Copyright
©The Author(s) 2023.
World J Gastrointest Surg. May 27, 2023; 15(5): 984-991
Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.984
Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.984
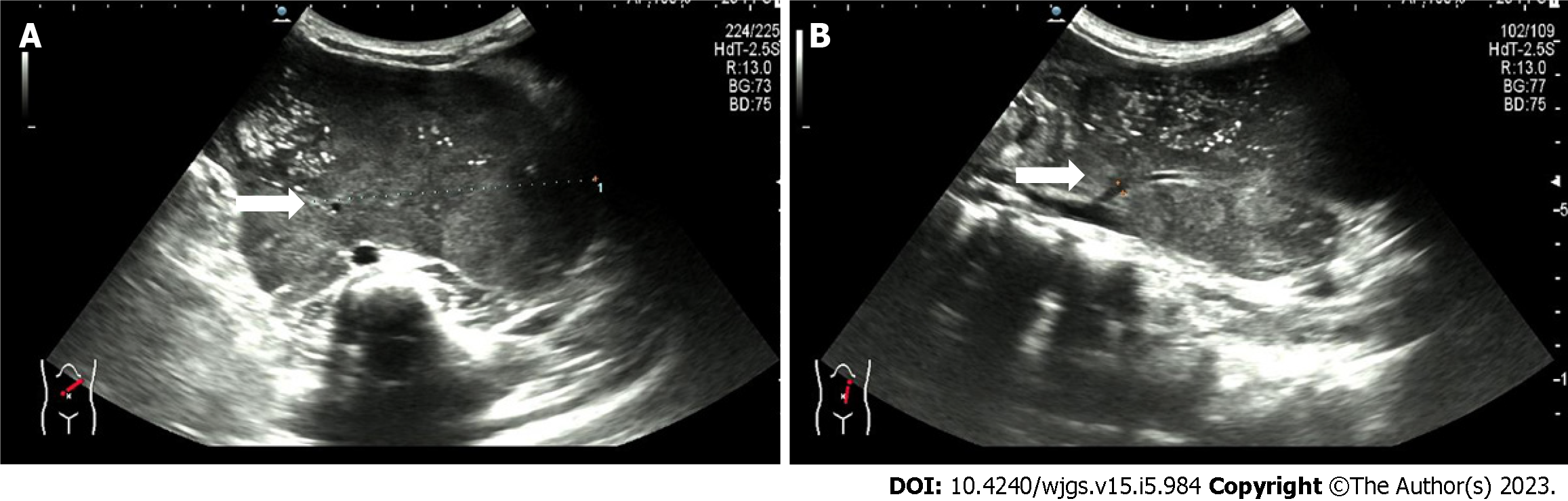
Figure 1 Ultrasound showing a retroperitoneal left-sided hypoechoic mass of 12.
4 cm × 10.5 cm × 6.3 cm with irregular morphology. A: A supply blood vessel with an internal diameter of 0.3 cm was present in the tumor; B: This supply blood is from the abdominal aorta.
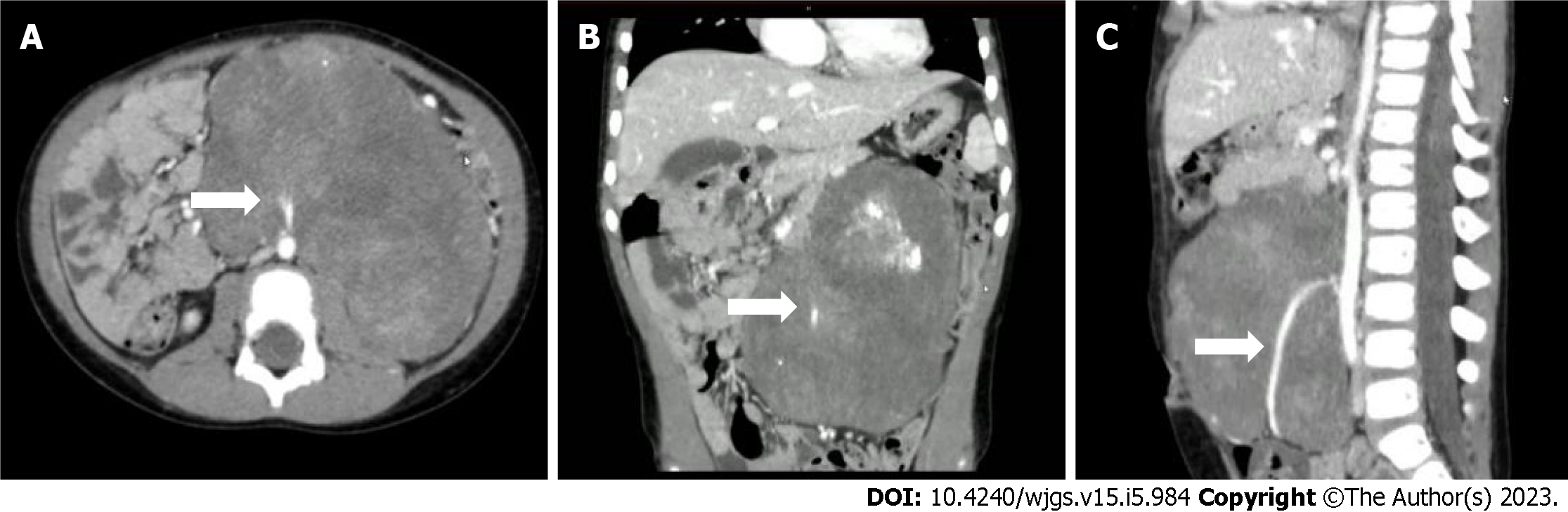
Figure 2 Enhanced computed tomography of the abdomen showing a huge mass-like mixed density lesion in the abdominal cavity with a maximum cross-section of about 123 mm × 85 mm and well-defined margins, encasing the inferior mesenteric artery.
A: Computed tomography (CT) transverse section view; B: CT coronal section view; C: CT sagittal section view. The arrow points to the inferior mesenteric artery.
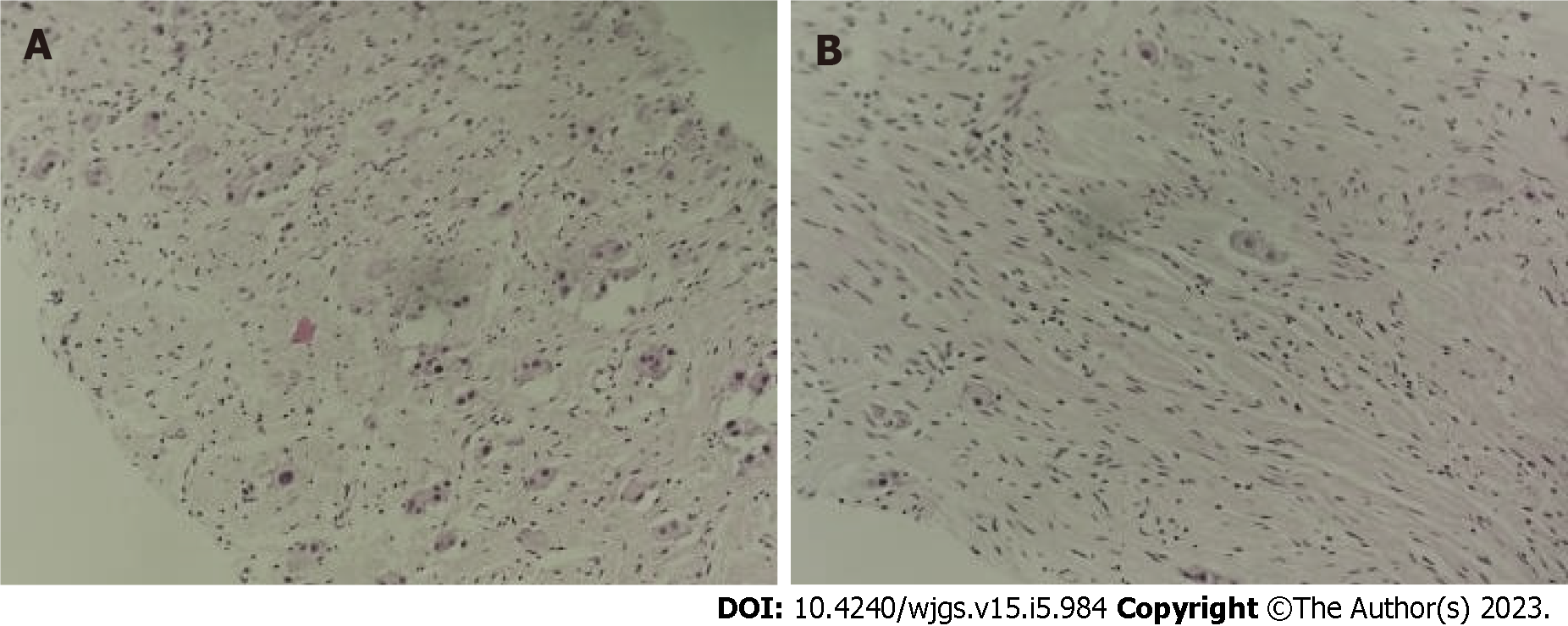
Figure 3 Ultrasound-guided abdominal mass puncture biopsy pathology of the tumor.
No definite neuroblastoma nesting mass was observed. A: Some of the cells were cytoplasm rich, nucleoli were visible, ganglion cell differentiation was suspected, and some areas appeared immature [hematoxylin & eosin (H&E), × 100]; B: Some cells were spindle-shaped, Schwann stroma was considered (H&E, × 100).
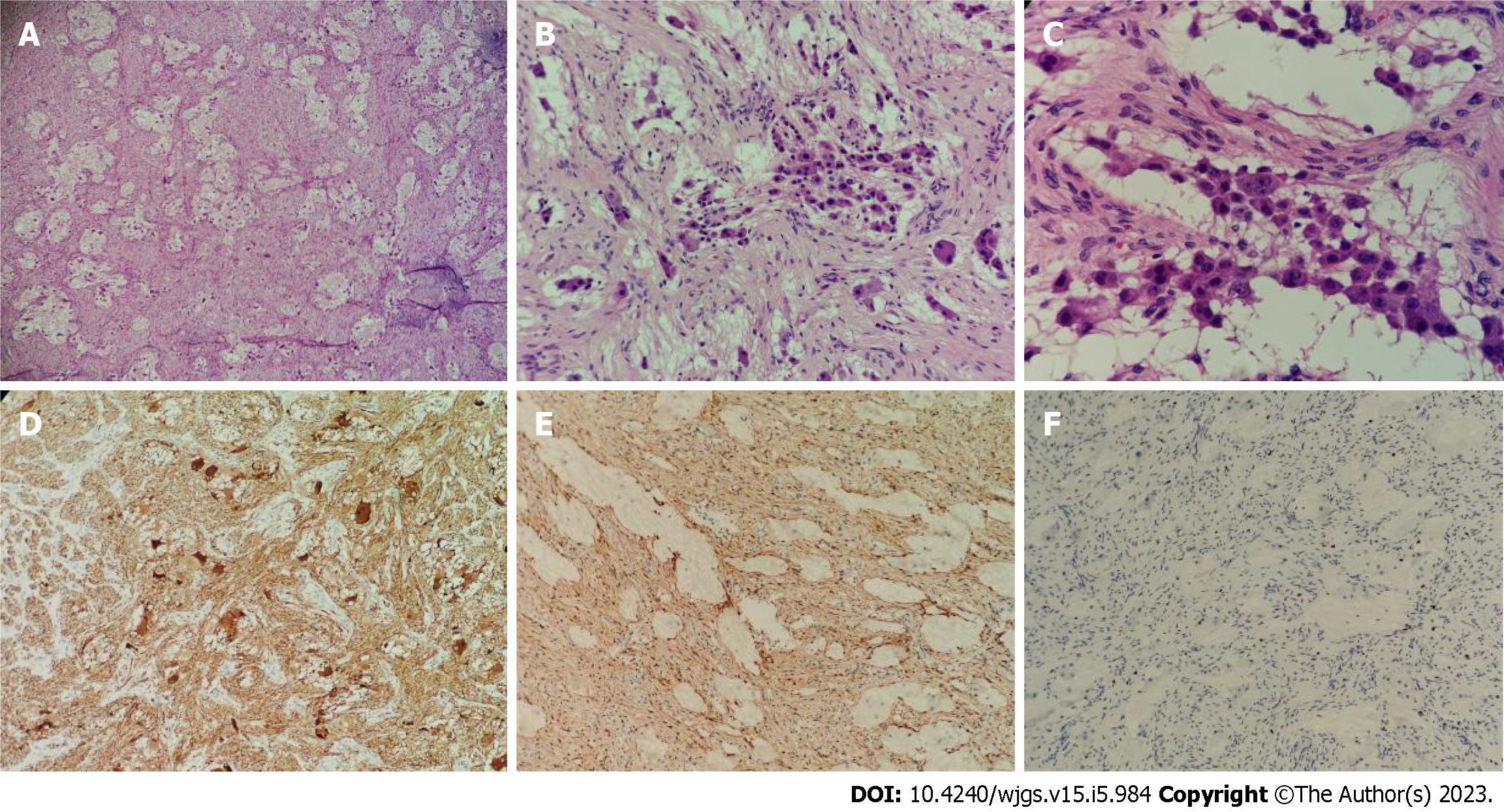
Figure 4 Pathology of the postoperative tumor specimen.
A: The tumor was mainly composed of nerve fibers and ganglion cells [hematoxylin & eosin (H&E), × 40]; B: Some ganglion cells did not mature in differentiation (H&E, × 100); C: Only a few scattered small round cells with deep-stained nuclei were seen locally, which were considered to be neuroblastoma cells, and these cells made up less than 10% of the tumor (H&E, × 200); D: Neuron specific enolase positive (H&E, × 40); E: S100 positive (H&E, × 40); F: Ki-67 positive (H&E, × 40).
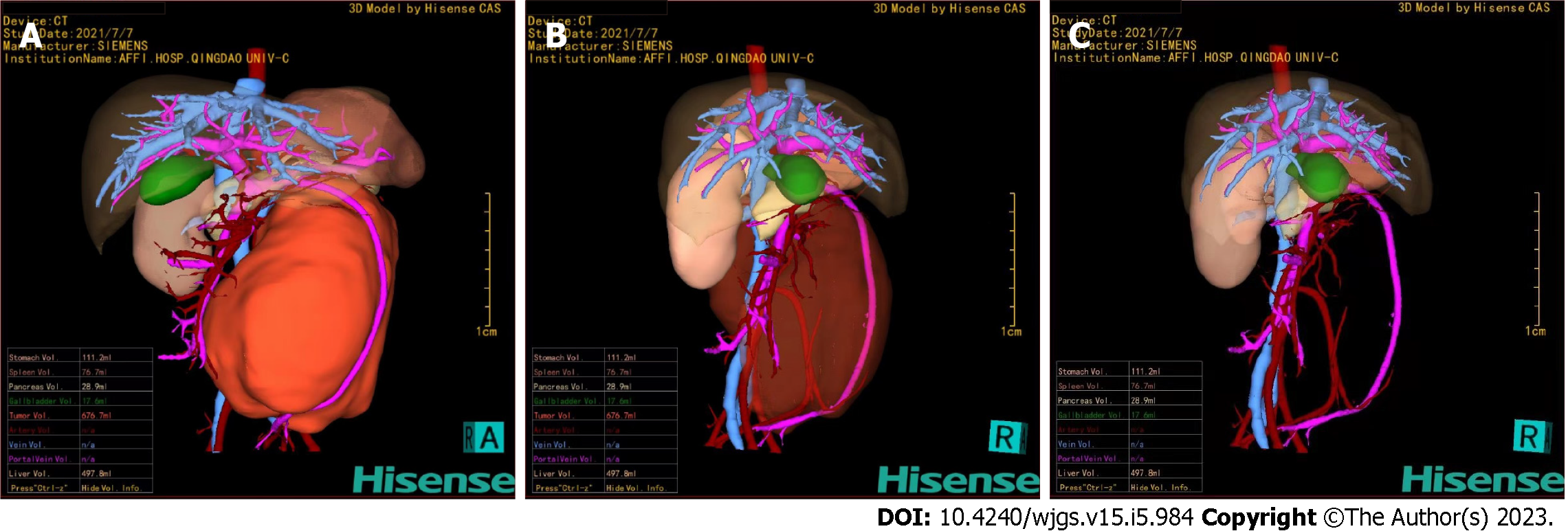
Figure 5 Hisense computer-assisted system three-dimensional reconstruction.
A: The tumor could be clearly located in the retroperitoneum and had a giant volume of 676.7 mL; B and C: Through the translucency and transparency function, the tumor was close to the abdominal aorta, and the superior mesenteric vein was pushed anteriorly. The inferior mesenteric artery passed through the tumor and was completely encased in the tumor.
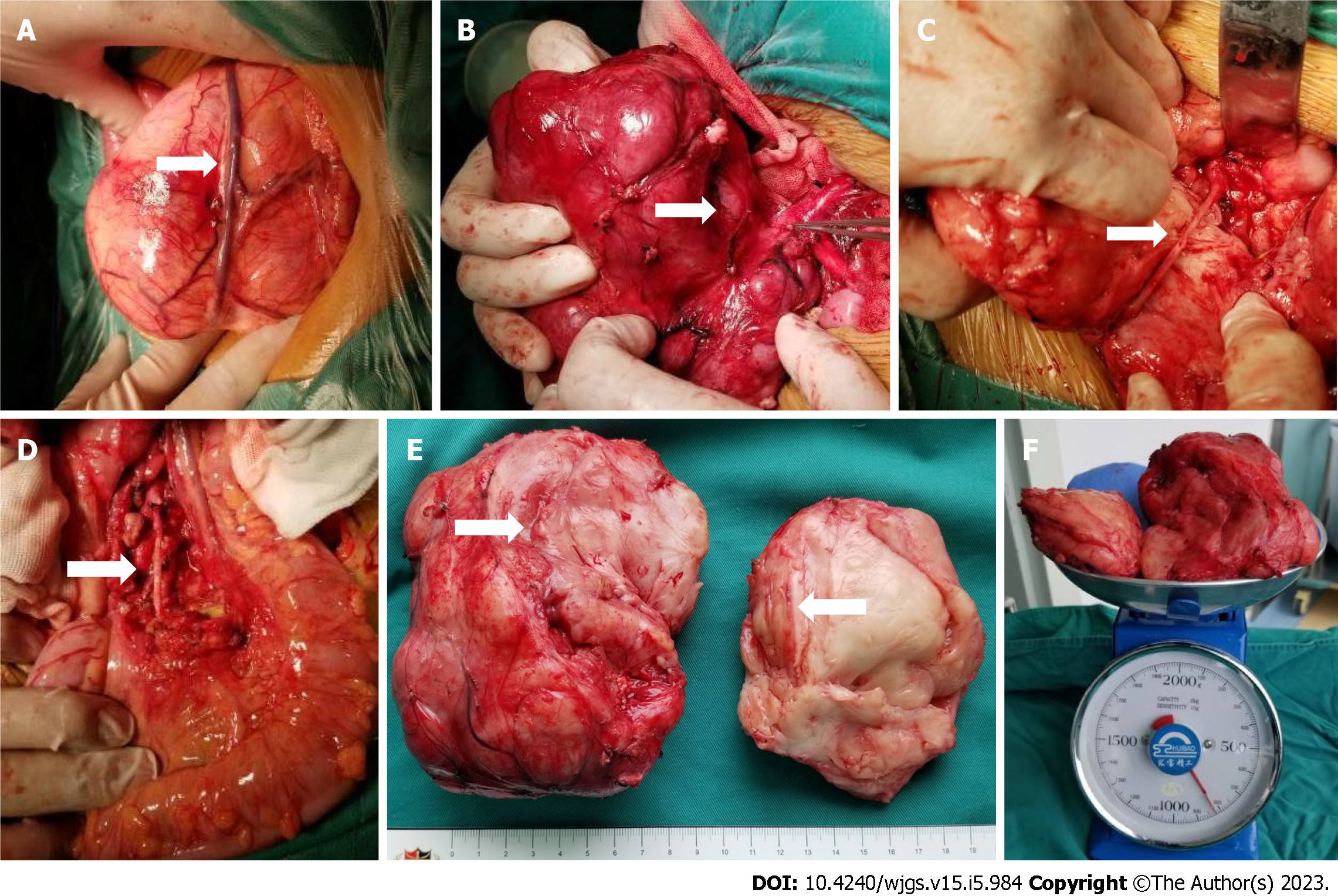
Figure 6 The intraoperative exploration was completely consistent with the preoperative three-dimensional evaluation, and the tumor had a relatively complete fibrous capsule.
A: The superior mesenteric vein was pushed to the front of the tumor; B: The tumor was close to the abdominal aorta, and the inferior mesenteric artery was penetrating the tumor; C: After splitting the tumor with a CUSA knife, the inferior mesenteric artery that was encased by the tumor could be seen; D: Arterial pulsation was seen in the exposed inferior mesenteric artery, and the distal sigmoid colon and rectum was ruddy; E: The tumor section was yellowish-white with a straight and intact vascular sheath; F: The preoperative tumor volume was 676.7 mL, and the postoperative tumor weight was 820 g.
- Citation: Xiu WL, Liu J, Zhang JL, Su N, Wang FJ, Hao XW, Wang FF, Dong Q. Computer-assisted rescue of the inferior mesenteric artery in a child with a giant ganglioneuroblastoma: A case report. World J Gastrointest Surg 2023; 15(5): 984-991
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/984.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.984