Peer-review started: August 16, 2016
First decision: September 2, 2016
Revised: September 17, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: January 15, 2017
Diabetic retinopathy affects a substantial proportion of patients with diabetes mellitus (DM) and is the leading cause of blindness in working-aged adults. Even though the incidence of diabetic retinopathy has declined in the last decades, its prevalence increased and is expected to rise further as a result of the increasing incidence of type 2 DM (T2DM) and the longer life expectancy of patients with DM. The pathogenesis of diabetic retinopathy is multifactorial. Some observational studies suggested an association between dyslipidemia and the development and progression of retinopathy in patients with DM but others did not confirm this association. Regarding lipid-lowering agents, studies that evaluated the role of statins in the management of these patients are mostly small and yielded discrepant results. Large randomized studies with statins in patients with T2DM showed no benefit of these agents on diabetic retinopathy but were not designed to address this effect. In contrast, both preclinical data and two large randomized controlled studies, the FIELD and the ACCORD trial, showed that fenofibrate delays the progression of diabetic retinopathy. Even though the mechanisms underpinning this favorable effect are not entirely clear, these findings suggest that fenofibrate might represent a useful tool for the management of diabetic retinopathy.
Core tip: Even though it is unclear whether dyslipidemia is implicated in the pathogenesis of diabetic retinopathy, both preclinical data and two large randomized controlled studies showed that treatment with fenofibrate delays the progression of diabetic retinopathy. In contrast, statins do not appear to play a role in the management of this complication despite the promising findings of animal studies.
- Citation: Ioannidou E, Tseriotis VS, Tziomalos K. Role of lipid-lowering agents in the management of diabetic retinopathy. World J Diabetes 2017; 8(1): 1-6
- URL: https://www.wjgnet.com/1948-9358/full/v8/i1/1.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i1.1
Diabetic retinopathy affects 27%-40% of patients with diabetes mellitus (DM)[1-4] and is the leading cause of blindness in working-aged adults[5]. Even though the incidence of diabetic retinopathy has declined in the last decades[6,7], its prevalence increased and is expected to rise further as a result of the increasing incidence of type 2 DM (T2DM) and the longer life expectancy of patients with DM[1,8].
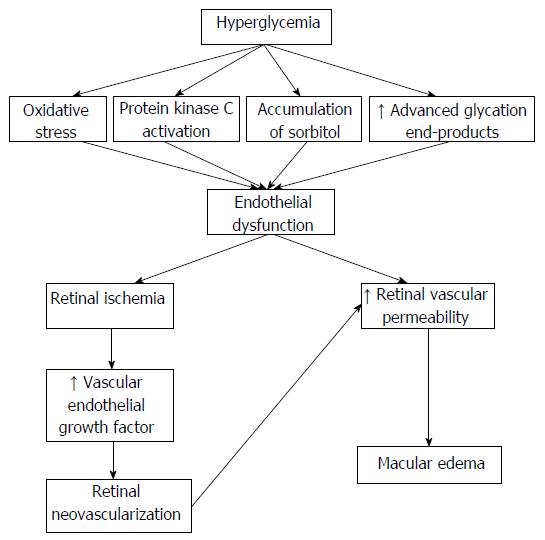
The pathogenesis of diabetic retinopathy is complex and involves many different pathways (Figure 1). Hyperglycemia is a major culprit and induces: (1) accumulation of advanced glycation end-products (AGE), which promote retinal pericyte loss; (2) inflammation, which increases vascular permeability and apoptosis of endothelial and neural cells; (3) protein kinase C (PKC) activation, which increases expression of matrix proteins and induces pericyte apoptosis; (4) accumulation of sorbitol through the polyol pathway, which damages endothelial cells and pericytes; (5) activation of the renin-angiotensin system, which induces vascular endothelial growth factor (VEGF) expression; and (6) oxidative stress, which further increases AGE accumulation and activation of PKC and the polyol pathway[9,10]. The above pathogenetic mechanisms result in endothelial dysfunction, which in turn induces retinal ischemia and increases retinal vascular permeability[9,10]. The former up-regulates the expression of VEGF, erythropoietin, carbonic anhydrase and growth hormone, which in turn promote neovascularization[9,10]. On the other hand, increased retinal vascular permeability might result in macular edema[9,10]. In addition to microvascular disease, neuroretinal damage is also implicated in the development and worsening of diabetic retinopathy, since increased neuronal apoptosis is observed in the retina in these patients[9,10]. Accordingly, tight glycemic control and aggressive blood pressure-lowering, particularly with blockers of the renin-angiotensin system, considerably reduce the risk for diabetic retinopathy[11-14]. However, only a minority of patients with DM achieves glycemic and blood pressure targets[15,16]. Moreover, hyperglycemia and hypertension only partly account for the risk of development and progression of diabetic retinopathy, suggesting that other pathogenetic mechanisms also play a role[9,10,17].
In this context, some observational studies suggested that elevated serum low-density lipoprotein cholesterol (LDL-C) levels are associated with increased incidence for diabetic retinopathy[18-20]. However, others did not confirm this association[21-23]. Elevated serum triglyceride levels also increased the risk for diabetic retinopathy in some reports[22,24] but not in others[23] whereas elevated high-density lipoprotein cholesterol levels were protective in some studies[22] but not in others[20,23]. In addition, perivascular deposition of lipid-laden macrophages has been reported in the retina of patients with diabetic retinopathy[25]. Moreover, small uncontrolled studies suggest that low-fat diet reduces hard exudates[26,27].
Statins act by inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase. This results in reduced serum LDL-C levels but also inhibits the mevalonate pathway, which results in reduced production of isoprenoids, including farnesyl pyrophosphate and geranylgeranyl pyrophosphate[28]. The latter leads to reduced prenylation (i.e., addition of farnesyl and geranylgeranyl to cysteine residues of proteins), which modulates a host of pathogenetic mechanisms involved in diabetic retinopathy, including inflammation, oxidative stress, angiogenesis and endothelial dysfunction[28]. In retinal endothelial cells, statins exhibit antiangiogenic actions by suppressing VEGF phosphorylation[29]. In retinal pigment epithelial cells, statins decrease the expression of matrix metalloproteinases (MMP), preventing the breakdown of the blood-retinal barrier[30]. Moreover, in animal models of diabetic retinopathy, treatment with statins prevented the upregulation of VEGF and preserved the blood-retinal barrier by exerting antioxidant[31-33] and anti-inflammatory effects[34,35]. In other preclinical studies, statins induced endothelium-dependent, nitric oxide-mediated vasodilation in retinal arteries[36].
In subjects without DM, statins improve endothelial function in the choroidal vasculature[37] and increase the blood flow in retinal arteries and veins[38]. In patients with diabetic retinopathy, statins reduce vascular resistance in the ophthalmic and central retinal arteries[39]. In addition, vitreous concentrations of VEGF, angiopoietin-2, MMP-9 and transforming growth factor β1 are lower in patients with diabetic retinopathy treated with statins[40].
A recent observational study showed that treatment with statins prior to vitrectomy is associated with greater improvement in visual acuity, particularly in patients who also underwent laser photocoagulation or received treatment with antibodies against VEGF and in those who had macular edema, vitreous hemorrhage, retinal detachment or proliferative retinopathy[41]. Treatment with statins also reduced the risk for repeat vitrectomy[41]. In a recent nationwide matched cohort study from Denmark (n = 62716), patients who were using statins prior to the diagnosis of DM had 40% lower risk for developing diabetic retinopathy[42]. However, other observational studies did not show a protective role of statins against retinopathy in patients with established DM[21,43].
Early case reports in patients with either type 1 DM (T1DM) or T2DM reported reduction of hard exudates and microaneurysms after treatment with statins[25,44]. Studies evaluating the effects of statins on diabetic retinopathy are shown in the Table 1. In a randomized study in 50 patients with T1DM or T2DM, treatment with simvastatin for 6 mo retarded the progression of retinopathy compared with placebo[45]. In an uncontrolled study in 18 patients with diabetic maculopathy, treatment with atorvastatin for 12 mo reduced hard exudates and fluorescein leakage[46]. In a randomized study in 30 patients with T2DM and macular edema, treatment with atorvastatin for 18 wk reduced the number of hard exudates compared with no lipid-lowering treatment but did not affect macular edema or visual acuity[47]. In addition, in a more recent randomized study in 30 patients with macular edema, treatment with atorvastatin for 6 mo had no effect on hard exudates, macular edema or visual acuity compared with no lipid-lowering treatment[48].
| Ref. | Type of diabetes mellitus | n | Treatment | Dose (mg/d) | Follow-up (mo) | Outcome |
| [45] | 1 and 2 | 50 | Simvastatin | 20 | 6 | Delayed progression of retinopathy |
| [46] | 2 | 18 | Atorvastatin | 20 | 12 | Reduction of hard exudates and fluorescein leakage |
| [47] | 2 | 30 | Atorvastatin | 10 | 4.5 | Reduction of hard exudates but no effect on macular edema or visual acuity |
| [48] | 2 | 30 | Atorvastatin | 20 | 6 | No effect on hard exudates, macular edema or visual acuity |
| [49] | 2 | 2838 | Atorvastatin | 10 | 47 | No effect on the incidence of laser treatment or progression of retinopathy |
| [50] | 2 | 5963 | Simvastatin | 40 | 58 | No effect on the incidence of laser treatment |
| [59] | 2 | 9795 | Fenofibrate | 200 | 60 | Reduction of the need for laser photocoagulation and the risk of progression of retinopathy but no effect on the development of retinopathy in patients without retinopathy at baseline or on the risk of deterioration of visual acuity |
| [60] | 2 | 2856 | Fenofibrate | 160 | 48 | Reduction of the rate of progression of retinopathy but no effect on the occurrence of moderate vision loss |
In addition to the conflicting results of these observational and small interventional studies, randomized, placebo-controlled trials did not show a beneficial effect of statins on diabetic retinopathy. In the Collaborative Atorvastatin Diabetes Study, treatment with atorvastatin 10 mg/d for 3.9 years had no effect on the risk of laser therapy or progression of retinopathy in 2838 patients with T2DM[49]. In the Heart Protection Study (n = 5963 patients with T2DM followed-up for 4.8 years), the incidence of laser treatment also did not differ between patients treated with simvastatin 40 mg/d and those who received placebo[50]. However, neither of these studies was designed to evaluate the effect of statins on diabetic retinopathy.
Fibrates act by inhibiting the nuclear receptor peroxisome proliferator-activated receptor-α (PPARα). PPARα activation not only mediates the lipid-lowering effects of fibrates but also results in inhibition of inflammation by suppression of nuclear factor κB and by direct binding to genes encoding proinflammatory cytokines[51]. Fenofibrate prevents the apoptosis of retinal endothelial cells[52] and of retinal pigment epithelial cells[53]. It also reduces retinal vascular permeability by exerting antiinflammatory effects and by suppressing the upregulation of fibronectin and collagen IV in the basal membrane of retinal capillaries[54,55]. Moreover, fenofibrate prevents the disruption of the retinal pigment epithelium[56]. Similar with statins, fenofibrate induces endothelium-dependent, nitric oxide-mediated vasodilation in retinal arteries[57].
Early studies reported a decrease in hard exudates after treatment with clofibrate[58]. More importantly, in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial (n = 9795 patients with T2DM), treatment with fenofibrate for 5 years reduced the need for laser photocoagulation by 31% (P = 0.002) and the risk of progression of retinopathy by 79% (P = 0.004) compared with placebo[59]. It was estimated that 17 patients with retinopathy had to be treated with fenofibrate for 5 years to prevent one laser treatment[59]. Interestingly, these benefits were apparent within 8 mo of initiation of fenofibrate treatment, suggesting that other mechanisms than lipid-lowering might be implicated[59]. However, fenofibrate had no effect on the development of retinopathy in patients without retinopathy at baseline and did not prevent the deterioration of visual acuity[59]. More recently, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye study (n = 2856 patients with T2DM), treatment with fenofibrate for 4 years reduced the rate of progression of retinopathy by 40% (P = 0.006) compared with placebo but again did not affect the occurrence of moderate vision loss[60].
In an early study, administration of omega-3 fatty acids to streptozotocin-induced diabetic rats did not affect pericyte loss and increased the formation of acellular, occluded capillaries in the retina[61]. However, in more recent animal studies, treatment with omega-3 fatty acids preserved retinal function[62,63]. However, there are no studies that evaluated the effects of omega-3 fatty acids on diabetic retinopathy in humans. There are also no studies evaluating the safety and efficacy of colesevelam or ezetimibe in animal models or patients with diabetic retinopathy.
It is unclear whether dyslipidemia is implicated in the pathogenesis of diabetic retinopathy. Observational studies reported conflicting findings regarding the association between lipids and development or progression of diabetic retinopathy. Moreover, studies that evaluated the role of statins in the management of these patients are mostly small and yielded discrepant results. Large randomized studies with statins in patients with T2DM showed no benefit of statins on diabetic retinopathy but were not designed to address this effect. In contrast, both preclinical data and two large randomized controlled studies, the FIELD and the ACCORD trial, showed that fenofibrate delay the progression of diabetic retinopathy. Even though the mechanisms underpinning this benefit are still not entirely clear, these findings suggest that fenofibrate might represent a useful tool for the management of this diabetic retinopathy.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beltowski J, Takebayashi K, Verrotti A S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF; Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 701] [Cited by in F6Publishing: 724] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 2. | Candrilli SD, Davis KL, Kan HJ, Lucero MA, Rousculp MD. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications. 2007;21:306-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2576] [Cited by in F6Publishing: 2724] [Article Influence: 227.0] [Reference Citation Analysis (3)] |
| 4. | Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 736] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 5. | Congdon N, O’Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1632] [Cited by in F6Publishing: 1708] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 6. | Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2010;117:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Klein R, Klein BE. Are individuals with diabetes seeing better?: a long-term epidemiological perspective. Diabetes. 2010;59:1853-1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in F6Publishing: 2812] [Article Influence: 281.2] [Reference Citation Analysis (1)] |
| 9. | Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1781] [Cited by in F6Publishing: 1915] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 10. | Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1061] [Cited by in F6Publishing: 1131] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 11. | The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17510] [Cited by in F6Publishing: 15822] [Article Influence: 510.4] [Reference Citation Analysis (3)] |
| 12. | Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 504] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 13. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [PubMed] [Cited in This Article: ] |
| 14. | Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713. [PubMed] [Cited in This Article: ] |
| 15. | Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group, Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, Orchard TJ. Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med. 2009;169:1307-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 429] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 16. | Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368:1613-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 708] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 17. | Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA. 2010;303:2291-2292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Chew EY, Klein ML, Ferris FL, Remaley NA, Murphy RP, Chantry K, Hoogwerf BJ, Miller D. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079-1084. [PubMed] [Cited in This Article: ] |
| 19. | Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53:2883-2892. [PubMed] [Cited in This Article: ] |
| 20. | Romero P, Salvat M, Fernández J, Baget M, Martinez I. Renal and retinal microangiopathy after 15 years of follow-up study in a sample of Type 1 diabetes mellitus patients. J Diabetes Complications. 2007;21:93-100. [PubMed] [Cited in This Article: ] |
| 21. | Klein BE, Myers CE, Howard KP, Klein R. Serum Lipids and Proliferative Diabetic Retinopathy and Macular Edema in Persons With Long-term Type 1 Diabetes Mellitus: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. JAMA Ophthalmol. 2015;133:503-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45:910-918. [PubMed] [Cited in This Article: ] |
| 23. | Cohen RA, Hennekens CH, Christen WG, Krolewski A, Nathan DM, Peterson MJ, LaMotte F, Manson JE. Determinants of retinopathy progression in type 1 diabetes mellitus. Am J Med. 1999;107:45-51. [PubMed] [Cited in This Article: ] |
| 24. | Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL, Knatterud GL. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998;39:233-252. [PubMed] [Cited in This Article: ] |
| 25. | Cusick M, Chew EY, Chan CC, Kruth HS, Murphy RP, Ferris FL. Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110:2126-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Ernest I, Linnér E, Svanborg A. Carbohydrate-rich, fat-poor diet in diabetes. Am J Med. 1965;39:594-600. [PubMed] [Cited in This Article: ] |
| 27. | Van Eck WF. The effect of a low fat diet on the serum lipids in diabetes and its significance in diabetic retinopathy. Am J Med. 1959;27:196-211. [PubMed] [Cited in This Article: ] |
| 28. | Athyros VG, Kakafika AI, Tziomalos K, Karagiannis A, Mikhailidis DP. Pleiotropic effects of statins--clinical evidence. Curr Pharm Des. 2009;15:479-489. [PubMed] [Cited in This Article: ] |
| 29. | Hata Y, Miura M, Asato R, Kita T, Oba K, Kawahara S, Arita R, Kohno R, Nakao S, Ishibashi T. Antiangiogenic mechanisms of simvastatin in retinal endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2010;248:667-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Dorecka M, Francuz T, Garczorz W, Siemianowicz K, Romaniuk W. The influence of elastin degradation products, glucose and atorvastatin on metalloproteinase-1, -2, -9 and tissue inhibitor of metalloproteinases-1, -2, -3 expression in human retinal pigment epithelial cells. Acta Biochim Pol. 2014;61:265-270. [PubMed] [Cited in This Article: ] |
| 31. | Fernandes R, Bento CF, Matafome P, Sena CM, Seiça RM, Pereira P. Atorvastatin-mediated protection of the retina in a model of diabetes with hyperlipidemia. Can J Physiol Pharmacol. 2014;92:1037-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231-3238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 34. | Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009;89:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Miyahara S, Kiryu J, Yamashiro K, Miyamoto K, Hirose F, Tamura H, Katsuta H, Nishijima K, Tsujikawa A, Honda Y. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004;164:1697-1706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Nagaoka T, Hein TW, Yoshida A, Kuo L. Simvastatin elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and mevalonate-rho kinase pathways. Invest Ophthalmol Vis Sci. 2007;48:825-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Bayerle-Eder M, Fuchsjäger-Mayrl G, Sieder A, Polska E, Roden M, Stulnig T, Bischof MG, Waldhäusl W, Schmetterer L, Wolzt M. Effect of pravastatin on responsiveness to N-monomethyl-L-arginine in patients with hypercholesterolaemia. Atherosclerosis. 2002;160:177-184. [PubMed] [Cited in This Article: ] |
| 38. | Nagaoka T, Takahashi A, Sato E, Izumi N, Hein TW, Kuo L, Yoshida A. Effect of systemic administration of simvastatin on retinal circulation. Arch Ophthalmol. 2006;124:665-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Ozkiris A, Erkiliç K, Koç A, Mistik S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br J Ophthalmol. 2007;91:69-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Tuuminen R, Sahanne S, Loukovaara S. Low intravitreal angiopoietin-2 and VEGF levels in vitrectomized diabetic patients with simvastatin treatment. Acta Ophthalmol. 2014;92:675-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Tuuminen R, Sahanne S, Haukka J, Loukovaara S. Improved outcome after primary vitrectomy in diabetic patients treated with statins. Eur J Ophthalmol. 2016;26:174-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2:894-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Zhang J, McGwin G. Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol. 2007;125:1096-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Gordon B, Chang S, Kavanagh M, Berrocal M, Yannuzzi L, Robertson C, Drexler A. The effects of lipid lowering on diabetic retinopathy. Am J Ophthalmol. 1991;112:385-391. [PubMed] [Cited in This Article: ] |
| 45. | Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56:1-11. [PubMed] [Cited in This Article: ] |
| 46. | Panagiotoglou TD, Ganotakis ES, Kymionis GD, Moschandreas JA, Fanti GN, Charisis SK, Malliaraki NE, Tsilimbaris MK. Atorvastatin for diabetic macular edema in patients with diabetes mellitus and elevated serum cholesterol. Ophthalmic Surg Lasers Imaging. 2010;41:316-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Narang S, Sood S, Kaur B, Singh R, Mallik A, Kaur J. Atorvastatin in clinically-significant macular edema in diabetics with a normal lipid profile. Nepal J Ophthalmol. 2012;4:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2728] [Cited by in F6Publishing: 2550] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 50. | Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005-2016. [PubMed] [Cited in This Article: ] |
| 51. | Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Anti-inflammatory effects of fibrates: an overview. Curr Med Chem. 2009;16:676-684. [PubMed] [Cited in This Article: ] |
| 52. | Kim J, Ahn JH, Kim JH, Yu YS, Kim HS, Ha J, Shinn SH, Oh YS. Fenofibrate regulates retinal endothelial cell survival through the AMPK signal transduction pathway. Exp Eye Res. 2007;84:886-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Miranda S, González-Rodríguez Á, García-Ramírez M, Revuelta-Cervantes J, Hernández C, Simó R, Valverde ÁM. Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J Cell Physiol. 2012;227:2352-2362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Trudeau K, Roy S, Guo W, Hernández C, Villarroel M, Simó R, Roy S. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci. 2011;52:6348-6354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Roy S, Kim D, Hernández C, Simó R, Roy S. Beneficial effects of fenofibric acid on overexpression of extracellular matrix components, COX-2, and impairment of endothelial permeability associated with diabetic retinopathy. Exp Eye Res. 2015;140:124-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Villarroel M, Garcia-Ramírez M, Corraliza L, Hernández C, Simó R. Fenofibric acid prevents retinal pigment epithelium disruption induced by interleukin-1β by suppressing AMP-activated protein kinase (AMPK) activation. Diabetologia. 2011;54:1543-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Omae T, Nagaoka T, Tanano I, Kamiya T, Yoshida A. Fenofibrate, an anti-dyslipidemia drug, elicits the dilation of isolated porcine retinal arterioles: role of nitric oxide and AMP-activated protein kinase. Invest Ophthalmol Vis Sci. 2012;53:2880-2886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Duncan LJ, Cullen JF, Ireland JT, Nolan J, Clarke BF, Oliver MF. A three-year trial of atromid therapy in exudative diabetic retinopathy. Diabetes. 1968;17:458-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 707] [Cited by in F6Publishing: 692] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 60. | ACCORD Study Group; ACCORD Eye Study Group, Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 821] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 61. | Hammes HP, Weiss A, Führer D, Krämer HJ, Papavassilis C, Grimminger F. Acceleration of experimental diabetic retinopathy in the rat by omega-3 fatty acids. Diabetologia. 1996;39:251-255. [PubMed] [Cited in This Article: ] |
| 62. | Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes. 2012;2:e36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Yee P, Weymouth AE, Fletcher EL, Vingrys AJ. A role for omega-3 polyunsaturated fatty acid supplements in diabetic neuropathy. Invest Ophthalmol Vis Sci. 2010;51:1755-1764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |