Published online Jan 15, 2018. doi: 10.4239/wjd.v9.i1.25
Peer-review started: February 12, 2017
First decision: May 17, 2017
Revised: November 17, 2017
Accepted: December 4, 2017
Article in press: December 4, 2017
Published online: January 15, 2018
Processing time: 337 Days and 7.1 Hours
To study complete dose-dependent effects of obestatin on lipolytic and glucose transport activities in human adipocyte preparations highly responsive to insulin.
Adipocytes were prepared by liberase digestion from subcutaneous abdominal adipose tissue obtained from overweight subjects undergoing plastic surgery. The index of lipolytic activity was the glycerol released in the incubation medium, while glucose transport was assessed by [3H]-2-deoxyglucose uptake assay.
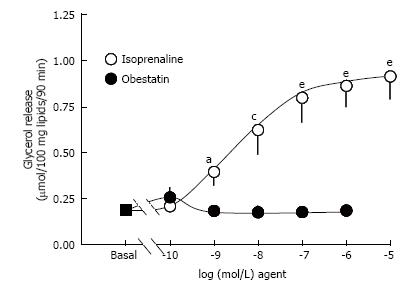
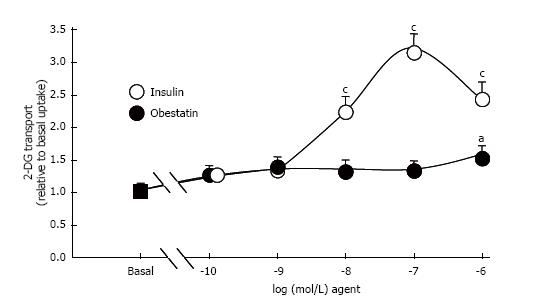
When tested from 0.1 nmol/L to 1 μmol/L, obestatin did not stimulate glycerol release; it did not inhibit the lipolytic effect of isoprenaline and did not alter the insulin antilipolytic effect. Obestatin hardly activated glucose transport at 1 μmol/L only. Moreover, the obestatin stimulation effect was clearly lower than the threefold increase induced by insulin 100 nmol/L.
Low doses of obestatin cannot directly influence lipolysis and glucose uptake in human fat cells.
Core tip: We have compared in adipocytes the well-known glucose uptake stimulation and lipolysis inhibition induced by insulin to the effects of obestatin, a gut peptide derived from ghrelin gene recently proposed to act on fat cells. Obestatin was much less efficient than insulin in adipocytes from human abdominal subcutaneous adipose tissue. Indeed, obestatin weakly activated hexose transport while it could not reproduce the antilipolytic effect of insulin at any tested concentration. We therefore propose that obestatin does not rapidly modulate lipogenesis and lipolysis and that its contribution to energy homeostasis depends on actions other than a direct control of adipocyte metabolism.
- Citation: Carpéné C, Les F, Estève D, Galitzky J. Short-term effects of obestatin on hexose uptake and triacylglycerol breakdown in human subcutaneous adipocytes. World J Diabetes 2018; 9(1): 25-32
- URL: https://www.wjgnet.com/1948-9358/full/v9/i1/25.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i1.25
Obestatin is a 23-amino acid peptide with highly conserved sequence among mammalian species that corresponds to the 76-98 segment of pre-proghrelin, a polypeptide of 117 residues, also generating by cleavage of its 24-51 segment the multifunctional hormone ghrelin. The receptor initially proposed for obestatin was the orphan G protein-coupled receptor GPR39[1]. However, this assumption has never been confirmed[2], and to our knowledge it remains unclear to what receptor can selectively bind obestatin.
Hardly clearer is the overall physiological action of obestatin, which suppresses food intake and decreases body-weight gain, and counteracts the appetite-stimulating properties of ghrelin. At the first glance, the anorectic and catabolic properties attributed to obestatin appear to be opposite to the insulin panel of actions. In fact, obestatin has been reported to limit food intake in rodents under special conditions only, such as fasting-refeeding challenges[1]. Then, it has been evidenced that obestatin failed to affect food intake and gut motility in ghrelin-deficient mice, and in further studies, obestatin administration did not exert clear-cut influence on food intake and body weight[3]. It is therefore currently suggested that obestatin is not a major regulator of satiety signalling[4] while it is still admitted that ghrelin and obestatin may have opposite effects on digestive physiology.
Similarly, the in vitro effects of obestatin directly measured on one of its targets, namely the adipose cell, are far from being univocally demonstrated. Several reports have evidenced that obestatin activates glucose uptake in 3T3-L1 cultured preadipocytes and in mature fat cells[5,6]. Accordingly, obestatin inhibited isoproterenol-induced lipolysis, promoted AMP-activated protein kinase phosphorylation, enhanced adiponectin secretion in both mice and human mature adipocytes. Obestatin also enhanced glucose uptake either in the absence or in the presence of insulin, promoted GLUT4 translocation and increased Akt phosphorylation, according to the studies of Granata and coworkers[6,7]. Also like insulin, obestatin promoted adipogenesis in rat[8] or murine[5] preadipocytes. However, other studies that described an antilipolytic action of obestatin on non-esterified fatty acid and glycerol release, failed to detect any influence on glucose transport[9]. Even a lack of obestatin effect was observed regarding glycerol release or adipogenesis in 3T3-L1 preadipocytes[10], while a pro-lipolytic action was evidenced in other models[11]. Such ability of obestatin to trigger lipid catabolism[12] was therefore hardly conceivable together with the above-reported insulin-like actions. Anyhow, such controversy was dealing with previous observations indicating that obestatin inhibits proliferation and differentiation of 3T3-L1 preadipocytes[3].
In this context, the putative ability of obestatin to modulate glucose uptake deserved to be verified in human native fat cells rather than in any additional engineered insulin-sensitive model. To this aim, and in order to also verify whether obestatin was able to acutely influence adipocyte lipolytic activity, we decided to study its acute effects on human subcutaneous adipocytes. Our approach was further justified by the fact that obestatin is proposed to belong to the large family of adipokines[13] secreted by adipose tissue[7]. A special attention was paid to use insulin-responsive fat cells, thereby to include human insulin as a positive control in our comparative study. Similarly, lipolytic agents such as isoprenaline (a β-adrenoceptor agonist also known as isoproterenol), atrial natriuretic peptide (ANP)[14] and antilipolytic factors such as UK14304 (α2-adrenoceptor agonist) were used as references for the fine regulation of lipolytic activity. Lastly, hydrogen peroxide (H2O2) was also used in our tests since it is known to activate glucose transport independently from insulin[15]. In the following results, we have therefore tested increasing doses of obestatin (0.1 nmol/L - 1 μmol/L) on human fat cells preparations highly responsive to insulin under conditions already validated to investigate the properties of other adipokines[16,17], drugs[18] or dietary components[19].
Recombinant human obestatin was purchased from Phoenix Pharmaceuticals Inc. (Belmont, CA, United States). Human insulin, bovine serum albumin, and other reagents were obtained from Sigma-Aldrich (Saint Quentin Fallavier, F). Liberase TM was from Roche Diagnostic (Indianapolis, IN, United States). [3H]-2-deoxyglucose was from Perkin Elmer (Boston, MA, United States). UK 14304 (bromoxidine) was a generous gift from late Dr Hervé Paris (INSERM, Toulouse, France).
Samples of subcutaneous adipose tissue (SCAT), were obtained from non-obese premenopausal women (age range 29-53 year) undergoing abdominal lipectomy at the plastic surgery department of Rangueil hospital (Toulouse, France) under the agreement of INSERM guidelines and the ethic committee for the protection of individuals under the reference DC-2008-452. The clinical characteristics of the donors and the biochemical profiles of the corresponding adipocyte preparations are described in Table 1. The removed pieces of fat depot were transferred in less than 30 min to the laboratory. SCAT was immediately treated by liberase digestion (15 μg/mL) in the presence of 3.5% of bovine serum albumin in the digestion buffer (Krebs-Ringer containing 15 mmol/L sodium bicarbonate, 10 mmol/L HEPES, 2 mmol/L pyruvate). Separation, washing and dilution of the buoyant adipocytes were performed in the same buffer without liberase as previously described[19], immediately prior biological assays.
| Clinical characteristics of SCAT donors | |
| BMI of subjects, kg/m2 | 26.1 ± 0.7 |
| Age, yr | 40 ± 3 |
| Biochemical features of adipocyte preparations | |
| Cell lipid content/lipolysis assay, mg (n) | 14.1 ± 1.3 (7) |
| Cell lipid content/glucose uptake assay, mg (n) | 15.9 ± 1.3 (10) |
| Lipolytic responsiveness (fold increase over basal glycerol release, n = 7) | |
| Basal | 1.00 ± 0.17 |
| Isoprenaline 10 μmol/L | 5.14 ± 0.67b |
| Human atrial natriuretic peptide 1 μmol/L | 5.16 ± 0.44b |
| Glucose transport capacity (fold increase over basal 2DG uptake, n = 10) | |
| Basal | 1.00 ± 0.13 |
| Insulin 100 nmol/L | 3.14 ± 0.28b |
| Hydrogen peroxide 1 mmol/L | 1.72 ± 0.27a |
Fat cells were diluted in around 10-fold their volume of buffer, and cell suspension was distributed into plastic vials. Lipolytic activity was assessed by the glycerol released by fat cells medium after a 90-min incubation in 400 μL final volume with the tested agents, as previously described[19]. Results were expressed as µmoles of glycerol released/100 mg cellular lipids/90 min, or as percentage of isoprenaline-induced stimulation.
For hexose uptake assays, incubations of the tested agents with fat cell suspensions lasted 45 min at 37 °C before 10 min exposure to 0.1 mmol/L [3H]-2-deoxyglucose (2-DG) as previously described[19]. Separation of internalized hexose was performed on 200 μL aliquots by centrifugation through dynonyl-phtalate silicon oil to separate buoyant intact fat cells from medium[17]. Lipid content was determined as previously reported[20,21]. Uptake was expressed as fold increase over basal uptake, which accounted for 0.30 ± 0.05 nmol 2-DG internalized/100 mg cellular lipids/10 min.
3T3 F442A cells were grown at 37 °C under 5% CO2 in DMEM supplemented with 10% foetal calf serum and antibiotic mixture (100 U/mL penicillin + 100 μg/mL streptomycin) until confluence. Contrarily to their parent cell line 3T3-L1, 3T3-F442A cells do not need isobutylmethylxanthine and dexamethasone to trigger adipogenic process and are in this regard only insulin-dependent[22]. Cells were therefore induced to differentiate by 50 nmol/L insulin for 8 d before being tested for 2-DG uptake.
Results are given as means ± SEM. Statistical significance was assessed by use of Student’s t-test or one-way ANOVA followed by Bonferroni test using Prism 5 for Mac OS X.
Since obestatin has been reported to activate glucose uptake in 3T3-L1 cultured preadipocytes, it was first verified whether our preparation could reproduce such insulin-like activity. However, our preliminary tests were performed on 3T3-F442A lineage, which is slightly distinct from 3T3-L1 cells since only requiring insulin to promote adipocyte differentiation. Eight days after confluence, cells were serum starved overnight and their basal [3H]-2-deoxyglucose uptake was activated by 1.79 ± 0.03 fold and by 1.21 ± 0.04 by 10 nmol/L insulin and 10 nmol/L obestatin, respectively (n = 3; P < 0.001 and P < 0.02). These preliminary observations indicated that obestatin preparation reproduced almost two-third of the insulin effect on glucose uptake and prompted us to treat human fat cells with obestatin.
As shown in Table 1, human adipocytes were isolated from subjects belonging to the normal-to-mild overweight class, according to the body mass index-based classification of obesity. From this group, constituted by a total 13 non-obese premenopausal women undergoing abdominal plastic surgery, there was sufficient SCAT material to test the influence of obestatin on triacylglycerol breakdown in seven cases while glucose uptake assays could be performed on 10 individual adipocyte preparations. When measuring glycerol release, one of the end-products of complete hydrolysis of triacylglycerols, the β-adrenergic agonist isoprenaline maximally stimulated fivefold the baseline, qualifying our test conditions as discriminative enough for studying the effects of any agent supposed to alter lipolytic activity. Other control conditions included atrial natriuretic peptide, which stimulated glycerol release as well as isoprenaline. Regarding glucose transport, human insulin induced a threefold increase of basal uptake (Table 1), which can be considered as a substantial stimulation for insulin-responsive cells. Hydrogen peroxide also significantly activated glucose transport.
While isoprenaline dose-dependently stimulated the lipolytic activity, obestatin did not modify basal lipolysis, when tested from 10-10 to 10-6 mol/L (Figure 1). In the same conditions, another peptide tested in parallel was able to maximally stimulate lipolysis to the same level than isoprenaline: Atrial natriuretic peptide 1 μmol/L (Table 1), indicating that diverse lipolytic agents other than isoprenaline could activate triglyceride breakdown in the tested preparations.
To check whether obestatin needed a pre-activated state of triglyceride breakdown to regulate lipolysis, we co-incubated obestatin with 5 nmol/L isoprenaline. The glycerol release provoked by such threshold dose of isoprenaline also enabled to observe antilipolytic actions. Lipolysis was not altered by obestatin at 10 or 100 nmol/L, indicating that the adipokine was not potentiating or inhibiting a moderate lipolytic activation (Figure 2). On the opposite, insulin, at 10-100 nmol/L, provoked a partial inhibition of the β-adrenergic-induced triglyceride breakdown. Obestatin did not significantly hamper or improve such antilipolytic action, clearly indicating that the adipokine was devoid of antilipolytic effect on its own, or unable to acutely enhance that of insulin. Further tests were performed in the presence of a higher, submaximal dose of isoprenaline. Again no clear-cut antilipolysis was found with obestatin while the α2-adrenergic agonist (UK 14304, also known as bromoxidine) impaired the lipolytic response to isoprenaline (Table 2).
| n | Treatment | Control | Obestatin 1 nmol/L | Obestatin 10 nmol/L | Obestatin 100 nmol/L | UK14304 1 μmol/L | |
| Lipolysis, µmol glycerol/ 100 mg lipid/90 min | 3 | Isoprenaline | 0.64 ± 0.10 | 0.61 ± 0.09 | 0.62 ± 0.10 | 0.62 ± 0.10 | 0.29 ± 0.04a |
| Glucose transport, nmol 2-DG/100 mg lipids/10 min | 10 | Insulin | 0.46 ± 0.14 | 0.45 ± 0.09 | 0.45 ± 0.09 | 0.43 ± 0.09 | ND |
Insulin dose-dependently stimulated the 2-DG uptake of human adipocytes, with a detectable effect at 10 nmol/L and a maximum at 100 nmol/L. A decline relative to the maximal insulin stimulation was observed at the high concentration of 1 μmol/L. By contrast, no clear-cut change in glucose uptake was observed in response to obestatin, save at this high micromolar dose (Figure 3). The significant stimulation of hexose uptake observed with 1 μmol/L obestatin was increasing baseline by 1.52 ± 0.19 fold. However such increase of hexose uptake by obestatin accounted for only 29% of the maximal response to insulin. During these tests, 1 mmol/L hydrogen peroxide also partially reproduced the insulin stimulation of hexose uptake (Table 1).
Since obestatin on its own was not able to fully mimic the insulin activation of glucose transport, it was further tested whether it was favouring the action of a threshold dose of the pancreatic hormone. However, obestatin, from 1 to 100 nmol/L, did not modify the 5 nmol/L insulin action (Table 2).
Taken together, our results indicate that obestatin does not act as a fast-acting antilipolytic agent or as a strong activator of glucose transport in human subcutaneous adipocytes.
Our observations are therefore in apparent contradiction with those of Granata and coworkers, who previously reported that obestatin inhibits lipolysis and activates glucose transport in 3T3-L1 murine preadipocytes, and in human omental and subcutaneous adipocytes[6]. However, in our study, the stimulation of glucose transport by insulin was equivalent to a threefold increase over basal uptake in adipocytes from overweight subjects, i.e., reaching a magnitude greater than the insulin responsiveness found in the human fat cell preparations used by Granata et al[6] or other research teams[23], which hardly reached a doubling of baseline. Indeed, when looking into details of glucose transport, the human fat cell preparations studied by Granata et al[6] were not overtly insulin-responsive: Insulin 100 nmol/L was activating basal hexose uptake by approximately a 1.3 fold factor. Consequently, it was feasible, for Granata et al[6], to conclude that obestatin largely reproduced the feeble action of insulin, while we observed here that 1 μmol/L obestatin concentration of peptide hardly induced one-third of the maximal response to insulin. Therefore, with a similar feeble activation of hexose uptake by obestatin, two distinct interpretations could be drawn since the difference lies mainly in the maximal activation by insulin, the “golden reference” for stimulation of glucose utilization. In fact, insulin responsiveness can dramatically decline until complete resistance when obesity is complicated with type 2 diabetes, making that the use of insulin-resistant fat cells is not a good tool to underscore insulin-mimicking factors. In this view, hydrogen peroxide, known as a partial insulin mimicker[15] regarding glucose uptake[24], was effective in human adipocytes under our conditions.
At this time, it is important to note that the lack of clear-cut stimulation of glucose uptake into human adipocytes reported here for 0.1-100 nmol/L obestatin totally agrees with a previous observation made on 3T3-L1 differentiated preadipocytes[9] and with its antiadipogenic properties found in the same cell lineage[3]. All these findings are therefore contrasting with the reported obestatin ability to improve insulin effect on glucose carrier translocation in several fat cell models[6]. Although the equipment in GPR 39, the controversial obestatin receptor[25], is less abundant in adipocytes from obese and diabetic subjects[26], it is difficult to support that such putative insulin-like effect of the adipokine on hexose uptake was improved in the insulin-resistant preparations and lowered in the insulin-sensitive ones.
Another amazing observation was that 1 μmol/L obestatin was able to stimulate glucose uptake weakly but significantly, while at lower doses it was unable to improve the submaximal action of insulin at 5 nmol/L. One could ask about the purity of our used preparation, but unfortunately we did not verify by chemical analyses the composition given by the furnisher. It could also be argued that the peptide was degraded before/during incubation with fat cells. Though we did not perform a before/after comparison of the incubation medium containing obestatin and fat cells, it can be assessed that the peptide preparation was correctly efficient on its own since it activated glucose uptake in 3T3-F442A preadipocytes. Moreover, in our hands, another peptide preparation, that of ANP, fully exhibited its recognized lipolytic action in human adipocytes[14]. Lastly, it is barely conceivable that a putative contaminant inhibited obestatin action and not that of insulin, since there was no impairment when obestatin preparation was tested in combination with insulin. Therefore, despite all the precautions that may be taken for the interpretation of our data, we propose that the only detected effect of obestatin on human adipocytes, occurring at 1 μmol/L, has to be considered as extraphysiological. This should also apply to the same micromolar dose of insulin, which also behaved strikingly, since less efficient than 100 nmol/L of the pancreatic hormone, the recognized reference for maximal activation of glucose uptake. Such assessment against the specificity of relatively high dosages does not mean that the maximal insulin action cannot be overpassed in adipocyte preparations. On the opposite, we confirmed in human fat cells, that the antilipolytic effect of the α2-adrenergic agonist (UK 14304) largely overpassed that of insulin. In contrast, no clear-cut antilipolytic action of obestatin could be detected when tested alone or even when combined with insulin. Again, our observations were not so different from those previous studies[6] in which only a modest antilipolytic effect of obestatin was observed, but without exhibiting a classical sigmoidal dose-dependent curve. Taken together, the data reported so far do not support that obestatin is directly regulating triglyceride breakdown in human adipocytes, at least during short-term incubations.
Our observations do not definitely close the characterization of the short-term insulin-like effects of obestatin, but prompt to recall the history of the insulin-like properties attributed transiently to visfatin by Shimomura and coworkers before a retraction of their original findings[27] and a lack of confirmation of such properties by various verification studies[16]. Thus, the capacity of obestatin to fully mimic short-term insulin-like actions (such as glucose transport activation or triglyceride breakdown inhibition) remains questionable owing to the small magnitude of the responses, if any. Obviously, it cannot be definitively ruled out that obestatin can promote some modulation of other lipolytic and lipogenic regulators, or act after longer exposure via other cells present in adipose tissue, therefore operating by mechanisms different from direct activation of fat cell receptors.
Anyhow, no insulin-like property is necessary for obestatin to exert a physiological adipokine role, together with other members of the ghrelin family. The concern is to clarify whether obestatin can be considered as a “fair” adipokine, like adiponectin, increasing insulin responsiveness and decreasing with obesity, or as a deleterious one, like many other pro-inflammatory cytokines linked to obesity-related insulin resistance.
In conclusion, our results did not confirm a direct biological regulatory effect of obestatin on glucose transport and triglyceride breakdown in fat cells from human subcutaneous adipose tissue, rendering questionable the occurrence of an obestatin-dependent modulation of lipogenic and lipolytic activities that might relay or help the defective responsiveness to insulin in pre-diabetic and diabetic states.
Obestatin is a gut hormone, derived from the same gene as ghrelin and involved in food intake regulation. This peptide, initially proposed to bind to the G protein-coupled receptor GPR39 is active in the digestive tract, pituitary and adipose tissues. Initially, obestatin was reported to inhibit triacylglycerol hydrolysis in cultured murine 3T3-L1 adipocytes and in human adipocytes. Another insulin-like property was added to the panel of obestatin actions: The stimulation of glucose transport into fat cells. However, several recent reports have indicated that obestatin may activate lipolysis and raised confusion about its role in the modulation of triacylglycerol storage/mobilization. Thus, it was of interest to verify whether processes that are exquisitely regulated by insulin (glucose utilisation and lipid mobilisation by adipocytes) were also modulated by obestatin in human adipocytes.
The study aimed at determining complete dose-dependent effects of human obestatin in human subcutaneous fat cells. Such approach brings additional evidence that obestatin cannot readily and rapidly reproduce the antilipolytic action of insulin, while it confirms that the α2-adrenergic agonist bromoxidine surpasses the insulin-induced inhibition of lipolysis in human fat cells. At 1 μmol/L, obestatin induces a moderate activation of hexose uptake in fat cells, the magnitude of which is too modest to assess definitively that the peptide acts as an insulin mimicker.
Although a direct regulatory action on adipocyte lipolysis/lipogenesis does not seem to contribute to the multifunctional in vivo actions of obestatin, our observations do not exclude a long-term influence of the peptide on adipocyte biology in healthy, obese or diabetic subjects. Whether such long-term actions might be beneficial to combat obesity and diabetes linked complications remains to be clarified.
Obestatin is primarily a gut hormone, derived from the same gene as ghrelin and should belong to the multiple steps linking digestive tract function and food intake regulation. Nevertheless, its apparent lack of direct action on target cells such as the adipocytes, which are involved in the regulation of energy balance and glucose handling, does not allow proposing novel obestatin-based therapeutic approaches in combating obesity and diabetes.
ANP: Atrial natriuretic peptide; SCAT: Subcutaneous adipose tissue; BMI: Body mass index SEM: Standard error of the mean; 2-DG: 2-deoxyglucose.
We thank the staff of plastic surgery of Rangueil Hospital (Toulouse, France) for providing us with surgical samples from abdominal lipectomy and Estelle Wanecq for technical assistance. The authors also thank Anne Bouloumié (I2MC, Toulouse, France) for helpful discussions and Anaïs Briot for improving the manuscript. In memoriam to Michel Berlan and to Jean Claude Murat.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hussain SAR, Mitra A, Zhao J S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 778] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 2. | Chartrel N, Alvear-Perez R, Leprince J, Iturrioz X, Reaux-Le Goazigo A, Audinot V, Chomarat P, Coge F, Nosjean O, Rodriguez M. Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake”. Science. 2007;315:766; author reply 766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Tang SQ, Jiang QY, Zhang YL, Zhu XT, Shu G, Gao P, Feng DY, Wang XQ, Dong XY. Obestatin: its physicochemical characteristics and physiological functions. Peptides. 2008;29:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Depoortere I, Thijs T, Moechars D, De Smet B, Ver Donck L, Peeters TL. Effect of peripheral obestatin on food intake and gastric emptying in ghrelin-knockout mice. Br J Pharmacol. 2008;153:1550-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Gurriarán-Rodríguez U, Al-Massadi O, Roca-Rivada A, Crujeiras AB, Gallego R, Pardo M, Seoane LM, Pazos Y, Casanueva FF, Camiña JP. Obestatin as a regulator of adipocyte metabolism and adipogenesis. J Cell Mol Med. 2011;15:1927-1940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Granata R, Gallo D, Luque RM, Baragli A, Scarlatti F, Grande C, Gesmundo I, Córdoba-Chacón J, Bergandi L, Settanni F. Obestatin regulates adipocyte function and protects against diet-induced insulin resistance and inflammation. FASEB J. 2012;26:3393-3411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Granata R, Ghigo E. Products of the ghrelin gene, the pancreatic β-cell and the adipocyte. Endocr Dev. 2013;25:144-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Wojciechowicz T, Skrzypski M, Kołodziejski PA, Szczepankiewicz D, Pruszyńska-Oszmałek E, Kaczmarek P, Strowski MZ, Nowak KW. Obestatin stimulates differentiation and regulates lipolysis and leptin secretion in rat preadipocytes. Mol Med Rep. 2015;12:8169-8175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Miegueu P, St Pierre D, Broglio F, Cianflone K. Effect of desacyl ghrelin, obestatin and related peptides on triglyceride storage, metabolism and GHSR signaling in 3T3-L1 adipocytes. J Cell Biochem. 2011;112:704-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Ren G, He Z, Cong P, Yu J, Qin Y, Chen Y, Liu X. Effect of TAT-obestatin on proliferation, differentiation, apoptosis and lipolysis in 3T3-L1 preadipocytes. J Pept Sci. 2013;19:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Pruszynska-Oszmalek E, Szczepankiewicz D, Hertig I, Skrzypski M, Sassek M, Kaczmarek P, Kolodziejski PA, Mackowiak P, Nowak KW, Strowski MZ. Obestatin inhibits lipogenesis and glucose uptake in isolated primary rat adipocytes. J Biol Regul Homeost Agents. 2013;27:23-33. [PubMed] |
| 12. | Nagaraj S, Raghavan AV, Rao SN, Manjappara UV. Obestatin and Nt8U influence glycerolipid metabolism and PPAR gamma signaling in mice. Int J Biochem Cell Biol. 2014;53:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Aktas B, Yilmaz Y, Eren F, Yonal O, Kurt R, Alahdab YO, Celikel CA, Ozdogan O, Imeryuz N, Kalayci C. Serum levels of vaspin, obestatin, and apelin-36 in patients with nonalcoholic fatty liver disease. Metabolism. 2011;60:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345-1351. [PubMed] |
| 15. | Ludvigsen C, Jarett L. Similarities between insulin, hydrogen peroxide, concanavalin A, and anti-insulin receptor antibody stimulated glucose transport: increase in the number of transport sites. Metabolism. 1982;31:284-287. [PubMed] |
| 16. | Wanecq E, Prévot D, Carpéné C. Lack of direct insulin-like action of visfatin/Nampt/PBEF1 in human adipocytes. J Physiol Biochem. 2009;65:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Carpéné C, Galitzky J, Saulnier-Blache JS. Short-term and rapid effects of lysophosphatidic acid on human adipose cell lipolytic and glucose uptake activities. AIMS Molec Sci. 2016;3:222-237. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Mercader J, Wanecq E, Chen J, Carpéné C. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J Physiol Biochem. 2011;67:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Gomez-Zorita S, Tréguer K, Mercader J, Carpéné C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J Physiol Biochem. 2013;69:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Bour S, Daviaud D, Gres S, Lefort C, Prévot D, Zorzano A, Wabitsch M, Saulnier-Blache JS, Valet P, Carpéné C. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie. 2007;89:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Atgié C, Sauvant P, Ambid L, Carpéné C. Possible mechanisms of weight loss of Siberian hamsters (Phodopus sungorus sungorus) exposed to short photoperiod. J Physiol Biochem. 2009;65:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Pairault J, Lasnier F. Control of the adipogenic differentiation of 3T3-F442A cells by retinoic acid, dexamethasone, and insulin: a topographic analysis. J Cell Physiol. 1987;132:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Sancho V, Nuche B, Arnés L, Cancelas J, González N, Díaz-Miguel M, Martín-Duce A, Valverde I, Villanueva-Peñacarrillo ML. The action of GLP-1 and exendins upon glucose transport in normal human adipocytes, and on kinase activity as compared to morbidly obese patients. Int J Mol Med. 2007;19:961-966. [PubMed] |
| 24. | May JM, de Haën C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J Biol Chem. 1979;254:9017-9021. [PubMed] |
| 25. | Dong XY, He JM, Tang SQ, Li HY, Jiang QY, Zou XT. Is GPR39 the natural receptor of obestatin? Peptides. 2009;30:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Catalán V, Gómez-Ambrosi J, Rotellar F, Silva C, Gil MJ, Rodríguez A, Cienfuegos JA, Salvador J, Frühbeck G. The obestatin receptor (GPR39) is expressed in human adipose tissue and is down-regulated in obesity-associated type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2007;66:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H. Retraction. Science. 2007;318:565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |