Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.534
Peer-review started: June 28, 2016
First decision: August 5, 2016
Revised: August 18, 2016
Accepted: September 7, 2016
Article in press: September 9, 2016
Published online: November 15, 2016
To study the effects of linagliptin on the structural signs of non-alcoholic fatty liver disease (NAFLD) in db/db mice.
Male diabetic db/db mice (BKS.Cg-Dock7m+/+Leprdb/J) aged 10 wk received the dipeptidyl peptidase 4 (DPP4) inhibitor linagliptin (10 mg/kg) or saline as a placebo once per day by gavage for 8 wk. Intact db/db mice served as controls. Structural changes in the liver were analyzed from light and electron microscopic images of sections from intact, placebo-treated and linagliptin-treated animals. We estimated the changes in hepatocytes, sinusoidal cells, liver microvasculature and lymphatic roots. Hepatic staining for lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) was assessed by immunohistochemistry.
In 18-wk-old diabetic mice, liver steatosis (predominantly microvesicular and mediovesicular steatosis) was accompanied by dilation of the roots of the lymphatic system, interlobular blood vessels and bile canaliculi. Compared to saline-treated mice, linagliptin-treated mice exhibited a reduction in the mean numeral densities of hepatocytes with lipid droplets (92.4% ± 1.7% vs 64.9% ± 5.8% per field of view, P = 0.0002) and a lower proportion of hepatocytes with a high density of lipid droplets (20.7% ± 3.6% vs 50.4% ± 3.1%, P = 0.0007). We observed heterogeneous hepatocytes and relatively preserved cell structures in the linagliptin group. Dilation of blood and lymphatic vessels, as well as ultrastructural changes in the hepatocyte endoplasmic reticulum and mitochondria, were alleviated by linagliptin treatment. In intact and placebo-treated mice, immunohistochemical staining for LYVE-1 was observed in the endothelial cells of interlobular lymphatic vessels and on the membranes of some endothelial sinusoidal cells. We observed an enlarged LYVE-1 reaction area in linagliptin-treated mice compared to intact and placebo-treated mice. The improvement in the structural parameters of the liver in linagliptin-treated mice was independent to changes in the plasma glucose levels.
The DPP4 inhibitor linagliptin alleviates liver steatosis and structural changes in the hepatic microvasculature and lymphatic roots in a model of NAFLD in diabetic db/db mice.
Core tip: Dipeptidyl peptidase 4 (DPP4) inhibitors are a relatively new class of hypoglycemic agents with multiple pleiotropic effects. In this study, we demonstrated that the DPP4 inhibitor linagliptin alleviates liver steatosis and diminishes structural changes in hepatic non-parenchymal compartments in db/db diabetic mice. The mechanism of the beneficial effect of linagliptin seems to be glucose-independent as no obvious hypoglycemic activity of the agent was observed in this model. The results of the study provide further evidence that linagliptin could be a promising agent for the treatment of non-alcoholic fatty liver disease in subjects with type 2 diabetes.
- Citation: Michurina SV, Ishenko IJ, Klimontov VV, Archipov SA, Myakina NE, Cherepanova MA, Zavjalov EL, Koncevaya GV, Konenkov VI. Linagliptin alleviates fatty liver disease in diabetic db/db mice. World J Diabetes 2016; 7(19): 534-546
- URL: https://www.wjgnet.com/1948-9358/full/v7/i19/534.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i19.534
Diabetes is associated with a spectrum of liver diseases, including non-alcoholic fatty liver disease (NAFLD) and steatohepatitis[1]. The current treatment for NAFLD primarily focuses on alleviating metabolic syndrome components via lifestyle modifications. However, the lack of success in their implementation and sustainment results in the need for effective pharmacological agents for the treatment of fatty liver[2]. Dipeptidyl peptidase 4 (DPP4) inhibitors are considered a new treatment option for NAFLD in patients with diabetes[3-5]. DPP4 inhibition reduces hepatic fat in experimental models of NAFLD[6-9], but the underlying mechanisms remain to be clarified. Several clinical trials are exploring the efficacy of DPP4 inhibitors for the treatment of NAFLD[5,10-12]. DPP4 inhibitors might have a beneficial effect on hepatic steatosis and serum transaminase activity, but the data regarding the effects of DPP4 inhibitors on liver histology are scarce.
Although DPP4 inhibitors have the same mode of action, they differ by some important pharmacokinetic and pharmacodynamic properties that may be clinically relevant. Linagliptin is a highly specific, potent inhibitor of DPP4 that is currently indicated for the treatment of type 2 diabetes (T2D). In clinical studies, linagliptin effectively reduced glycated hemoglobin (HbA1c) levels in patients with T2D and exhibited a placebo-like safety and tolerability profile[13]. Linagliptin has an interesting pharmacokinetic profile in terms of its predominantly non-renal elimination. Fecal excretion is the dominant excretion pathway of linagliptin[14]. This DPP4 inhibitor is mainly excreted unchanged via bile, but is also excreted directly into the gut independent of biliary excretion[15]. Linagliptin also accumulates in hepatic tissue and exhibits both anti-inflammatory and anti-steatotic activity in a model of non-alcoholic steatohepatitis in streptozotocin-treated neonatal mice on a high-fat diet[8]. Long-term linagliptin treatment reduces liver fat content in mice with diet-induced hepatic steatosis and insulin resistance[6].
Histopathological changes that occur with NAFLD are not limited by changes in the hepatic parenchyma. Involvement of other cell types (sinusoidal endothelial cells, Kupffer cells, and stellate cells) and the recruitment of inflammatory cells and platelets lead to abnormal microcirculation and impaired intrahepatic fluid transport[16,17]. Despite the accumulating data on the favorable influence of DPP4 inhibitors on liver steatosis, the effects of these agents on non-parenchymal cells, bile transport, microcirculation and lymphatic drainage in the liver remain unknown. Therefore, we studied the long-term effects of the DPP4 inhibitor linagliptin on structural changes in hepatocytes, endothelial sinusoidal cells, and the interstitial compartments of the liver in db/db mice with obesity and T2D.
Twenty-four specific pathogen free (SPF) male db/db mice (BKS.Cg-Dock7m+/+Leprdb/J) were utilized for the experiments. Mice homozygous for the diabetes spontaneous mutation (Leprdb) became identifiably polyphagic and obese at approximately 3 to 4 wk of age and exhibited elevated blood glucose from 4-8 wk. The animals were acclimatized to laboratory conditions for two weeks prior to experimentation. The mice were housed in individually ventilated cages (Animal Care Systems, Colorado, United States) in groups of one to four animals per cage with ad libitum food (Ssniff, Soest, Germany) and water. The mice were housed in a room within an SPF animal facility with a regular 14/10 h light/dark cycle (lights on 02:00 AM), a constant room temperature of 24 °C ± 2 °C, and a relative humidity of approximately 45% ± 10%.
After randomization, the experimental group of animals (n = 8) received linagliptin (Boeringer Inghelheim) at a dose of 10 mg/kg of body weight diluted in 200 μL of saline. Mice randomized to the “рlacebo” treatment (n = 8) received 200 μL of saline under the same scheme. Linagliptin or placebo was administered by gavage once per day for 56 d from the 10th to 18th week of age. Intragastric gavage administration was performed with conscious animals using straight gavage needles appropriate for the animal size. The control group was comprised of intact db/db male mice (n = 8).
At the 18th week, all mice were sacrificed by cervical dislocation under anesthesia. Liver samples were obtained for histological assessments, ultrastructural examinations and immunohistochemistry.
All mice were weighed weekly during the experiment using electronic scales. Blood samples were obtained from the retro-orbital sinus of linagliptin-treated and placebo-treated mice at the 10th, 14th and 18th weeks. No stress-inducible procedures, including blood sample collections, were performed in intact animals. Blood samples were centrifuged to obtain plasma that was stored at -20 °C until analysis. The levels of glucose, triglycerides, total cholesterol, alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT) in the blood plasma were measured using automatic clinical chemistry system (Dade Behring Inc, United States) and commercially available cartridges according to the manufacturer’s instructions (Dimension Clinical kit, Siemens, United States).
Liver samples for the light-optical studies were fixed in 10% formalin (pH = 7.4), dehydrated in alcohol at increasing concentrations and embedded in Histomix material (BioVitrum, Russia). Sections 3-4 microns thick were prepared on a microtome LEICA RM2155 (Germany, Switzerland) and were stained with Mayer’s hematoxylin and eosin (H and E). Liver samples for electron microscopy were fixed in a 4% solution of paraformaldegid with 0.1 mol/L phosphate buffer (PB, рН = 7.4) followed by 1% OsO4. The samples were then dehydrated and embedded in Epon-812. Using the LEICA TM UC7 ultratom (Germany), semi-thin sections (1 micron thick) were prepared and stained with toluidine blue. Liver sections 35-45 nm thick were contrasted with aqueous uranyl acetate solution and lead citrate and were studied with the JEOL JEM-1400 electron microscope (Japan).
A morphometric analysis of computed digital images of semi-thin sections from the livers of placebo-treated and linagliptin-treated mice was used to evaluate liver steatosis. Specifically, we calculated the proportion of hepatocytes containing lipid droplets and the distribution of hepatocytes with different lipid droplet densities. Hepatocytes were attributed to a cell population with a high density of lipid inclusions if more than 15 lipid droplets were revealed in the cytoplasm. Low lipid accumulation density was defined as hepatocytes containing less than five droplets. Microvesicular steatosis was defined by the presence of small cytoplasmic lipid droplets around a centrally positioned nucleus. Steatosis was considered mediovesicular when several medium-sized lipid vacuoles were present in the cytoplasm of the hepatocytes[18]. Macrovesicular steatosis was recorded when the diameter of the lipid droplets exceeded half of the hepatocyte nucleus diameter. We also calculated the numeral density of hepatocytes with different sized lipid droplets and estimated the proportions of cells with micro-sized, middle-sized and macro-sized lipid droplets in the cytoplasm. For cases in which the lipid droplets were of different sizes, each cell was taken into account twice or thrice.
Immunohistochemical detection of the lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) marker was performed on 3-mm thick sections from the livers of intact, placebo-treated and linagliptin-treated mice using an indirect avidin-biotin ABC-peroxidase method with the VECTASTAIN Universal Quick Kit (Vector Laboratories, United States). Blocking of endogenous peroxidase was performed by incubating the sections in a 0.3% H2O2 solution for 10 min with a subsequent incubation in normal horse non-immune blocking serum for 20 min. Next, the sections were incubated for one hour at room temperature with anti-LYVE-1 (Isotype: Rabbit polyclonal, bs-1311R; Bioss) at a final dilution of 5 mg/mL; washed in 3 changes of phosphate buffer for 3 min; and further incubated for 30 min at room temperature with a biotinylated second antibody followed by washing in 3 changes of phosphate buffer for 5 min. Incubation with the ABC-peroxidase complex was performed for 30 min at room temperature followed by washing in 3 changes of phosphate buffer for 5 min. Immunohistochemical staining of the sections was performed with a chromogenic substrate (ImmPACT DAB, Vector Laboratories, United States). To quantify the LYVE-1 staining, computed morphometric analysis of the digital images was performed using the “VideoTest Morpho 3.2” program.
All animal experiments were performed in compliance with the protocols and recommendations for the proper use and care of laboratory animals (ECC Directive 86/609/EEC). The protocol was approved by the Ethics Committee of Institute of Clinical and Experimental Lymphology (Protocol Number 1/2, April 1, 2014), and by the Inter-Institutional Animal Ethics Committee based on the Institute of Cytology and Genetics SB RAS (Permission Number: 21, April 1, 2014). All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Russian National Center of Genetic Resources of Laboratory Animals based on the SPF Vivarium of Institute of Cytology and Genetics SB RAS, Novosibirsk, Russia (Permit Number: 246, April 8, 2014). All efforts were made to minimize the number of animals used and their pain or discomfort.
Statistical processing of the results was performed using the STATISTICA software package 10 (StatSoft Inc., United States). A statistical review of the study was performed by a biomedical statistician. The Shapiro-Wilk test was used for testing normality. For the analysis of normally distributed quantitative data, the mean (M) and standard error of the mean (SEM) were calculated. The significance of differences between the groups was assessed by Student’s t-test. Non-normally distributed data (body weights and biochemical parameters) are presented as medians with minimum and maximum values; the significance of differences was determined using the non-parametric Mann-Whitney U-test or Wilcoxon signed rank test for repeated measurements. The differences were considered significant at P < 0.05.
As expected, db/db mice became obese by week 10. The weight of the animals remained stable throughout experiment (Table 1). All animals had severe hyperglycemia at the 10th week with plasma glucose levels of 506 mg/dL (28.1 mmol/L) or more. The glucose levels remained elevated throughout the experiment in both the linagliptin and placebo groups. No significant differences in the levels of glucose, triglycerides, total cholesterol, ALT and GGT were observed between the groups at week 10, 14 or 18.
| Parameters | Placebo group (n = 8) | Linagliptin group (n = 8) | ||||
| 10 wk | 14 wk | 18 wk | 10 wk | 14 wk | 18 wk | |
| Body weight, g | 35.1 (25.6-47.7) | 33.2 (24.6-50.9) | 37.3 (27.3-51.6) | 37.6 (34.5-44.2) | 39.9 (30.0-42.3) | 41.7 (31.5-45.0) |
| Glucose, mg/dL | 637 (549-678) | 579 (551-671) | 610 (506-683) | 651 (631-693) | 588 (520-640) | 625 (601-646) |
| Triglycerides, mg/dL | 415 (209-510) | 324 (209-336) | 316 (149-555) | 385 (262-637) | 279 (251-315) | 391 (238-480) |
| Total cholesterol, mg/dL | 129 (94-156) | 100 (39-140) | 131 (22-156) | 112 (28-132) | 104 (24-124) | 71 (18-120) |
| ALT, U/L | 132 (105-375) | 126 (72-369) | 170 (69-306) | 146 (84-255) | 185 (118-225) | 203 (80-294) |
| GGT, U/L | 16.7 (8.2-28.1) | 13 (8.5-25.1) | 14.6 (10.5-16.5) | 14.4 (8.9-18.7) | 12.5 (7.1-22) | 13.6 (10.5-22.7) |
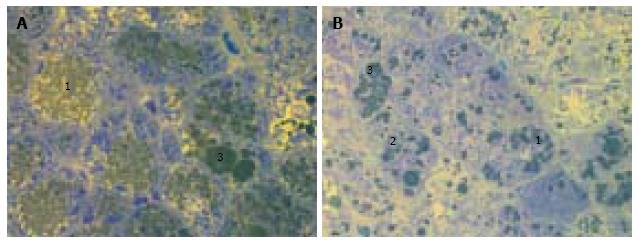
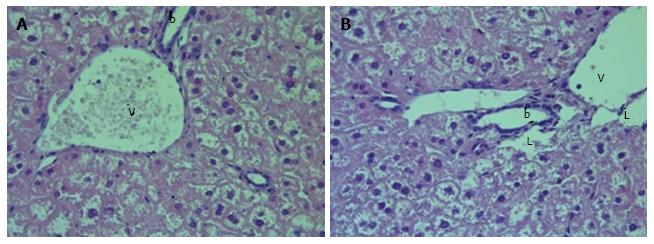
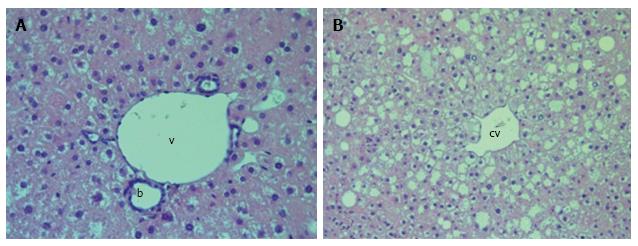
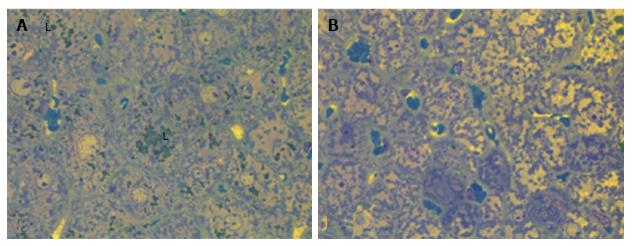
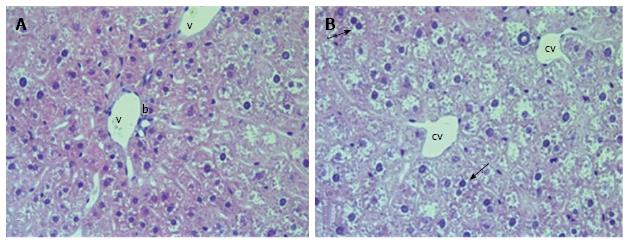
We observed diffuse lipid accumulation in the livers of all 18-wk-old db/db diabetic mice. Lipid droplets were found in 92.4% ± 1.7% of hepatocytes per field of view. Microvesicular and mediovesicular steatosis was the principal morphological finding, although sporadic large lipid droplets were also observed (Figure 1). Vacuolar degeneration was found in the pericentral and intermediate zones of predominantly hepatic lobuli. In some cells, glycogenized nuclei were noticed. The dilation of interlobular arteries and veins, central and sublobular veins, lymphatic vessels and bile canaliculi was present in most of the histological preparations (Figure 2). These changes were accompanied by edema in the connective tissue layers. The sludge of erythrocytes was found in intralobular sinusoidal capillaries. We detected no signs of inflammatory infiltration or interstitial fibrosis.
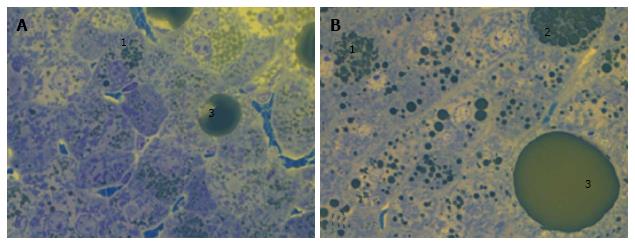
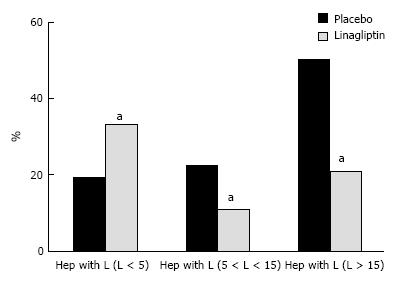
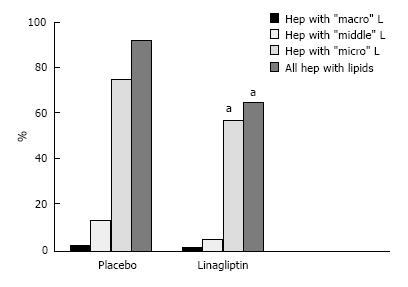
The liver histology in placebo-treated mice was very similar to intact animals (Figures 3 and 4). We observed heterogeneous hepatocytes in mice treated with linagliptin. Although lipid infiltration was present in some hepatocytes, other cells demonstrated preserved morphology (Figure 5). In the periportal zones, numerous diplocariocytes were found, which may be interpreted as a regenerative sign. In linagliptin-treated mice, compared to intact or placebo-treated mice, the dilation of blood and lymphatic vessels of the portal tracts, sublobular and central veins was less profound, and edema of the perisinusoidal lymphatic spaces was diminished (Figure 6). The severity of liver steatosis in the linagliptin group was alleviated. Specifically, the proportion of hepatocytes with a high numeral density of lipid droplets (> 15 per cell) was reduced significantly in the linagliptin group compared to the placebo group (20.7% ± 3.6% and 50.4% ± 3.1%, respectively, P = 0.0007; Figure 7). The mean percent of hepatocytes with lipid droplets per field of view was also decreased (linagliptin: 64.9% ± 5.8%, placebo: 92.4% ± 1.7%, P = 0.0002), mostly due to the reduction of microvesicular and mediovesicular lipid accumulation (Figure 8).
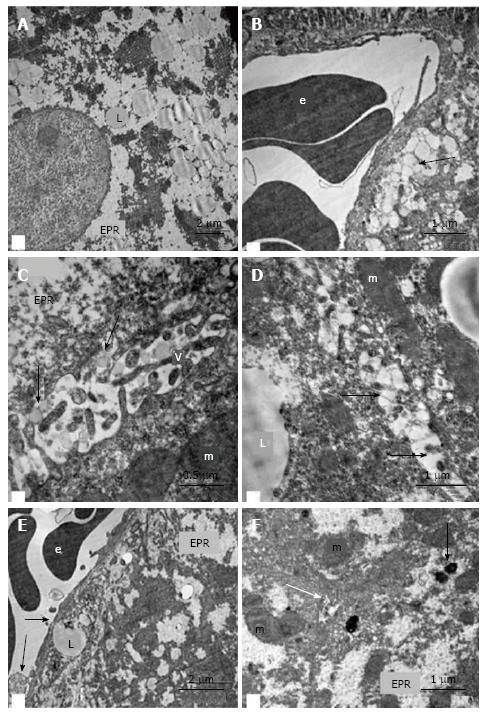
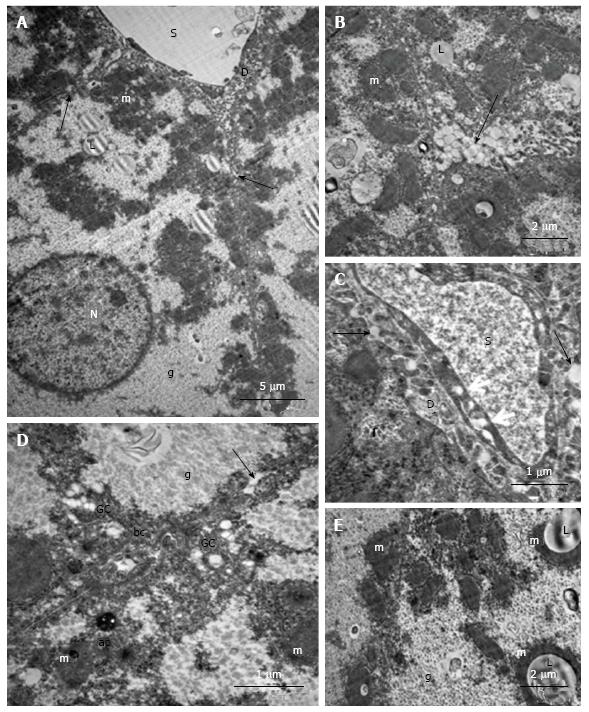
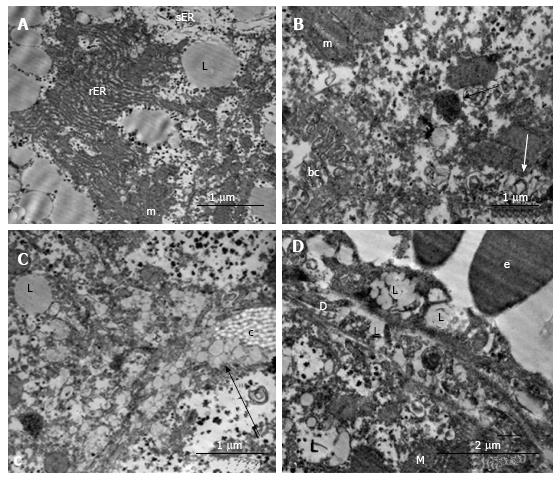
In the hepatocytes of 18-wk-old intact mice, areas of hyperplasia of the smooth endoplasmic reticulum (ER) and lipid inclusions, predominantly small ones, were found via electron microscopy. We observed intense exocytosis of lipids into the Disse space and interstitial areas between hepatocytes. The hyperplasia of the microvilli on the vascular poles of hepatocytes was in concordance with enhanced lipid transport from the cells. The mitochondria were concentrated on the bile poles of hepatocytes and appeared condensed, with increased matrix density and indistinct cristae. Compartmentalization of the complexes of mitochondria and rough ER was found in many cells. We observed 1-2 active Golgi complexes, residual bodies and autophagosomes in addition to bile capillaries (Figure 9).
Ultrastructural changes in the placebo-treated mice were similar to those in intact animals. We observed microvesicular and mediovesicular lipid inclusions, numerous compartments of mitochondria-ER complexes, as well as marked hyperplasia of the microvilli on the vascular and lateral poles of hepatocytes. The Disse space and gaps between hepatocytes were enlarged (Figure 10A). Intense exocytosis of small lipid droplets into the gaps between hepatocytes was observed (Figure 10B). Additionally, we found the exocytosis of lipid-containing vacuoles into the enlarged Disse space (Figure 10C). In the peribiliar areas of some hepatocytes, we observed 1-3 active Golgi complexes and autophagosomes with dense content and ribosomes (Figure 10D). Mitochondria complexes with lipid inclusions were also present (Figure 10E).
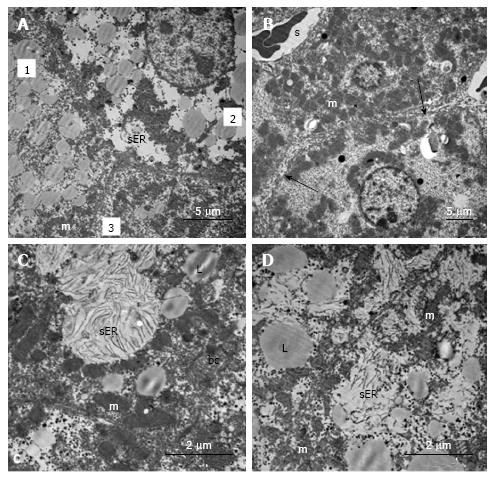
In the livers of linagliptin-treated mice, we observed heterogeneous ultrastructural changes. There were parenchymal cells with lipid accumulation and hyperplasia of the smooth ER (Figure 11А and B). Some hepatocytes demonstrated preserved (almost normal) cellular organization. In the cytoplasm of other cells, we observed zones of destructive ER membranes and quantities of free ribosomes and polyribosomes (Figure 11C and D). Aggregates from mitochondria, rough ER and lipids were present in some images (Figure 12А). In the peribiliary zones of some hepatocytes, we found myelin structures, vacuoles of Golgi complex and autophagosomes (Figure 12B). Hepatocytes with no ER hyperplasia and a homogenous distribution of mitochondria were observed in the livers from linagliptin-treated mice. The presence of large vacuoles with lipid content in the cytoplasm of endothelial sinusoidal cells was another structural feature of this group (Figure 12D).
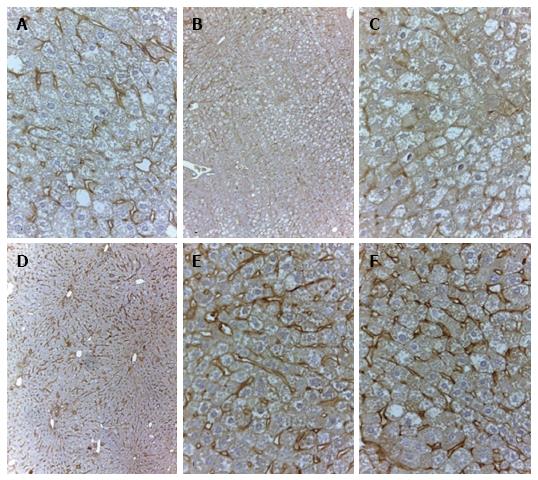
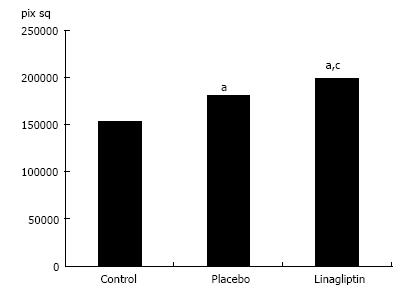
In 18-wk-old intact or placebo-treated diabetic mice, we detected immunohistochemical staining for LYVE-1 in the endothelial cells of interlobular lymphatic vessels and on the membranes of some endothelial sinusoidal cells. The LYVE-1 staining was intensified in linagliptin-treated animals compared to intact or saline-treated animals (Figure 13). An enlarged LYVE-1 reaction area was observed in the linagliptin group as revealed by morphometric analysis (Figure 14).
DPP4 inhibitors are a relatively new class of hypoglycemic agents that have a broad application for the treatment of diabetes worldwide. A growing body of evidence indicates that DPP4 inhibitors could produce multiple pleiotropic effects independent of lowering glucose levels[19,20]. In this study, we demonstrated the beneficial effects of the DPP4 inhibitor linagliptin on both parenchymal and non-parenchymal hepatic cells in T2D db/db mice (BKS.Cg-Dockmм+/+Leprdb/J). Our results demonstrate the protective effects of linagliptin on hepatocytes, sinusoidal cells and the roots of the hepatic lymphatic system in a T2D model.
Expectedly, lipid accumulation in the liver was the principal morphological finding characterizing the development of NAFLD in db/db mice. Specifically, microvesicular and mediovesicular steatosis were prevalent. However, we observed no evident signs of inflammation or fibrosis.
Structural changes in the ER and mitochondria were found in hepatocytes by electron microscopy. In particular, we observed compartmentalization of the complexes of mitochondria and rough ER. Although the ER and mitochondria play distinct cellular roles, these organelles also form physical interactions with each other at sites defined as mitochondria-associated ER membranes, which are essential for calcium, lipid and metabolite exchange. In the liver, obesity leads to a marked reorganization of mitochondria-associated ER membranes resulting in mitochondrial calcium overload, compromised mitochondrial oxidative capacity and augmented oxidative stress[21]. Mitochondrial dysfunction and ER stress or the unfolded protein response contribute to hepatocyte cell death during alterations of lipid and fatty acid metabolism[22]. An association between microvesicular steatosis and apoptosis was demonstrated recently in an NAFLD diabetic model[18].
Consistent with the findings of another research group[6,8], we documented the amelioration of liver steatosis in linagliptin-treated animals. The phenomenon of hepatocyte heterogeneity with the emergence of a relatively preserved cell structure was observed in the linagliptin group. Additionally, the ultrastructural changes in hepatocyte ER and mitochondria were alleviated by linagliptin treatment. Because we observed a preserving effect of the DPP4 inhibitor on ER and mitochondria structure, we anticipate improvement of hepatocyte synthetic function and energy expenditure. Modulation of mitochondrial function upon DPP4 inhibition has been recently described. In a model of Western-diet induced liver steatosis, DPP4 inhibitor MK0626 significantly reduced mitochondrial incomplete palmitate oxidation and increased the indices of pyruvate dehydrogenase activity[9].
As far as we know, we provide here the first detailed description of the morphological changes in the hepatic interstitium of db/db mice. The data indicate deviations in the structure of the interlobular blood vessels, hematolymphatic barrier and intrahepatic lymphatic collectors. Dilation of the roots of the lymphatic system, venous collectors and bile ducts provide morphological evidence of the impairment of lymphatic drainage and bile collection in this model of NAFLD. We also observed morphological signs of enhanced lipid transport into the interstitial tissue between hepatocytes and into the Disse space in db/db mice. Because hepatocyte homeostasis is intimately associated with blood microcirculation and lymphatic drainage, the changes in parenchymal cells and non-parenchymal compartments of the liver in subjects with diabetes could be mutually deteriorated.
We observed imminohistochemical staining for LYVE-1 in the endothelial cells of interlobular lymphatic vessels and on the membranes of endothelial sinusoidal cells in intact and saline-treated db/db mice. The LYVE-1 molecule is considered the primary immunohistochemical marker of lymphatic endothelial cells[23]. Nevertheless, LYVE-1 can be expressed by other cell types, including sinusoidal cells in the liver[24,25]. As a transmembrane receptor, LYVE-1 is involved in the transport and turnover of hyaluronan and may play a role in lymphangiogenesis[26]. The reduced expression of LYVE-1 in sinusoidal cells was reported previously in human chronic hepatitis and liver cirrhosis. A loss of fenestrae in the sinusoidal endothelium was observed in the damaged areas with low LYVE-1 expression. Interestingly, LYVE-1 attenuation in the sinusoidal endothelium is one of the manifestations of capillarization and is associated with hepatic disease progression[25]. We report here that linagliptin potently enhances the expression of LYVE-1 in the endothelial cells of interlobular lymphatic vessels and on the membranes of endothelial sinusoidal cells. Considering the previously mentioned data, we speculate that this phenomenon is associated with the activation of transendothelial transport and lymphatic drainage.
Importantly, the liver histology in linagliptin-treated mice improved significantly despite the absence of an obvious effect on hyperglycemia. Other authors also observed no significant effects of linagliptin on the glucose metabolism parameters of diabetic db/db mice[27]. Nevertheless, it has been documented that a protective effect of linagliptin on the kidneys could be achieved independent of the hypoglycemic action in this model of diabetes[27,28]. Although some of the effects of DPP4 inhibitors could be due to an overall improvement in the metabolic parameters, no data support improvements independent of weight loss or via direct effects on hepatocytes in vitro. In experimental and clinical diabetes, DPP4 activity in the blood serum and liver does not correlate with mean glucose or glycated hemoglobin A1c levels, which are both related to hepatic lipogenesis and liver damage[29]. The glucose-independent action of linagliptin in NAFLD could be mediated, at least partially, via the prolongation of the GLP-1 half-life and the extending GLP-1 effects in the liver. Multiple hepatocyte signal transduction pathways appear to be activated by GLP-1 and its analogues, and both cAMP-activated protein kinase and Akt are proposed key players in improving hepatic steatosis[3,30].
DPP4 itself might be an important target molecule in NAFLD. The liver expresses high levels of DPP4, and recent accumulating data suggest that DPP4 is involved in the development of various chronic liver diseases, such as NAFLD, hepatitis C virus infection, and hepatocellular carcinoma. In addition to its peptidase activity, DPP4 is associated with immune stimulation, binding to and the degradation of the extracellular matrix, resistance to anti-cancer agents, and lipid accumulation. Furthermore, DPP4 is expressed in hepatic stem cells and plays a crucial role in hepatic regeneration[29]. Normal and high fat diet fed DPP4-deficient rats exhibited reduced hepatic triglycerides, accompanied by the down-regulation of lipogenesis enzymes and the parallel up-regulation of carnitine palmitoyltransferase-1, a key enzyme in fatty acid β-oxidation[30]. Rats with DPP4 deficiency have improved bile secretory function in a high fat diet-induced steatosis model[7]. In patients with T2D and/or morbid obesity, circulating DPP4 activity is associated with current apoptosis and liver fibrosis[31].
Thus, it is highly plausible that the observed improvement in liver histology following linagliptin treatment could be mediated by both the prolongation of GLP-1 effects and the inhibition of hepatic DPP4 activity per se.
The results demonstrate the favorable effect of long-term linagliptin treatment on the liver structure of obese db/db mice with T2D. In this model of NAFLD, linagliptin alleviates structural signs of steatosis, and disturbances in microcirculation and lymphatic drainage. The improvement in the structural parameters of the liver in linagliptin-treated mice was independent to changes in the plasma glucose levels.
We are grateful to the staff of the Center of Electron and Light Microscopy, Research Institute of Physiology and Fundamental Medicine, Novosibirsk, for technical support.
Dipeptidyl peptidase 4 (DPP4) inhibitors are a relatively new class of hypoglycemic agents with multiple pleiotropic effects. The ability of DPP4 inhibitors to modify the development of diabetic complications remains unclear. It was recently demonstrated that some DPP4 inhibitors result in hepatic fat reduction in experimental models of non-alcoholic fatty liver disease (NAFLD). Preliminary data indicate that DPP4 inhibitors might have a beneficial effect on hepatic steatosis and serum transaminase activity, but the data on their effects on liver histology are limited.
Despite the accumulating data on the favorable influence of DPP4 inhibitors on liver steatosis, the effects of these agents on non-parenchymal cells, bile transport, microcirculation and lymphatic draining in the liver remain unknown.
In this study, the authors demonstrated for the first time that the DPP4 inhibitor linagliptin not only alleviates liver steatosis but also diminishes structural changes in hepatic non-parenchymal compartments in db/db diabetic mice. Incremental changes in the lymphatic vessel endothelial hyaluronan receptor-1 expression in the endothelial cells of interlobular lymphatic vessels and on the membranes of some endothelial sinusoidal cells under linagliptin treatment may improve impaired lymphatic drainage and sinusoid function in NAFLD. The mechanism of the beneficial effect of linagliptin seems to be glucose-independent as no obvious hypoglycemic effect of the agent was observed in this model.
The results of this study provide further evidence that linagliptin could be a promising agent for the treatment of NAFLD in subjects with type 2 diabetes. Further studies regarding the effects of DPP4 inhibitors on liver structure and function in diabetes are urgently needed.
Sinusoidal cells, a non-parenchymal cell population in the liver that includes sinusoidal endothelial cells, Kupffer cells, Ito cells and Pit cells. Lymphatic vessel endothelial hyaluronan receptor-1, a transmembrane receptor for the extracellular matrix glycosaminoglycan hyaluronan.
The investigation by Michurina et al aimed to study the effects of Linagliptin on the structural signs of non-alcoholic fatty liver disease in db/db mice. This is an interesting work form a basic science point of view, that may have clinical practice consequences.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ali O, Chen X, García-Mayor RV S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Ahmadieh H, Azar ST. Liver disease and diabetes: association, pathophysiology, and management. Diabetes Res Clin Pract. 2014;104:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Blaslov K, Bulum T, Zibar K, Duvnjak L. Incretin based therapies: a novel treatment approach for non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:7356-7365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Samson SL, Bajaj M. Potential of incretin-based therapies for non-alcoholic fatty liver disease. J Diabetes Complications. 2013;27:401-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Nakouti T, Karagiannis AK, Tziomalos K, Cholongitas E. Incretin-Based Antidiabetic Agents for the Management of Non-Alcoholic Fatty Liver Disease. Curr Vasc Pharmacol. 2015;13:649-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Carbone LJ, Angus PW, Yeomans ND. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Kern M, Klöting N, Niessen HG, Thomas L, Stiller D, Mark M, Klein T, Blüher M. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One. 2012;7:e38744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Ben-Shlomo S, Zvibel I, Rabinowich L, Goldiner I, Shlomai A, Santo EM, Halpern Z, Oren R, Fishman S. Dipeptidyl peptidase 4-deficient rats have improved bile secretory function in high fat diet-induced steatosis. Dig Dis Sci. 2013;58:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Klein T, Fujii M, Sandel J, Shibazaki Y, Wakamatsu K, Mark M, Yoneyama H. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol. 2014;47:137-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Aroor AR, Habibi J, Ford DA, Nistala R, Lastra G, Manrique C, Dunham MM, Ford KD, Thyfault JP, Parks EJ. Dipeptidyl peptidase-4 inhibition ameliorates Western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function. Diabetes. 2015;64:1988-2001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Kanazawa I, Tanaka K, Sugimoto T. DPP-4 inhibitors improve liver dysfunction in type 2 diabetes mellitus. Med Sci Monit. 2014;20:1662-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Macauley M, Hollingsworth KG, Smith FE, Thelwall PE, Al-Mrabeh A, Schweizer A, Foley JE, Taylor R. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab. 2015;100:1578-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Mashitani T, Noguchi R, Okura Y, Namisaki T, Mitoro A, Ishii H, Nakatani T, Kikuchi E, Moriyasu H, Matsumoto M. Efficacy of alogliptin in preventing non-alcoholic fatty liver disease progression in patients with type 2 diabetes. Biomed Rep. 2016;4:183-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Guedes EP, Hohl A, de Melo TG, Lauand F. Linagliptin: farmacology, efficacy and safety in type 2 diabetes treatment. Diabetol Metab Syndr. 2013;5:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Blech S, Ludwig-Schwellinger E, Gräfe-Mody EU, Withopf B, Wagner K. The metabolism and disposition of the oral dipeptidyl peptidase-4 inhibitor, linagliptin, in humans. Drug Metab Dispos. 2010;38:667-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Fuchs H, Runge F, Held HD. Excretion of the dipeptidyl peptidase-4 inhibitor linagliptin in rats is primarily by biliary excretion and P-gp-mediated efflux. Eur J Pharm Sci. 2012;45:533-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | McCuskey RS, Ito Y, Robertson GR, McCuskey MK, Perry M, Farrell GC. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken). 2008;291:684-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Trak-Smayra V, Paradis V, Massart J, Nasser S, Jebara V, Fromenty B. Pathology of the liver in obese and diabetic ob/ob and db/db mice fed a standard or high-calorie diet. Int J Exp Pathol. 2011;92:413-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Avogaro A, Fadini GP. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care. 2014;37:2884-2894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Korbut AI, Klimontov VV. Incretin-based therapy: renal effects. Diabetes Mellitus. 2016;19:53-63. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427-1435. [PubMed] [Cited in This Article: ] |
| 22. | Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 23. | Akishima Y, Ito K, Zhang L, Ishikawa Y, Orikasa H, Kiguchi H, Akasaka Y, Komiyama K, Ishii T. Immunohistochemical detection of human small lymphatic vessels under normal and pathological conditions using the LYVE-1 antibody. Virchows Arch. 2004;444:153-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079-8084. [PubMed] [Cited in This Article: ] |
| 25. | Arimoto J, Ikura Y, Suekane T, Nakagawa M, Kitabayashi C, Iwasa Y, Sugioka K, Naruko T, Arakawa T, Ueda M. Expression of LYVE-1 in sinusoidal endothelium is reduced in chronically inflamed human livers. J Gastroenterol. 2010;45:317-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yu M, Zhang H, Liu Y, He Y, Yang C, Du Y, Wu M, Zhang G, Gao F. The cooperative role of S1P3 with LYVE-1 in LMW-HA-induced lymphangiogenesis. Exp Cell Res. 2015;336:150-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Sharkovska Y, Reichetzeder C, Alter M, Tsuprykov O, Bachmann S, Secher T, Klein T, Hocher B. Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase-4 inhibition in a mouse model of type-2 diabetic nephropathy. J Hypertens. 2014;32:2211-2223; discussion 2223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Klimontov VV, Bgatova NP, Gavrilova JuS, Ischenko IJu, Myakina NE, Michurina SV, Zavjalov EL. Linagliptin allieviate renal injury in a model of type 2 diabetic nephropathy. Diabetes. 2015;64:A144. [DOI] [Cited in This Article: ] |
| 29. | Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol. 2013;19:2298-2306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 129] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 30. | Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Williams KH, Vieira De Ribeiro AJ, Prakoso E, Veillard AS, Shackel NA, Brooks B, Bu Y, Cavanagh E, Raleigh J, McLennan SV. Circulating dipeptidyl peptidase-4 activity correlates with measures of hepatocyte apoptosis and fibrosis in non-alcoholic fatty liver disease in type 2 diabetes mellitus and obesity: A dual cohort cross-sectional study. J Diabetes. 2015;7:809-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |