Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1180
Peer-review started: May 24, 2021
First decision: June 17, 2021
Revised: July 2, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: October 27, 2021
Endoscopic submucosal dissection was introduced in Japan for the mini-invasive treatment of early gastric cancer, as part of national screening program considering high prevalence of disease in these latitudes. This technique allows en-bloc curative oncological excision and to obtain in a single step R0-resection, characterization, histological staging and potential cure of the tumor with a very high cost-benefit balance. Over the years, Western endoscopists have adopted endoscopic submucosal dissection, achieving good rates of efficacy, long-term improved outcomes and safety, with low risk of local recurrence comparable to those obtained in Asian institutes. However, according to some authors, the excellent outcomes from East country could not be representative of the Western experience. Despite epidemiological differences of early gastric cancer, scant volume data and limitations in training opportunities between Western and Eastern countries, European Society of Gastrointestinal Endoscopy have adopted Japanese guidelines and developed a European core curriculum for endoscopic submucosal dissection training. Endoscopists should be able to estimate the probability of performing a curative resection by considering the benefit/risk relationship case-by-case in order to implement a correct decision-making process.
Core Tip: In Western countries, endoscopic submucosal dissection (ESD) is an accepted first-line therapy of superficial gastric neoplasia, including dysplastic and recurrent lesions. This technique allows a high rate of curative resection and a good safety profile compared with other therapeutic approaches, including surgery, which can be reserved as a rescue therapy. Despite there certainly being some obstacles to its diffusion in the West, European Society of Gastrointestinal Endoscopy has developed a European core curriculum for ESD practice across Europe, with the aim of high quality ESD training. Probably nowadays, Western endoscopists are slowly reaching the same level of expertise and proficiency of the colleagues from the East.
- Citation: De Luca L, Di Berardino M, Mangiavillano B, Repici A. Gastric endoscopic submucosal dissection in Western countries: Indications, applications, efficacy and training perspective. World J Gastrointest Surg 2021; 13(10): 1180-1189
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1180.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1180
Resection of early-stage gastric neoplasia, including dysplatic lesions and tumors limited to the mucosa or submucosa, is the core business of endoscopists in Eastern world, unlike their Western colleagues who are more involved in the removal of colon lesions. Although from 1970 to 2015 Europe observed a reduction in incidence and mortality rates from gastric cancer, its prevalence has increased and represents a problem in our continent. Adapting to the Eastern countries, the turning point in early gastric cancer (EGC) study occurred in 1998 with the "Vienna Classification" of gastrointestinal (GI) epithelial tumors for predicting lymph node metastases. Obviously, endoscopic resection should be reserved for patients with negligible nodal risk metastases; nowadays, there are two recognized techniques for minimally invasive treatment of early GI neoplastic lesions: endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Introduction of the EMR technique showed good results compared to traditional surgery with lower risk of adverse events. However, EMR has the disadvantage of not facilitating en-bloc removal of gastric lesions greater than 15-20 mm and non-lifting or flat lesions, increasing the risk of residual/recurrence adenomatous tissue. Furthermore, piecemeal EMR carries the risk of missing areas with deeper invasion, and of an inadequate histology assessment[1].
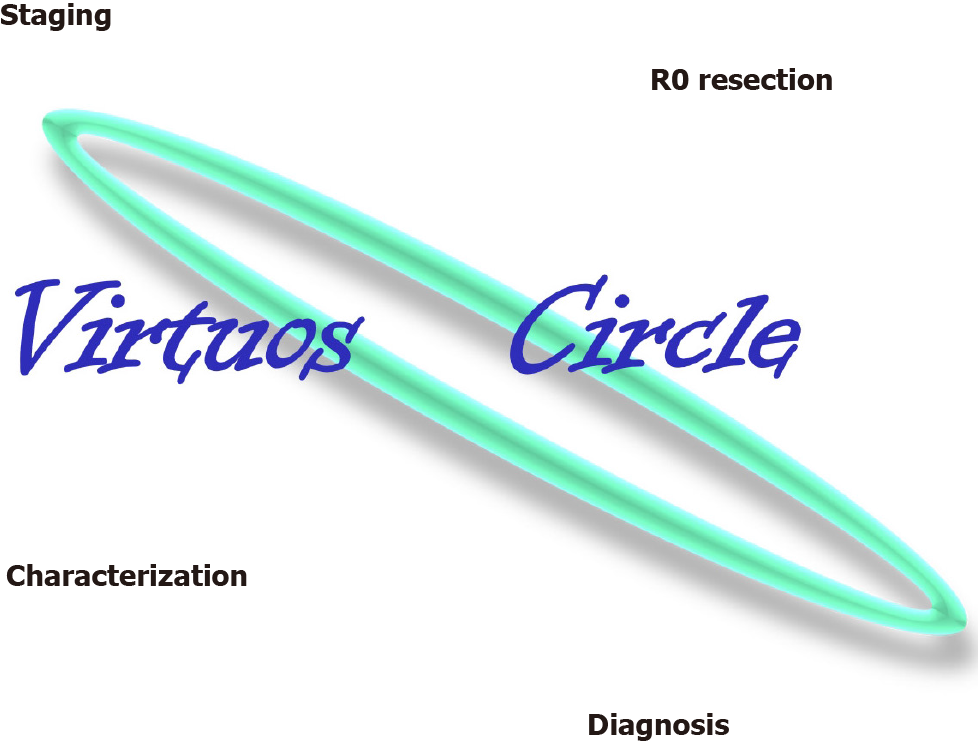
ESD was first described in 1999 by Gotoda as "... a new EMR"[2] for rectal flat lesions, now rapidly spreading around the globe, to overcome the limits of EMR, reducing the rates of en-bloc unresectable lesions and leading to radically oncological removal[3]. This process is able to trigger a “virtuous circle”, as in a single-step, it is possible to obtain R0-resection, characterization, diagnosis and histological staging of the lesion with a very high cost-benefit balance, which has almost no comparison in any other medical procedure (Figure 1). In an editorial published in 2014, Professor Lightdale of Columbia University says that: “In the history of gastrointestinal endoscopy, every once in a while a new therapeutic method comes to the fore that seems difficult and risky, yet so elegant and dramatic in its benefits and possibilities that it fires the desire of interventional endoscopists worldwide to perform it. One such technique is ESD”[4]. Nevertheless, EMR and ESD should be considered complementary.
Therefore, if it is endoscopically feasible, en-bloc excision incorporating marking of the lesion within the resection specimen is the only acceptable therapeutic outcome to achieve the possibility of endoscopic cure. In most cases, gastric EMR lacks this level of precision and ESD is undoubtedly more exact and effective.
In the following sections, we will focus on the Western gastric ESD with respect to the technique, indications, efficacy, long-term outcomes and training considerations.
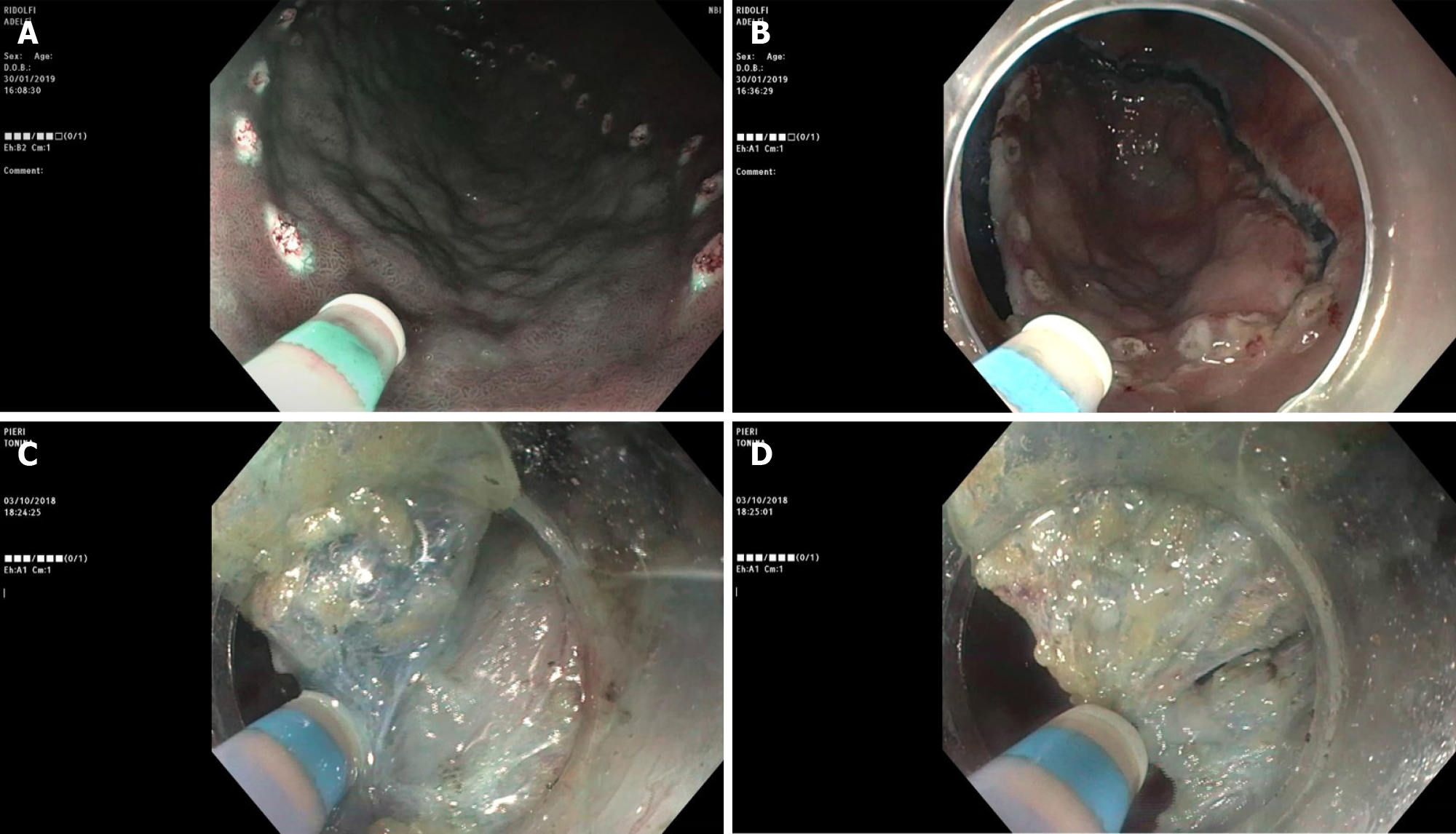
Diagnostic strategic planning is performed as a separate session before the treatment procedure and without any hurry. Endoscopic differentiation of a dysplastic focal lesion from EGC, or prediction of submucosal invasion, is more difficult and less accurate than in the colon, especially in Western countries. Furthermore, with conventional imaging, lesion margins are less distinct and this carries a risk of incomplete resection. The inspection of the lesion using (vital or virtual) chromoendoscopy plus magnification is crucial for evaluating the margins of the lesions[5], macroscopic features, submucosal scarring and target biopsies in order to establish the feasibility of endoscopic resection and an appropriate “working therapeutic project”. A very useful tool for evaluating the lesions, spread in Eastern countries is represented by The Magnifying Endoscopy Simple Diagnostic Algorithm for gastric cancer (MESDA-G), based upon evaluation of the microvascular and micro-surfaces irregularities of the lesion[6].
This process involves a broad photographic documentation. Findings associated with irregular surface, marked marginal elevation, and clubbing, or fusion of converging folds are suggestive of submucosal disease and therefore ESD probably is not feasible. Although in the West the endoscopists should be familiar with the macroscopic and pit pattern classification of superficial neoplastic lesions (i.e. Paris classification), biopsies in the stomach are always mandatory. Endoscopic ultrasonography (EUS), abdominal computer tomography or other procedures are not routinely recommended. EUS can be reserved for few selected cases. It is recommended a discussion on the therapeutic proposal with the patient, based on the endoscopic findings, as it provides a more solid base for the informed consent process and discussion of risks, benefits and alternatives to ESD.
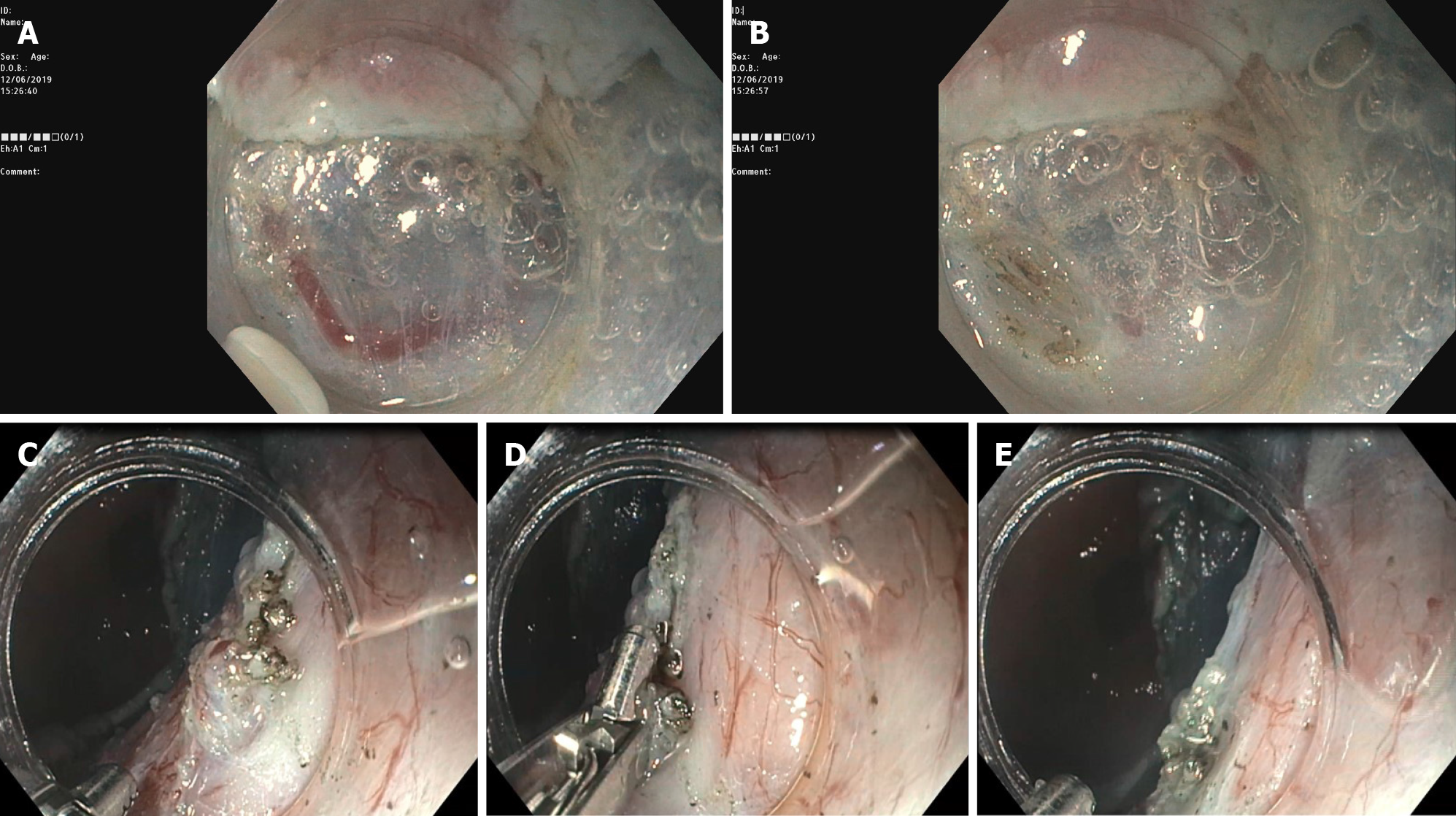
ESD can be carried out with a wide range of knives, which have been developed over time. Three types of knives are currently available, namely the needle, insulated and scissors types. The scissors are neither currently available in the United States nor in routine use in Western countries. Among the needle type knives, we must remember the most used and approved in the Western countries like the Dual knife (KD-650L and KD-650U; Olympus America, Center Valley, PA, United States), Hybrid knife (ERBE USA, Marietta, GA, United States), and IT 2 and IT nano (Olympus America) -- these last two are insulated tip knives --, and the Hook knife and triangular tip knife (Olympus America)[7]. In addition, multitasking devices are commercially available, such as the single-use electrosurgical knife with the water-jet function; it enables the user to effectively perform multiple procedure steps, to reduce procedural time and costs and mark the target lesion, submucosal injection, mucosal incision and submucosal dissection (Figure 2).
Although during ESD general rules need to be followed, technical steps may vary among endoscopists and differ significantly according to devices confidence, type (i.e. degree of fibrosis, histology), morphology (i.e. depressed/ulcerated), and size and location (i.e. proximal third of the stomach) of the lesion. After marking the lesion to isolate the area, lifting agents (different solutions have been used, i.e. saline, sodium hyaluronate, 10% glycerin) are injected in order to create a submucosal fluid cushion. In most of the cases, partial incision should be preferred, in order to avoid fluid escape from the submucosal layer and, therefore, to obtain long-lasting lift, resulting in fewer reinjection, and much safer dissection. The submucosal dissection is the most difficult phase and must take place through small accurate, coordinated movements, mostly in a retrograde direction with twist on the longitudinal axis scope, remaining with the endoscopic tip constantly close to the lesion. The need for a certain force of countertraction to assist ESD has been attempted in different ways by many authors; this factor can improve the dissection in difficult locations and in several lesions with a prevalent fibrosis component and can also result in time-saving during the procedure; although, this latter aspect did not result in a statistically significant outcome[8]. One of the most used and simple is the traction technique, so-called “clip-with-line”. It consists of attaching the distal part or oral side of the lesion to a clip with line and applying a reasonable countertraction in order to do a dissection in a forward plane instead of a reverse position, that in some sites, like pylorus for example or cardia, become difficult to obtain[9]; this technique is useful both for gastric ESD and esophageal as well as colonic ESD. Different types of the intraluminal traction method are reported; the tools most generally used to obtain the countertraction, in order to pull up the lesion and open the resection margins, are the two clips attached to a rubber ring or line, a so-called “medical ring”[10], the stainless spring-assisted ESD[11]. Other more complex methods are percutaneous traction, such as the forceps traction method (e.g., forceps traction method, magnet anchor method, and second-endoscope method) and the double-channel endoscope method.
The use of the cap on the endoscopic tip is very important. In fact, the distal attachment represents the operating "second arm", and helps to anchor the lesion, maintaining scope stability and opening the submucosal gap (otherwise, it is as if a surgeon operated with one hand!). The ESD knife should exit slightly from the distal cap and the submucosal plane should be dissected parallel to the muscular layer.
Submucosal vessels should be preferably visualized before the area is cut through. Once a vessel has been identified, a prophylactic hemostatic maneuver should be carried out with the ESD knife or with hemostatic forceps (Figure 3). The number and thickness of blood vessels vary in different gastric segments. Indeed, in the gastric antrum and in the lesser curvature such density is low; thus, the dissection is usually clear and quite easy. On the contrary, in the greater curvature, the anterior/posterior wall of the stomach, the number of submucosal vessels is high and, therefore, the dissection must be carried out very carefully. After resection, visible blood vessels on the artificial ulcer must be coagulated by using coagrasper to prevent delayed bleeding.
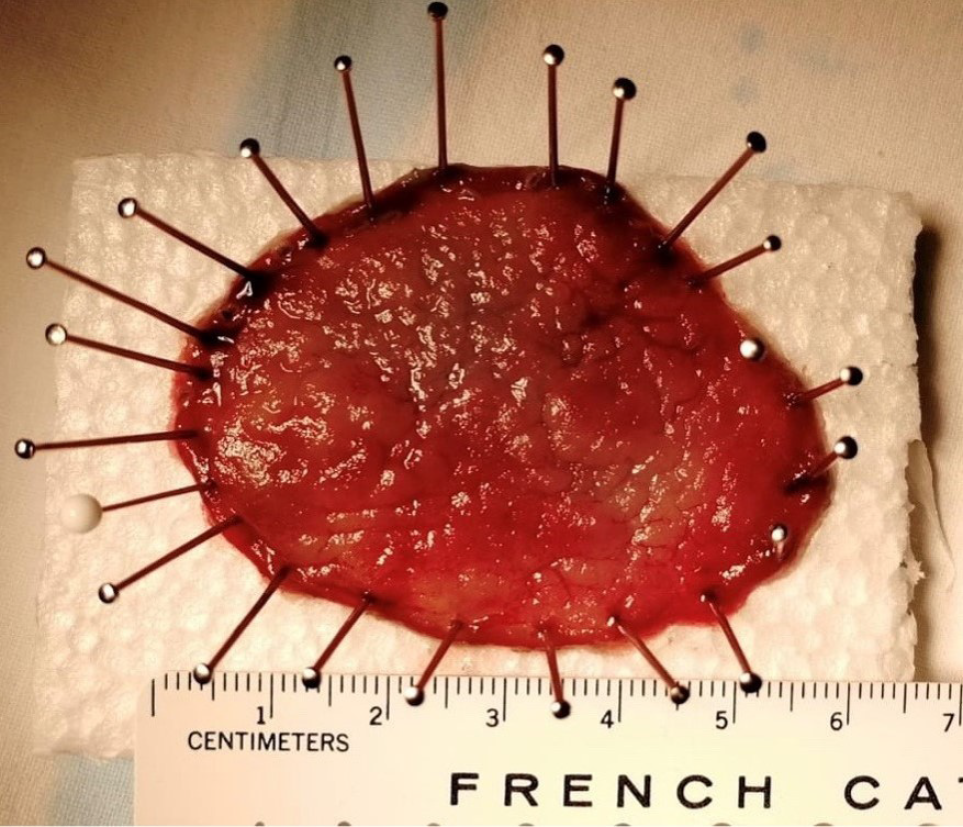
Keeping the whole resected specimen for an accurate pathological evaluation represents an ESD crucial key point to identify high-risk features requiring surgery. Indeed, tumor infiltration depth is statistically correlated to the lymph node metastases whose incidence is negligible if the lesion is confined to the shallow submucosal layer (sm1, ≤ 500 μm). ESD showed significantly higher rates of en-bloc R0 curative and histologically complete resection, as well as lower recurrence frequency, in comparison to a piece-meal technique[12]. The lesion is pinned down on a polystyrene or cork block and then placed in formalin (Figure 4).
Despite the lowest prevalence of gastric cancer, the scant volume data and the limited experience in both Western and Eastern countries, endoscopists have adopted Japanese guidelines[13]. ESD has to be considered in case of a dysplastic lesion measuring > 20 mm in diameter or with high suspicion of superficial submucosal invasion that, otherwise, cannot be radically removed by snare-based techniques. The European Society of Gastrointestinal Endoscopy (ESGE) guidelines strongly recommend endoscopic resection for the treatment of gastric superficial neoplastic lesions that possess a very low risk of lymph node metastasis or when the risk of metastasis is lower compared to the risk of mortality possibly related to surgery[14] (Table 1). Regardless of the technique adopted, the goal is to have a curative resection according to the criteria of the Japanese Gastric Cancer Association (JGCA); the intramucosal neoplasia must be well-differentiated, lateral (> 2 mm) and vertical (> 500 μm) margins free from neoplasia (R0) and with absence of lymphovascular invasion[15].
| Absolute criteria | Expanded criteria | |||||
| A | B | C | D | |||
| Histology | Dysplasia | Differentiated | Differentiated | Differentiated | Undifferentiated | Differentiated |
| Tumor size in mm | Any | ≤ 20 | > 20 | ≤ 30 | ≤ 20 | ≤ 30 |
| Ulceration | Negative | Negative | Negative | Positive | Negative | Negative |
| Depth invasion | None | T1a | T1a | T1a | T1a | sm1, ≤ 500 μm |
Several comparative Asian studies indicate that clinical outcomes of ESD in EGC are comparable when absolute and expanded criteria are considered[16]. A recent meta-analysis[17] of a large data set from surgically resected specimens suggests that the various components of the expanded criteria do not carry equal prognostic significance. In the largest series to date on ESD for EGC in the Western world, Probst et al[18] compared their results favorably with the previous Japanese ESD series in terms of efficacy and safety. The authors reported high rates of curative treatment using the expanded criteria and recommend ESD as treatment of choice not only for guideline criteria EGCs but also for intramucosal non-ulcerated EGCs, regardless of their diameter. Real-life experience shows that ESD is feasible, effective and safe in Western settings and affords the best chance for ECG treatment[19]. ESD instead of surgery is now recommended by both the JGCA and ESGE for expanded criteria lesions[20].
Italian single-center studies substantiated that ESD outcomes were comparable to those achieved in Asian institutions. In a prospective study, Repici et al[21] showed en-bloc removal of early gastric lesions was successful in 100% of cases, whereas R0 was achieved in 92.8% of patients. Clinical results from another study[22] based on a retrospective collected database of gastric ESD procedures between 2005 and 2014 confirmed high rates of complete, curative and en-bloc resection among the study periods. From a recently published multi-center Italian series[23] on ESD procedures for gastric neoplastic lesion, en-bloc and R0 resection rates were very high, with low incidence of adverse events, even though the curative rate for EGC needs to be improved to achieve Eastern results. Similarly, Pagano et al[24] showed the same favorable therapeutic results.
Unfortunately, only two Western clinical trials have assessed the long-term results of EGC. In a Portuguese series[25], ESD was performed in 194 lesions between 2005 and 2014. En-bloc and complete resection rates were 95.3% and 93.8%, respectively, and the patients’ overall survival rates (median follow-up of 40 mo) were 95% and 90% at 1 and 3 years, respectively. A German study[18] showed a high en-bloc/R0 resection, and low adverse event and low local recurrence rates. No gastric cancer-related death was observed and long-term survival was comparable among patients who meet the absolute and expanded criteria. A systematic review and meta-analysis[26] that included 238 publications and 84318 patients (although there was an unequal distribution of studies between both groups) showed that ESD performed in Eastern countries is associated with better outcomes compared to Western countries with regard to R0, en-bloc and curative resection rates.
Many comparative studies between ESD and EMR have been published in the literature in order to balance ECG overall benefit costs. In Western meta-analysis[27] including 10 retrospective studies (8 full text and 2 abstracts), overall data on 4328 lesions, 1916 in the ESD and 2412 in the EMR group, were pooled and analyzed. ESD showed a superior efficacy with respect to EMR in terms of en-bloc and histological complete resection rates, as well as a lower recurrence frequency. However, a higher adverse event rate was observed in the ESD group but this could be also justified by anatomical features (i.e. the gastric wall thickness means that submucosal infiltration is partially performed with poor lifting, unlike other GI tract regions where the mucosa is less tenaciously attached to the underlying structures).
There are few comparative data between ESD and surgery. Overall, retrospective data did not show statistically significant differences in oncological outcomes with respect to en-bloc resection, recurrence and overall survival[28,29]. However, the results showed that surgery required a longer operative time, longer and more expensive patients’ hospital stay, and higher adverse event rate, altering native organ and digestive function.
Taken together, these data indicate that ESD should be the first-line therapy for all potentially endoscopically resectable superficial gastric neoplasia. Surgery can be reserved and used as a rescue therapy.
The limited number of ESDs for EGC in the West is due above all to the lower incidence of this pathology. On the other hand, the high incidence of gastric neoplasia in the East led to the implementation of screening programs and surveillance, consequently developing and improving the ESD technique. Although there is a significant awareness of the technique, its diffusion in Europe remains limited by a whole series of factors, including cultural, logistical, aptitude, technological and, last but not least, remunerative. Eastern endoscopists have greater manual skills and dexterity (it is possible that use of handling chopstick in Asian culinary traditions can facilitate!), have vast technological capabilities that offer greater flexibility as well as meticulous training to achieve expertise. Some of the operators have a surgical background and training that makes them more comfortable in taking risk and less afraid of adverse events, overall resulting in better cost-performance. Training programs differ substantially between the East and West. In Japan, ESD training is well documented and established. Trainees first obtain a formal didactic teaching in ESD, followed by a careful observation by senior and renowned endoscopists. Then, they assist in ESD procedures, and they finally perform ESD under expert supervision on less challenging lesions, usually in the gastric antrum. This process can take years to achieve. ESD training for Western endoscopists may follow the pathway of observership in a high-volume Eastern center, practice on animal models, followed by preceptorship with an expert mentor, beginning with resection of smaller gastric antral lesions. A step-up training protocol recently developed in a German study[30] has shown that ESD training can lead to a high level of competency with a low adverse event rate within a short period of time, at least for easier locations in the rectum and lower stomach. In a recent Italian national survey, it has been observed that endoscopists performing ESD have achieved a good competence level, even if there is a high degree of variability in training protocols, initial supervision of procedures and practice settings[31]. Despite the differences and various proposed training algorithms to Western endoscopists[12], ESD has become increasingly popular, to the point that ESGE has developed a European core curriculum for ESD practice across Europe with the aim of high-quality ESD training[32].
Besides the lower incidence of EGC in Western countries and a widespread use of proton pump inhibitors that could mask gastric cancers still in the early stages, the reasons for the low ability to diagnose in our latitudes compared to the Asian ones are essentially attributable to methodological problems. Unfortunately, gastroscopy is performed in a few minutes and therefore more prone to lesion and characterization oversight. It is advisable for all the GI endoscopist fellows to (1) perform an advanced endoscopy diagnostic practice before starting ESD training, (2) gain proficiency to perform basic techniques in EMR (loop polypectomy, standard EMR, etc.), and (3) train in managing adverse events, such as bleeding, perforations etc. Moreover, trainees have to learn and become proficient at the surveillance strategy after endoscopic treatment.
ESD is widely accepted as a treatment of gastric superficial epithelial tumors and recurrent neoplasia with scars after previous resection, without risk of lymph node metastases, allowing a high rate of curative resection with a good safety profile compared with other therapeutic approaches, including surgery. Promising Western data were reported from centers with a higher case volume. Instead, poor results of ESD have to be considered at the beginning of the learning curve and from lower volume case centers. Over the years, the ESD technique has increasingly expanded in Western countries, achieving a good efficacy and safety standards according to European guidelines, and opening new frontiers of mini-invasive oncological resection[33]. There are certainly some obstacles to its diffusion, including the low incidence of suitable gastric lesions (reference centers will deal with no more than 20 cases/year!)[34], essential requirements for beginner ESD endoscopists, the poor familiarity of endoscopists to detect and characterize early gastric lesions, and the overall lack of qualified trainers[35]. Furthermore, it would also be important to have national registers in order to optimize resources. Although in gastric ESD studies there were no meaningful differences between Western and Eastern endoscopists by evaluating the main endpoints such as curative resection and adverse events, outcomes from centers in Asia could not be representative of the Western experience[12,34]. The optimal treatment strategy must be modulated on a case-by-case basis according to the characteristics of lesions, to the patient's condition and to the level of local experience and expertise available.
From an ethical point of view, patients should be referred to specialized institutes where advanced diagnostic and therapeutic endoscopy is standardized, there are multidisciplinary teams for appropriate management, and certified training programs are implemented for a limited number of junior endoscopists. Therefore, when it is asked whether Western endoscopists are reaching the same level of expertise and proficiency of the colleagues from the East, “the answer is probably yes, but slowly, and only in high-volume centers”[20].
Cecilia Scimia, (MD, PhD - Boston, United States) provided critical English language review of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Esaki M, Kim GH, Noh CK S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Liu JH
| 1. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 330] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1844] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 4. | Lightdale CJ. Endoscopic submucosal dissection: on the rise from East to West. Gastrointest Endosc Clin N Am. 2014;24:xiii-xxiv. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Ling T, Wu L, Fu Y, Xu Q, An P, Zhang J, Hu S, Chen Y, He X, Wang J, Chen X, Zhou J, Xu Y, Zou X, Yu H. A deep learning-based system for identifying differentiation status and delineating the margins of early gastric cancer in magnifying narrow-band imaging endoscopy. Endoscopy. 2021;53:469-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Muto M, Yao K, Kaise M, Kato M, Uedo N, Yagi K, Tajiri H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc. 2016;28:379-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc. 2013;78:677-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Imaeda H, Hosoe N, Kashiwagi K, Ohmori T, Yahagi N, Kanai T, Ogata H. Advanced endoscopic submucosal dissection with traction. World J Gastrointest Endosc. 2014;6:286-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Sasaki S, Nishikawa J, Sakaida I. Clip-with-line traction method allows efficient endoscopic submucosal dissection of an early gastric cancer spreading across the pyloric ring. Dig Endosc. 2020;32:e36-e37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Matsumoto K, Nagahara A, Sakamoto N, Suyama M, Konuma H, Morimoto T, Sagawa E, Ueyama H, Takahashi T, Beppu K, Shibuya T, Osada T, Yoshizawa T, Ogihara T, Watanabe S. A new traction device for facilitating endoscopic submucosal dissection (ESD) for early gastric cancer: the "medical ring". Endoscopy. 2011;43 Suppl 2 UCTN:E67-E68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Sakurazawa N, Kato S, Miyashita M, Kiyama T, Fujita I, Yamashita N, Saitou Y, Tajiri T, Uchida E. An innovative technique for endoscopic submucosal dissection of early gastric cancer using a new spring device. Endoscopy. 2009;41:929-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Ma MX, Bourke MJ. Endoscopic submucosal dissection in the West: Current status and future directions. Dig Endosc. 2018;30:310-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 205] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 14. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 852] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 735] [Cited by in F6Publishing: 1098] [Article Influence: 366.0] [Reference Citation Analysis (0)] |
| 16. | Tanabe S, Hirabayashi S, Oda I, Ono H, Nashimoto A, Isobe Y, Miyashiro I, Tsujitani S, Seto Y, Fukagawa T, Nunobe S, Furukawa H, Kodera Y, Kaminishi M, Katai H. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer. 2017;20:834-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Abdelfatah MM, Barakat M, Lee H, Kim JJ, Uedo N, Grimm I, Othman MO. The incidence of lymph node metastasis in early gastric cancer according to the expanded criteria in comparison with the absolute criteria of the Japanese Gastric Cancer Association: a systematic review of the literature and meta-analysis. Gastrointest Endosc. 2018;87:338-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Probst A, Schneider A, Schaller T, Anthuber M, Ebigbo A, Messmann H. Endoscopic submucosal dissection for early gastric cancer: are expanded resection criteria safe for Western patients? Endoscopy. 2017;49:855-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Santos-Antunes J, Baldaque-Silva F, Marques M, Lopes J, Carneiro F, Macedo G. Real-life evaluation of the safety, efficacy and therapeutic outcomes of endoscopic submucosal dissection in a Western tertiary centre. United European Gastroenterol J. 2018;6:702-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Ciocîrlan M. Is the West finally reaching the East in endoscopic submucosal dissection? Endoscopy. 2017;49:837-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Repici A, Zullo A, Hassan C, Spaggiari P, Strangio G, Vitetta E, Ferrara E, Malesci A. Endoscopic submucosal dissection of early gastric neoplastic lesions: a western series. Eur J Gastroenterol Hepatol. 2013;25:1261-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Catalano F, Mengardo V, Trecca A, Tomezzoli A, Rodella L, Cerofolini A, Verlato G, de Manzoni G. The impact of experience on short- and long-term outcomes on gastric ESD: a western series. Updates Surg. 2019;71:359-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Manta R, Galloro G, Pugliese F, Angeletti S, Caruso A, Zito FP, Mangiafico S, Marmo R, Zullo A, Esposito G, Annibale B, Mutignani M, Conigliaro R. Endoscopic Submucosal Dissection of Gastric Neoplastic Lesions: An Italian, Multicenter Study. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Pagano N, Frazzoni L, La Porta M, Fuccio L, Bazzoli F, Zagari RM. Endoscopic submucosal dissection for superficial premalignant and malignant epithelial neoplasms of the digestive tract: a real-life experience in Italy. Eur Rev Med Pharmacol Sci. 2019;23:8354-8359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 25. | Libânio D, Pimentel-Nunes P, Afonso LP, Henrique R, Dinis-Ribeiro M. Long-Term Outcomes of Gastric Endoscopic Submucosal Dissection: Focus on Metachronous and Non-Curative Resection Management. GE Port J Gastroenterol. 2017;24:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Daoud DC, Suter N, Durand M, Bouin M, Faulques B, von Renteln D. Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: A systematic review and meta-analysis. World J Gastroenterol. 2018;24:2518-2536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 27. | Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 110] [Cited by in F6Publishing: 107] [Article Influence: 10.7] [Reference Citation Analysis (2)] |
| 28. | Cho JH, Cha SW, Kim HG, Lee TH, Cho JY, Ko WJ, Jin SY, Park S. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc. 2016;30:3762-3773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Shin DW, Hwang HY, Jeon SW. Comparison of Endoscopic Submucosal Dissection and Surgery for Differentiated Type Early Gastric Cancer within the Expanded Criteria. Clin Endosc. 2017;50:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Ebigbo A, Probst A, Römmele C, Messmann H. Step-up training for colorectal and gastric ESD and the challenge of ESD training in the proximal colon: results from a German Center. Endosc Int Open. 2018;6:E524-E530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Maselli R, Iacopini F, Azzolini F, Petruzziello L, Manno M, De Luca L, Cecinato P, Fiori G, Staiano T, Rosa Rizzotto E, Angeletti S, Caruso A, Coppola F, Andrisani G, Viale E, Missale G, Panarese A, Mazzocchi A, Cesaro P, Campanale M, Occhipinti P, Tarantino O, Crosta C, Brosolo P, Sferrazza S, Rondonotti E, Amato A, Fuccio L, Costamagna G, Repici A. Endoscopic submucosal dissection: Italian national survey on current practices, training and outcomes. Dig Liver Dis. 2020;52:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Pimentel-Nunes P, Pioche M, Albéniz E, Berr F, Deprez P, Ebigbo A, Dewint P, Haji A, Panarese A, Weusten BLAM, Dekker E, East JE, Sanders DS, Johnson G, Arvanitakis M, Ponchon T, Dinis-Ribeiro M, Bisschops R. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2019;51:980-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 33. | Araújo-Martins M, Pimentel-Nunes P, Libânio D, Borges-Canha M, Dinis-Ribeiro M. How Is Endoscopic Submucosal Dissection for Gastrointestinal Lesions Being Implemented? GE Port J Gastroenterol. 2020;27:1-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic Submucosal Dissection: Indications and Application in Western Endoscopy Practice. Gastroenterology. 2018;154:1887-1900.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Saito Y, Yamada M, So E, Abe S, Sakamoto T, Nakajima T, Otake Y, Ono A, Matsuda T. Colorectal endoscopic submucosal dissection: Technical advantages compared to endoscopic mucosal resection and minimally invasive surgery. Dig Endosc. 2014;26 Suppl 1:52-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |