopic cholecystectomy (LC) has become the gold standard operation for treating symptomatic gallbladder stones[8,9]. According to relevant studies, during LC, the incidence of bile duct injury (BDI) was 0.86% from 1995 to 2002[10], 0.46% from 2000 to 2011[11], and 0.19% from 2012 to 2016[12]. With the improvements in preoperative examination and the refinement of intraoperative techniques, the incidence of BDI has decreased, but this condition has always existed. BDI is a constant topic, and how to reduce the occurrence of BDI is an area of study. Reducing the occurrence relies on our accurate identification of the anatomy of the CD before LC, especially when there are anatomical variations in the CD. We believe that the morphology of the CD deserves our attention. During LC, a tortuous gallbladder duct is more likely to cause misjudgment, which can lead to BDI.
The CD connects the gallbladder to the extrahepatic bile duct (EHBD). The CD usually measures 2-4 cm in length and enters the EHBD from the right lateral aspect in 49.9% of patients[13]. Anatomic variants of CD are common and many researchers have proposed their own anatomical classification according to the CD insertion site to the EHBD[14-16]. A new classification system for EHBD according to the percentile distribution of the length ratio between CD insertion and the duodenal papilla (CDDP)/EHBD was designed, and the following categories were obtained: Type 1 (below the 25th percentile) for a CDDP/EHBD ratio ≤ 50%; type 2 (25th to 75th percentile) for a CDDP/EHBD ratio 51%-75%; and type 3 (above the 75th percentile) for CDDP/EHBD ratio > 75%[14]. According to the angle and morphology of the convergence of the CD into the EHBD, CD patterns were divided into 3 patterns: Type I (85.4%), located on the right and angled up; type II (3.1%), located on the right and angled down; and type III (11.5%), located on the left and angled up[15]. Several researchers have also broadly classified the configuration of the CD into four types: Angular (44%), linear (40%), spiral (11%) and complex (5%)[16]. However, not all CDs insert into the EHBD, and some CDs insert into the left hepatic duct (LHD) or right hepatic duct (RHD)[17]. We should not focus only on the insertion of the CD to classify its anatomical variants. Perhaps we can start from the morphology of the CD and propose new classification criteria.
Magnetic resonance cholangiopancreatography (MRCP) is a well-established and noninvasive diagnostic technique for visualizing the pancreatic and biliary duct systems without the adverse effects of injecting a contrast agent[18,19]. MRCP has excellent overall sensitivity and specificity for demonstrating the severity and presence of biliary obstruction[20]. MRCP can clearly show the anatomy of the intrahepatic and EHBDs. The alignment and morphology of CDs can be determined via MRCP. At present, MRCP has become a routine examination before LC.
Therefore, the primary aim of this study was to propose a new classification system for the morphology of CD for improved clinical application. The secondary aim was to explore the relationship between anatomic variants of the CD and gallstones.
We retrospectively collected data from patients who underwent MRCP for disease diagnosis and treatment between October 2021 and January 2022 at our hospital. The observation group (gallstone group) met the following criteria for inclusion: (1) Had preoperative MRCP and abdominal ultrasonography confirmed gallstones or postoperative pathology suggested gallstones; and (2) Had CD anatomy that was completely and clearly visualized via MRCP. Patients who met the following criteria were excluded: (1) Had other gallbladder diseases, such as gallbladder polyps, gallbladder adenomyosis, or gallbladder cancer; (2) Had CBD stones, stricture or obstruction; (3) Had their gallbladder removed; and (4) Had incomplete clinical information. The control group (nongallstone group) met the following criteria for inclusion: (1) Had MRCP or pathology suggesting hepatic hemangioma, hepatocellular carcinoma, or pancreatic tumor; and (2) Had MRCP clearly revealing the structure and morphology of the intrahepatic and EHBDs. Patients who met the following criteria were excluded: (1) Had gallbladder diseases such as gallstones, gallbladder polyps, gallbladder adenomyosis, or gallbladder cancer; and (2) Had the remaining exclusion criteria detailed in (2) to (4) of the exclusion criteria for the gallstone group. The following data were collected for each patient: Age, sex, body mass index (BMI), cholesterol, and triglycerides. This study was approved by the ethics committee of our hospital and was conducted in accordance with the Declaration of Helsinki.
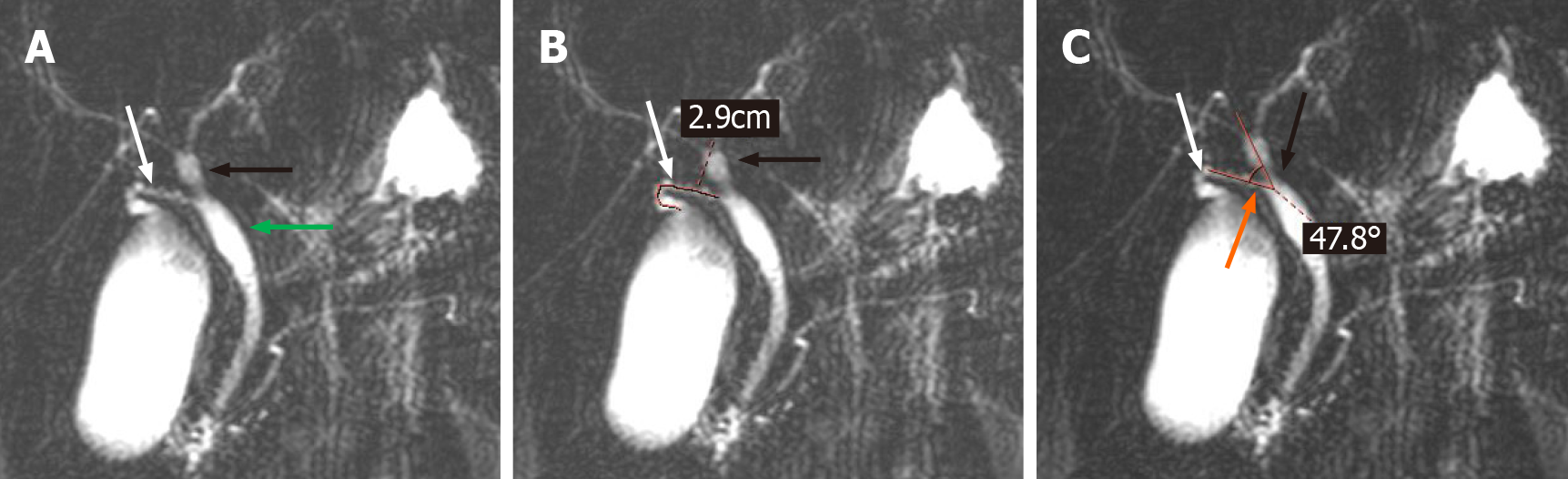
All magnetic resonance imaging (MRIs) were performed on a 1.5 T MRI superconductive scanner (Siemens Sonata) by using an abdominal coil. The imaging parameters for T2W-TSE slices: TR 4500 ms, TE 985 ms, 150 flip angle, 256 × 512 matrix, 28 cm × 28 cm field of view, 265 Hz bandwidth, and 60 mm slice thickness. The collection of evaluation indicators for CD was performed by 2 researchers who were proficient in MRCP. When disagreements arose, a third researcher participated in the discussion, and a consensus was finally reached. The following data were collected for each MRCP examination: The morphology of the CD, the site of CD insertion into the EHBD, the length of the CD, and the angle between the common hepatic duct and the CD (< CHD) (Figure 1). In addition, the anatomy of the intrahepatic bile ducts was also observed and documented in the present study.
The statistical analyses were performed using SPSS version 25.0 (Chicago, IL, United States). Continuous variables are expressed as the mean ± SD or median and interquartile ranges (IQRs). Categorical variables are expressed as counts and percentages. The Mann-Whitney U test was performed to compare continuous variables. Categorical variables were compared using the chi-quare test or Fisher’s exact test. Multivariate analysis was performed using the logistic regression analyses to identify the independent risk factors using variables significant in the univariate analysis. P < 0.05 was considered to indicate statistical significance.
Among the 300 patients enrolled in this study, 200 patients (66.7%) were in the gallstone group, and 100 patients (33.3%) were in the nongallstone group. The mean age was 48.1 ± 13.3 years; 142 (47.3%) were male and 158 (52.7%) were female. A total of 55.7% of the patients had a BMI over 24 kg/m2. In addition, in the study population, cholesterol and triglyceride levels were 4.8 mmol/L (IQR: 4.1-5.4 mmol/L) and 1.6 mmol/L (IQR: 1.1-2.2 mmol/L), respectively. These results are detailed in Table 1.
| Variables | Study population (n = 300) |
| Sex, n (%) | |
| Male | 142 (47.3) |
| Female | 158 (52.7) |
| Age, mean ± SD, years | 48.1 ± 13.3 |
| BMI, kg/m2, n (%) | |
| < 24 | 133 (44.3) |
| ≥ 24 | 167 (55.7) |
| Cholesterol, median (IQR), mmol/L | 4.8 (4.1-5.4) |
| Triglyceride, median (IQR), mmol/L | 1.6 (1.1-2.2) |
| Morphology of CD, n (%) | |
| Linear | 117 (39) |
| Tortuosity | 183 (61) |
| Site of the CD insertion into the EHBD, n (%) | |
| Right | 265 (88.3) |
| Nonright | 35 (11.7) |
| Length of CD, median (IQR), mm | 23.4 (17.3-29.8) |
| < CHD, median (IQR), degrees | 31.6 (21.8-45.7) |
| Intrahepatic biliary anatomy, n (%) | |
| Typical | 270 (66.7) |
| Atypical | 30 (33.3) |
| Group, n (%) | |
| Gallstone | 200 (66.7) |
| Nongallstone | 100 (33.3) |
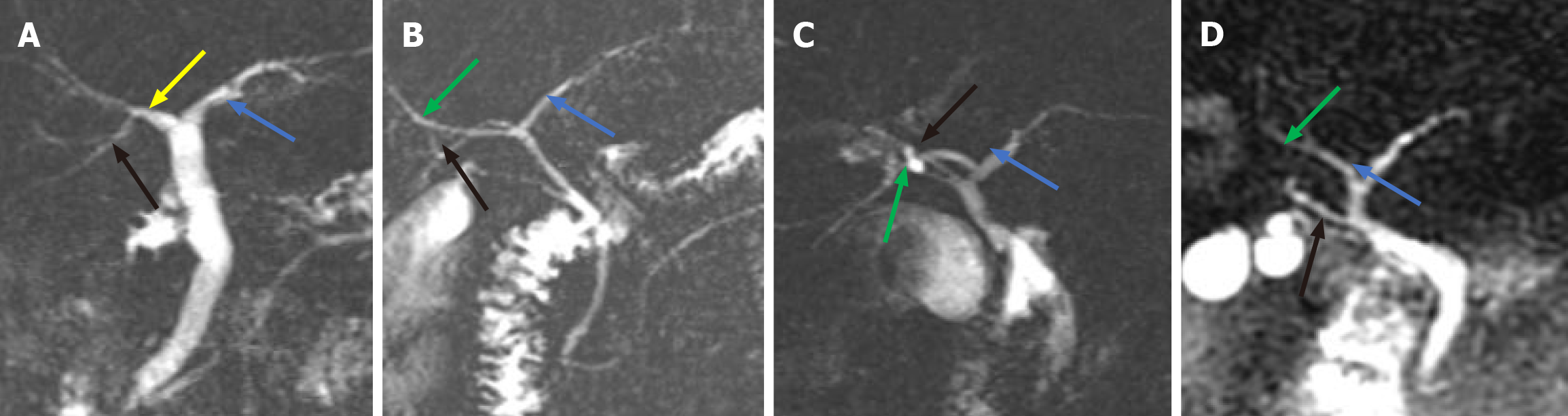
We found that the anatomical variations in the intrahepatic bile ducts were mainly in the right posterior duct (RPD). We classified the different locations at which the RPD converged into the intrahepatic and EHBDs into 4 types (Figure 2): Type A, the right RPD converges into the RHD (Figure 2A); Type B, the right RPD converges into the junction of the right and LHDs (Figure 2B); and Type C, the right RPD converges into the LHD (Figure 2C); and Type D, the right RPD converges into the EHBD (Figure 2D). The typical anatomy of intrahepatic bile ducts (type A) was observed in patients (66.7%), and the atypical anatomy (type B, type C, and type D) was observed in 30 patients (33.3%) (Table 1).
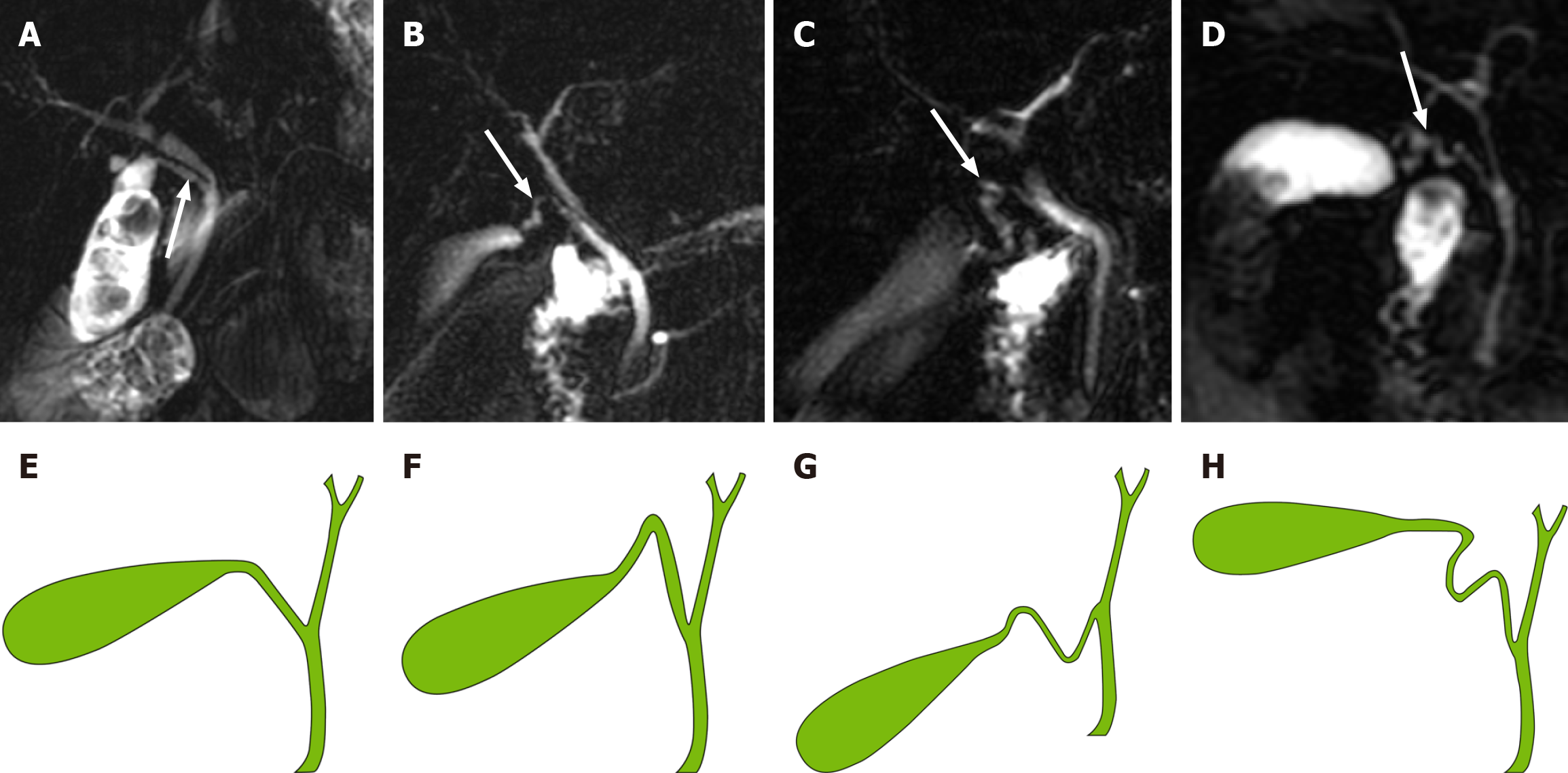
A novel classification system for CD based on the anatomical morphology of the CD was proposed. It is classified into four types (Figure 3): (1) Type I: Linear, the CD is straight and enters straight into the EHBD with no tortuosity (Figure 3A and E); (2) Type II: n-shaped, the CD has a bend in the shape of an n (Figure 3B and F); (3) Type III: S-shaped, the CD converges in an S-shape into the EHBD (Figure 3C and G); and (4) Type IV: W-shaped, the CD converges into the EHBD in a W-shape (Figure 3D and H).
In 300 patients, the CD length was 23.4 mm (IQR: 17.3-29.8 mm), 88.3% of the CDs converged to the right of the EHBD, the < CHD was 31.6 degrees (IQR: 21.8-45.7 degrees), and only 117 (39%) CDs converged to the EHBD in a straight line (Table 1). Univariate analysis revealed a correlation between gallstone formation and anatomical variation in the CD (Table 2). Among all the anatomical factors, there were significant differences in the morphology of CDs between the gallstone group and the non-gallstone group [linear: 43 (21.5%) vs 74 (74%); n-shaped: 108 (54%) vs 15 (15%); S-shaped: 28 (14%) vs 8 (8%); W-shaped: 21 (10.5%) vs 3 (3%), P < 0.001]. The gallstone group had a significantly longer CD length [24.5 (19.6-32.4) mm vs 18.9 (13.5-27.1) mm, P < 0.001]. There was also a significant difference in the site of CD insertion into the EHBD between the two groups [gallstone vs nongallstone: Right: 177 (88.5%) vs 99 (99%); left: 16 (8%) vs 1 (1%); front: 2 (1%) vs 0 (0%); back: 5 (2.5%) vs 0 (0%), P = 0.011]. There was no difference in < CHD between the gallstone and nongallstone groups [31.0 (21.9-44.9) degrees vs 33.4 (21.2-50.8) degrees, P = 0.576]. Moreover, there was no difference in intrahepatic biliary anatomy in the gallstone and the nongallstone groups [typical: 178 (89.0%) vs 22 (11.0%), P = 0.414]. In addition, there were significant differences in sex distribution, BMI, cholesterol, and triglyceride levels between the two groups. The gallstone group had more women than nongallstone (P < 0.001), and the gallstone group had larger BMI (P = 0.002), higher cholesterol [4.9 (4.4-5.4) mmol/L vs 4.5 (3.8-5.3) mmol/L, P =0.01], and higher triglyceride [1.7 (1.2-2.3) mmol/L vs 1.5 (1.0-2.0) mmol/L, P = 0.025] (Table 2).
| Variables | Univariate | Multivariate | |||
| Gallstone group (n = 200) | Nongallstone group (n = 100) | P value | OR (95%CI) | P value | |
| Sex, n (%) | |||||
| Male | 79 (39.5) | 63 (63) | < 0.001 | ||
| Female | 121 (60.5) | 37 (37) | 3.32 (1.77-6.21) | < 0.001 | |
| Age, median (IQR), years | 49.00 (38-59) | 48 (41.00-56.8) | 0.857 | ||
| BMI, kg/m2, n (%) | |||||
| < 24 | 76 (38) | 57 (57) | 0.002 | ||
| ≥ 24 | 124 (62) | 43 (43) | 2.51 (1.35-4.66) | 0.004 | |
| Cholesterol, median (IQR), mmol/L | 4.9 (4.3-5.4) | 4.5 (3.8-5.3) | 0.01 | ||
| Triglyceride, median (IQR), mmol/L | 1.67 (1.2-2.3) | 1.5 (1.0-2.0) | 0.025 | ||
| Morphology of CD, n (%) | |||||
| Linear | 43 (21.5) | 74 (74) | < 0.001 | ||
| n-shaped | 108 (54) | 15 (15) | 10.97 (5.22-23.07) | < 0.001 | |
| S-shaped | 28 (14) | 8 (8) | 4.43 (1.64-11.95) | 0.003 | |
| W-shaped | 21 (10.5) | 3 (3) | 7.74 (1.88-31.78) | 0.005 | |
| Site of the CD insertion into the EHBD, n (%) | |||||
| Right | 177 (88.5) | 99 (99) | 0.011 | ||
| Left | 16 (8) | 1 (1) | |||
| Front | 2 (1) | 0 (0) | |||
| Back | 5 (2.5) | 0 (0) | |||
| Length of CD, median (IQR), mm | 24.5 (19.6-32.4) | 18.9 (13.5-27.1) | < 0.001 | ||
| < CHD, median (IQR), degrees | 31.0 (21.9-44.9) | 33.4 (21.2-50.8) | 0.576 | ||
| Intrahepatic biliary anatomy, n (%) | |||||
| Typical | 178 (89) | 92 (92) | 0.414 | ||
| Atypical | 22 (11) | 8 (8) | |||
According to our multivariate analysis, female [odds ratio (OR) = 3.32, 95% confidence interval (95%CI): 1.77-6.21; P < 0.001], BMI (≥ 24 kg/m2) (OR = 2.51, 95%CI: 1.35-4.66; P = 0.004), and CD morphology were found to be independent risk factors for gallstone formation. The odds ratios for the n-shaped, S-shaped, and W-shaped variables were 10.97 (95%CI: 5.22-23.07, P < 0.001), 4.43 (95%CI: 1.64-11.95, P = 0.003), and 7.74 (95%CI: 1.88-31.78, P = 0.005), respectively (Table 2).
Gallstones are a frequent health problem. The overall prevalence of gallstones ranges from 10% to 15% in adults in Europe and the United States[3], and from 4.2% to 12.1% in the Chinese population[21]. The incidence rate of gallstones in the general population was found to be 0.60% per year[2]. Gallstones can cause various complications, such as secondary choledocholithiasis, cholangitis, acute biliary pancreatitis, gallstone ileus, and even life-threatening complications including severe acute pancreatitis, gallbladder cancer, and recurrent pyogenic cholangitis, leading to an increased health care burden[22,23]. Currently, LC is the first treatment for symptomatic gallstones. Preoperatively, we need to accurately predict the morphology of CDs based on MRCP, especially those with anatomical variants, which are otherwise prone to BDI. However, the anatomical morphology of CD has not been adequately studied to date. Therefore, we formulated a classification system for the morphology of CD for improved clinical application.
Anatomic variations in the biliary system are highly prevalent and might be observed in more than 30% of cases[24]. Operative findings of LC revealed variations in 61 (20.33%) patients, mainly involving the cystic artery (10.67%), CD (4.33%), right hepatic artery (2.67%) and gallbladder (2%)[25]. Turner and Fulcher[13] indicated that variations in CD insertion are common, and occur in 18%-23% of cases[13]. Sureka et al[26] attempted to simplify the classification of CD anatomy and ultimately classified it into 8 types[26]. Al-Muhanna et al[24] found only four variants, Type (A), Type (B), Type (C), and Type (D) , based on the classification system of Sureka et al[26]. Renzulli et al[14] proposed an anatomical classification of CD based on the ratio of the length of the CDB to the length of the EHBD. According to the CD take-off, Cao et al[15] divided 226 CD patterns into 3 patterns, of which type I was divided into three subtypes: Line type, S type, and α type. Garg et al[16] broadly classified gliomas into four subtypes, angular, linear, spiral and complex, which were subsequently divided into numerous subtypes. On the one hand, these anatomical typologies are too complex for clinical application; on the other hand, some CD confluence flows into the intrahepatic bile duct (Figure 3), while our new classification focuses on the morphology of CD.
We believe that the residual CD length should be ≤ 0.5 cm when LC is performed. The long CDs may develop secondary or residual stones after LC, further leading to postcholecystectomy syndrome[27,28]. Patients in this group often require reoperation to remove the overlong CD. The incidence of residual gallstones following cholecystectomy is < 2.5%[29,30]. However, the pursuit of shorter CDs is usually accompanied by an increased risk of BDI. Moreover, the length of the residual CD is not always less than 0.5 cm each time LC is undertaken, especially when there are anatomical variants of the CD, such as convergence to the left side of the EHBD, or convergence to the intrahepatic bile duct, or if the classification of the morphology of CD if type II, III, or IV. The length of the residual CD should be further investigated and explored. Near-infrared imaging with indocyanine green (ICG) may be the answer to this contradiction. The use of near-infrared imaging with the ICG technique provides good overall visualization of the CD, CBD, CHD and CD junction regions prior to and following dissection of Calot’s triangle[31,32]. Therefore, the use of near-infrared imaging with the ICG technique for cholecystectomy should be considered, especially in patients with anatomical variants of CD.
Based on a study of MRCP in 300 patients, we classified the anatomy of the intrahepatic bile ducts into 4 types: Type A, type B, type C, and type D. Type D was defined when the right RPD converged into the EHBD. For type D, we observed that the RPD converged into the common hepatic duct in our study (Figure 2D). Sarawagi et al[33] found that in 4% of subjects, the RPD drained into the common hepatic duct, whereas in 0.8% of subjects, the RPD drained into the CD in their MRCP-based study. Choi et al[34] intraoperative cholangiography of 300 liver transplant donors revealed that the anatomy of the intrahepatic bile ducts was typical in 63% of patients (n = 188), that of the RPD into the common hepatic duct in 6% (n = 19), and that of the RPD was anomalous into the CD in 2% (n = 6). In this study, we did not observe the convergence of the RPD into the CD, but this anatomical variation in the intrahepatic bile duct is not uncommon. Gupta et al[35] found it in 4.4% of MRCPs; Chaib et al[36] showed it in 6.1% of the study population based on endoscopic retrograde cholangiography and intraoperative cholangiography. In the present study, 22% of the patients with gallstones had anatomical variations in their intrahepatic bile ducts. When the anatomical classification of intrahepatic bile ducts is type D, especially when the RPD into the CD, we tend to misidentify the RPD as the CD, resulting in BDI. Accurate assessment of the anatomical variations in the intrahepatic bile ducts prior to LC is also essential for ensuring the safety of surgery. In our clinical practice, we sometimes observe a phenomenon in which the CD converges into the intrahepatic bile duct (Figure 4). Before LC, both the anatomical morphology of the CD and the anatomical variations in the intrahepatic bile ducts need to be considered.
Several studies have shown that advanced age, female gender, race, obesity, rapid weight loss, diet and a family history of gallstone disease are risk factors for the gallstone development[3-5,37,38]. Univariate analysis revealed that sex, BMI, triglycerides and cholesterol were associated with the formation of gallstones. Multivariable logistics analysis suggested that sex and BMI are independent risk factors for gallstones. A population-based study in China revealed that the risk of gallstones increases markedly with age[39]. Our study did not show that age is a risk factor for gallstones. With the awakening of health awareness, people undergo regular medical checkups, which can lead to an earlier age at gallstone diagnosis. BMI (≥ 24 kg/m2) is an independent risk factor for gallstones. A recent study revealed that there have been steady increases in the mean BMI among all age groups in China[40]. Rapid increases in BMI may accelerate the formation of gallbladder stones and thus weaken the correlation between gallbladder stones and age.
In addition, our study showed that the anatomical morphology of the CD is an independent risk factor for gallstone formation, which means that tortuous CD is an important cause of gallstone formation. When the gallbladder is not functioning properly, the components of the bile are supersaturated leading to the formation of solid crystals, called gallstones[41]. Gallbladders with tortuous CDs are prone to gallstone formation for the following reasons:
(1) Bile stasis: Bile stasis may cause cholesterol supersaturation and allow the formation of cholesterol stones[41]. Studies suggest that flow resistance is affected by CD morphology, which plays a dominant role[42,43]. Tortuous CDs can lead to increased flow resistance, resulting in bile stasis, which can promote the development of gallstones. From the aspect of conservation of energy, the gallbladder can be considered to be a pump, that pumps bile flow through the CD into the CBD. The pump function of the gallbladder provides bile with kinetic energy that enables the bile to flow. The tortuous CD increases the flow resistance, which eliminates some of the kinetic energy. With this reduction in kinetic energy, the bile cannot be completely drained, resulting in bile stasis, which contributes to the formation of gallstones.
(2) Increased bile viscosity: Increased viscosity of gallbladder bile plays an important role in the pathogenesis of gallstones[41]. The tortuous CD increases flow resistance, which leads to reduced bile flux. According to Poiseuille’s law, bile flux through the CD is inversely correlated with bile viscosity[43]. A decrease in bile flux increases bile viscosity, which leads to the development of gallstone.
And (3) Mucous membrane repair: The bile flowing through the tortuous CD constantly impinges on the tortuous points of the duct, which may cause damage to the mucosa in CD. On the one hand, under the stimulation of inflammation and mucosal repair, gallstones may form by damaging the mucosa as an eruption point. On the other hand, repair of the mucosa may cause narrowing of the CD, possibly resulting in bile stasis[41,43].
Univariate analysis revealed that the length of the CD (P < 0.001) and nonright lateral confluence into the EHBD (P = 0.011) were risk factors for gallstone formation. The group with gallstones had a longer or narrower CD than those without calculi[44,45]. The longer the CD is, the more likely it is to become tortuous in a relative space. When the CD converges into the anterior, posterior or bypasses the anterior or posterior EHBD to the left side, the length of the CD increases, and the filling of the EHBD may squeeze the CD, causing relative narrowing and leading to bile stasis.
There are certain limitations of our study. First, this study is retrospective and may need further validation by prospective studies. Second, we excluded patients who were unable to undergo MRCP, which could have led us to miss certain rare CD morphologies. Third, we performed only an imaging study, and we can use near-infrared imaging with the ICG technique for further in-depth study and validation in LC.
In conclusion, our study provides comprehensive knowledge of the spatial anatomical morphology of CDs and suggests a better clinically applicable classification of the morphology of CDs. It has also been confirmed that CD tortuosity is an independent risk factor for gallstones. In the future, we will construct predictive models based on the risk factors for gallstones identified in our study to provide individualized follow-up strategies for high-risk patients.
At present, there is no optimal classification of the morphology of the cystic duct (CD) applicable to clinical practice, and the relationship between anatomical variation of the CD and gallstone remains to be explored.
Classification of anatomical morphology of CD can be applied to clinical practice to reduce the occurrence of bile duct injury, and we also found that CD tortuosity is an independent risk factor for gallstone. In the future, we will construct predictive models based on the risk factors for gallstone identified in our study to provide individualized follow-up strategies for high-risk groups.
To create a more comprehensive clinically applicable classification of the morphology of the CD and to explore the correlations between anatomic variants of the CD and gallstone.
This was a case-control study. We retrospectively collected data on patients underwent magnetic resonance cholangiopancreatography with (without) gallstones at the Second Affiliated Hospital of Kunming Medical University, Yunnan, China. 300 patients with (without) gallstones identified by abdominal ultrasound and magnetic resonance cholangiopancreatography were enrolled from October 2021 to January 2022. They were divided into two groups: The gallstone group and the non-gallstone group. Data such as sex, age and body mass index were collected.
Of the 300 patients enrolled in this study, 200 (66.7%) of them had gallstones. The mean age was 48.10 ± 13.30 years, of which 142 (47.3%) were male and 158 (52.7%) were female. 55.7% of the patients had a body mass index (BMI) ≥ 24 kg/m2. Based on the magnetic resonance cholangiopancreatography, the CD anatomical typology is divided into four types: Type I: Linear, type II: n-shaped, type III: S-shaped, and type IV: W-shaped. Univariate analysis showed differences between the gallstone and non-gallstone groups in relation to sex, BMI, cholesterol, triglyceride, morphology of CD, site of the CD insertion into the extrahepatic bile duct, length of CD, the angle between the common hepatic duct and CD. In the multivariate analysis, female, BMI (≥ 24 kg/m2), and morphology of CD [n-shaped: Odds ratio (OR) = 10.97, 95% confidence interval (95%CI): 5.22-23.07, P < 0.001; S-shaped: OR = 4.43, 95%CI: 1.64-11.95, P = 0.003; W-shaped: OR = 7.74, 95%CI: 1.88-31.78, P = 0.005] were significantly associated with gallstone.
This present study details the morphological variation of the CD and confirms that CD tortuosity is an independent risk factor for gallstones.
Basic and clinical research of diseases in hepatopancreatobiliary surgery.
We thank all the medical staff and technicians who agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H, Japan; Boscarelli A, Italy; Isogai M, Japan S-Editor: Fan JR L-Editor: A P-Editor:Zhao YQ
| 1. | Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, Shaheen NJ, Sandler RS. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004;126:1448-1453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Shabanzadeh DM, Sørensen LT, Jørgensen T. Determinants for gallstone formation - a new data cohort study and a systematic review with meta-analysis. Scand J Gastroenterol. 2016;51:1239-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 428] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 4. | Nakeeb A, Comuzzie AG, Martin L, Sonnenberg GE, Swartz-Basile D, Kissebah AH, Pitt HA. Gallstones: genetics vs environment. Ann Surg. 2002;235:842-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Marschall HU, Einarsson C. Gallstone disease. J Intern Med. 2007;261:529-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Sipahi M, Erkoç MF, Serin HI, Börekçi H, Banlı O. A novel approach for differentiating etiology of gallstone formation: sistocholedochal angle. Eur Rev Med Pharmacol Sci. 2015;19:1063-1067. [PubMed] [Cited in This Article: ] |
| 7. | Park JS, Lee DH, Lim JH, Jeong S, Jeon YS. Morphologic factors of biliary trees are associated with gallstone-related biliary events. World J Gastroenterol. 2015;21:276-282. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 8. | Warttig S, Ward S, Rogers G; Guideline Development Group. Diagnosis and management of gallstone disease: summary of NICE guidance. BMJ. 2014;349:g6241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Baron TH, Grimm IS, Swanstrom LL. Interventional Approaches to Gallbladder Disease. N Engl J Med. 2015;373:357-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Karvonen J, Gullichsen R, Laine S, Salminen P, Grönroos JM. Bile duct injuries during laparoscopic cholecystectomy: primary and long-term results from a single institution. Surg Endosc. 2007;21:1069-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Martin D, Uldry E, Demartines N, Halkic N. Bile duct injuries after laparoscopic cholecystectomy: 11-year experience in a tertiary center. Biosci Trends. 2016;10:197-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Mangieri CW, Hendren BP, Strode MA, Bandera BC, Faler BJ. Bile duct injuries (BDI) in the advanced laparoscopic cholecystectomy era. Surg Endosc. 2019;33:724-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Turner MA, Fulcher AS. The cystic duct: normal anatomy and disease processes. Radiographics. 2001;21:3-22; questionnaire 288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Renzulli M, Brocchi S, Marasco G, Spinelli D, Balacchi C, Barakat M, Pettinari I, Golfieri R. A New Quantitative Classification of the Extrahepatic Biliary Tract Related to Cystic Duct Implantation. J Gastrointest Surg. 2021;25:2268-2279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Cao J, Ding X, Wu H, Shen Y, Zheng R, Peng C, Wang L, Zou X. Classification of the cystic duct patterns and endoscopic transpapillary cannulation of the gallbladder to prevent post-ERCP cholecystitis. BMC Gastroenterol. 2019;19:139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Garg S, Dutta U, Chaluvashetty SB, Kumar KH, Kalra N, Sahni D, Aggarwal A. The anatomy of the cystic duct and its association with cholelithiasis: MR cholangiopancreatographic study. Clin Anat. 2022;35:847-854. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Zhu J, Shao H, Zou H. A New Quantitative Classification of the Extrahepatic Biliary Tract Related to Cystic Duct Implantation. J Gastrointest Surg. 2022;26:2414-2415. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 18. | Bülow R, Simon P, Thiel R, Thamm P, Messner P, Lerch MM, Mayerle J, Völzke H, Hosten N, Kühn JP. Anatomic variants of the pancreatic duct and their clinical relevance: an MR-guided study in the general population. Eur Radiol. 2014;24:3142-3149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Renzulli M, Biselli M, Fabbri E, Caretti D, Sergenti A, Modestino F, Giannone FA, Storchi M, Pierotti L, Golfieri R. What is the best fruit juice to use as a negative oral contrast agent in magnetic resonance cholangiopancreatography? Clin Radiol. 2019;74:220-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 376] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 21. | Zhu L, Aili A, Zhang C, Saiding A, Abudureyimu K. Prevalence of and risk factors for gallstones in Uighur and Han Chinese. World J Gastroenterol. 2014;20:14942-14949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Song ST, Shi J, Wang XH, Guo YB, Hu PF, Zhu F, Zeng X, Xie WF. Prevalence and risk factors for gallstone disease: A population-based cross-sectional study. J Dig Dis. 2020;21:237-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Portincasa P, Di Ciaula A, de Bari O, Garruti G, Palmieri VO, Wang DQ. Management of gallstones and its related complications. Expert Rev Gastroenterol Hepatol. 2016;10:93-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Al-Muhanna AF, Lutfi AM, Al-Abdulwahhab AH, Al-Sharydah AM, Al-Quorain A, Al-Muhanna AF, Al-Dhaferi BF. Magnetic resonance and retrograde endoscopic cholangiopancreatography-based identification of biliary tree variants: are there type-related variabilities among the Saudi population? Surg Radiol Anat. 2019;41:869-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Talpur KA, Laghari AA, Yousfani SA, Malik AM, Memon AI, Khan SA. Anatomical variations and congenital anomalies of extra hepatic biliary system encountered during laparoscopic cholecystectomy. J Pak Med Assoc. 2010;60:89-93. [PubMed] [Cited in This Article: ] |
| 26. | Sureka B, Bansal K, Patidar Y, Arora A. Magnetic resonance cholangiographic evaluation of intrahepatic and extrahepatic bile duct variations. Indian J Radiol Imaging. 2016;26:22-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 27. | Kim JY, Kim KW, Ahn CS, Hwang S, Lee YJ, Shin YM, Lee MG. Spectrum of biliary and nonbiliary complications after laparoscopic cholecystectomy: radiologic findings. AJR Am J Roentgenol. 2008;191:783-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Upchurch CP, Haas NL, Magnone G, Compton J. Symptomatic Cholelithiasis of a Remnant Gallbladder after Open Cholecystectomy. J Emerg Med. 2018;55:e71-e73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Chowbey P, Soni V, Sharma A, Khullar R, Baijal M. Residual gallstone disease - Laparoscopic management. Indian J Surg. 2010;72:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Popescu RC, Leopa N, Dumitru A, Dan C, Dosa A, Bosneagu R, Iordache IE, Botea F. Residual Gallbladder and Cystic Duct Stump Stone after Cholecystectomy: Laparoscopic Management. Chirurgia (Bucur). 2021;116:484-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Serban D, Badiu DC, Davitoiu D, Tanasescu C, Tudosie MS, Sabau AD, Dascalu AM, Tudor C, Balasescu SA, Socea B, Costea DO, Zgura A, Costea AC, Tribus LC, Smarandache CG. Systematic review of the role of indocyanine green near-infrared fluorescence in safe laparoscopic cholecystectomy (Review). Exp Ther Med. 2022;23:187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Vlek SL, van Dam DA, Rubinstein SM, de Lange-de Klerk ESM, Schoonmade LJ, Tuynman JB, Meijerink WJHJ, Ankersmit M. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc. 2017;31:2731-2742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Sarawagi R, Sundar S, Raghuvanshi S, Gupta SK, Jayaraman G. Common and Uncommon Anatomical Variants of Intrahepatic Bile Ducts in Magnetic Resonance Cholangiopancreatography and its Clinical Implication. Pol J Radiol. 2016;81:250-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Choi JW, Kim TK, Kim KW, Kim AY, Kim PN, Ha HK, Lee MG. Anatomic variation in intrahepatic bile ducts: an analysis of intraoperative cholangiograms in 300 consecutive donors for living donor liver transplantation. Korean J Radiol. 2003;4:85-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Gupta A, Rai P, Singh V, Gupta RK, Saraswat VA. Intrahepatic biliary duct branching patterns, cystic duct anomalies, and pancreas divisum in a tertiary referral center: A magnetic resonance cholangiopancreaticographic study. Indian J Gastroenterol. 2016;35:379-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Chaib E, Kanas AF, Galvão FH, D'Albuquerque LA. Bile duct confluence: anatomic variations and its classification. Surg Radiol Anat. 2014;36:105-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Lam R, Zakko A, Petrov JC, Kumar P, Duffy AJ, Muniraj T. Gallbladder Disorders: A Comprehensive Review. Dis Mon. 2021;67:101130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 424] [Article Influence: 23.6] [Reference Citation Analysis (5)] |
| 39. | Wang J, Shen S, Wang B, Ni X, Liu H, Yu R, Suo T. Serum lipid levels are the risk factors of gallbladder stones: a population-based study in China. Lipids Health Dis. 2020;19:50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 543] [Article Influence: 181.0] [Reference Citation Analysis (0)] |
| 41. | Luo X, Li W, Bird N, Chin SB, Hill NA, Johnson AG. On the mechanical behavior of the human biliary system. World J Gastroenterol. 2007;13:1384-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Pitt HA, Roslyn JJ, Kuchenbecker SL, Doty JE, Denbesten L. The role of cystic duct resistance in the pathogenesis of cholesterol gallstones. J Surg Res. 1981;30:508-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Pitt HA, Doty JE, DenBesten L, Kuchenbecker SL. Stasis before gallstone formation: altered gallbladder compliance or cystic duct resistance? Am J Surg. 1982;143:144-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Deenitchin GP, Yoshida J, Chijiiwa K, Tanaka M. Complex cystic duct is associated with cholelithiasis. HPB Surg. 1998;11:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Bird NC, Ooi RC, Luo XY, Chin SB, Johnson AG. Investigation of the functional three-dimensional anatomy of the human cystic duct: a single helix? Clin Anat. 2006;19:528-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |