INTRODUCTION
Obesity related diseases including type 2 diabetes and non-alcoholic fatty liver disease have become a major health problem. Inappropriate insulin production, insulin resistance and dyslipidemia are commonly associated with obesity. It is widely accepted that adipose tissue dysfunction is the major underlying reason for metabolic diseases in obesity[1-3].
White adipose tissue is a highly dynamic organ which rapidly responds to nutrient excess and shortage. In obesity adipose tissue expands by hypertrophy and hyperplasia[2,4]. In epididymal fat of rodents fed a high fat diet adipogenesis is detected after four weeks feeding while subcutaneous fat expands by hypertrophy for up to twelve weeks[5]. Distinct adipose tissue depots also differ in gene expression, adipokine release and function[4,6]. Accumulation of visceral adipose tissue is an independent risk factor for metabolic diseases while gain of subcutaneous fat may even be protective[2,4]. The mechanisms regulating fat pad weight and distribution of body fat are, however, not well understood.
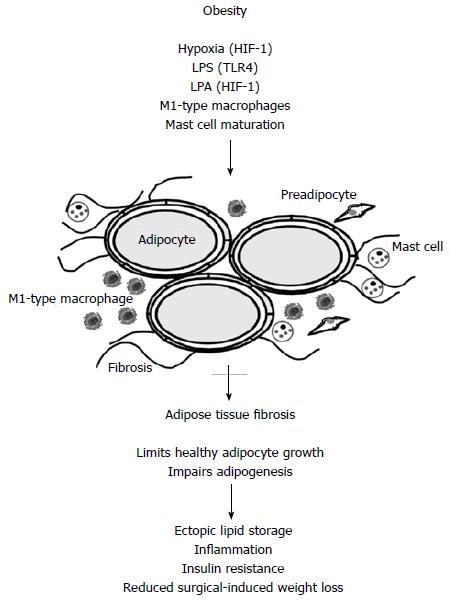
Macrophages are localized in adipose tissues and their number is strongly increased in obesity[2,4]. Macrophages are classified as M1 and M2 types which is a very simplified approach in view of the high diversity of these cells[7]. M1 cells express proinflammatory factors and M2 cells anti-inflammatory proteins. Adipose tissue resident macrophages in the lean state are polarized to the M2 type and in the obese state to the M1 type (Figure 1). Various studies demonstrate a close association between adipose tissue resident macrophages and insulin resistance[8].
Figure 1 In obese adipose tissue hypoxia, lipopolysaccharide, lysophosphatidic acid, M1-type macrophages and maturated mast cells contribute to adipose tissue fibrogenesis.
Hypoxia and LPA mediated effects involve activation of HIF-1 and LPS activates TLR4. Fibrosis impairs adipogenesis and healthy adipocyte growth. Lipids are therefore stored in peripheral tissues like the liver. This is associated with impaired insulin sensitivity and inflammation. Adipose tissue fibrosis negatively affects surgery-induced weight loss. LPS: Lipopolysaccharide; LPA: Lysophosphatidic acid; HIF-1: Hypoxia inducible factor 1.
Adipocyte inflammatory pathways are, however, essential for adipose tissue growth. Fat tissue expression of: (1) a dominant-negative tumor necrosis factor (TNF); (2) RIDα/β, an adenoviral protein complex that inhibits proinflammatory signaling pathways like toll-like receptor 4 (TLR4)-, TNF- and IL-1 beta-mediated signaling; and (3) a mutated human IκBalpha which inhibits NFκB pathway, impairs adipogenesis and intestinal barrier function and favors ectopic lipid storage, systemic inflammation and insulin resistance. Therefore, adipocyte inflammation may be an adaption to an increased fat storage demand[9].
Not all of the obese suffer from metabolic diseases. Obese people protected from metabolic complications display reduced adipocyte stress, lower inflammation and less accumulation of central fat than obese insulin-resistant individuals. Serum adiponectin is similar to levels in normal-weight controls. Adipose tissue growth of these individuals does not provoke adipocyte dysfunction, inflammation and fibrosis[10]. Evaluation of the mechanisms underlying healthy and unhealthy obesity will help to identify the pathways associated with metabolic disturbances.
Research during the last 20 years revealed that rapid adipose tissue expansion is linked to adipocyte dysfunction[2,3,11]. Adipose tissue inflammation, adipocyte death, low adiponectin, systemic inflammation, increased lipolysis and more recently adipose tissue fibrosis have been identified in obesity and are clearly associated with metabolic disturbances[2,3,11].
ADIPOSE TISSUE EXTRACELLULAR MATRIX
Extracellular matrix proteins in adipose tissues regulate mechanical properties, adipogenesis and lipid droplet growth[12,13]. Disruption of collagens impairs triglyceride storage during adipocyte differentiation and collagen 5 (COL5) and COL6 are essential for proper adipogenesis[13]. High flexibility of the extracellular matrix guarantees healthy adipose tissue expansion. Inappropriately increased and rigid extracellular matrix hinders adipose tissue growth and promotes local and systemic pathologies associated with obesity[3,14].
Fibrosis has been extensively studied in the liver. Liver injury activates “quiescent” hepatic stellate cells and these cells start to proliferate, synthesize connective tissue growth factor (CTGF) and extracellular matrix proteins. Transforming growth factor beta (TGF-β) is the main profibrotic factor in liver fibrosis and upregulates CTGF. CTGF stimulates binding of TGF-β to its receptor and thereby enhances TGF-β activity. CTGF is induced by TGF-β indicating an autocrine or paracrine loop that mutually enhances synthesis of both proteins[1]. TGF-β also upregulates CTGF in adipocytes which has been shown to inhibit adipogenesis[15]. TGF-β correlates with adiposity in humans and rodents. Blockage of TGF-β signaling protects from obesity, insulin resistance and fatty liver. Beneficial effects are partly explained by browning of white adipose tissue[16]. In fat tissue, preadipocytes, adipocytes and macrophages produce collagens demonstrating differences in adipose tissue and liver fibrosis where alpha-smooth muscle actin and collagens are mainly synthesized by activated hepatic stellate cells[1,2,17,18].
HYPOXIA IN ADIPOSE TISSUE FIBROSIS
Adipose tissue grows by hyperplasia and hypertrophy which leads to a hypoxic state. Oxygen levels are markedly reduced in white fat of obese rodents and are also lower in white adipose tissues of humans. In obese adipose tissue capillary density is reduced and more large vessels are detected[19].
Hypoxia-inducible factor 1 (HIF-1) is activated when oxygen is low. Hypoxia is supposed to induce tissue fibrosis, and collagen, type I, alpha 1 (COL1A1), COL3A1 and the enzyme lysyl oxidase with a central role in collagen cross-linking are increased when mice are exposed to low oxygen. The HIF-1α inhibitor PX-478 and expression of dominant negative HIF-1α block high fat diet induced HIF-1α activation, lower body weight gain and antagonize the development of metabolic diseases. Adipose tissue fibrosis and inflammation are improved[20]. Therefore, hypoxia mediated activation of HIF-1 seems to be critically involved in limiting healthy adipose tissue growth (Figure 1).
Hypoxia and HIF-1 activation is also believed to significantly contribute to fibrogenic progression of chronic liver diseases and HIF-mediated processes independent of hypoxia have also been described to be involved herein[21].
Hypoxia stimulates cytokine and chemokine release from adipose tissue resident macrophages and inhibits preadipocyte differentiation by lowering peroxisome proliferator-activated receptor gamma (PPARγ) in these cells. Therefore, hypoxia is suggested to link adipose tissue growth, inflammation and adipose tissue fibrosis. Improving adipose tissue angiogenesis lowers hypoxia, HIF-1α, TGF-β pathway and fibrogenesis[22].
COL6 IN ADIPOSE TISSUE FIBROSIS
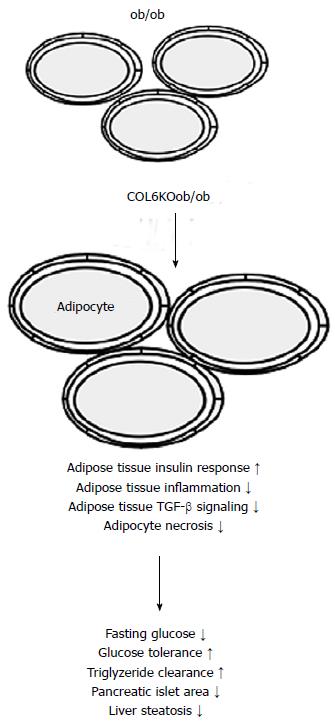
Inflammation and adipocyte death are reduced by the absence of COL6 which is a highly abundant extracellular matrix component of rodent fat tissues. The increased flexibility of the extracellular matrix enables healthy adipocyte growth, lowers local and systemic inflammation and improves metabolic disease (Figure 2). PPARγ agonist and adiponectin reduce adipose tissue collagens in mice demonstrating that improvement of adipose tissue extracellular matrix composition is one of their beneficial features[23].
Figure 2 Mice with leptin deficiency (ob/ob mice) have large adipocytes and their size further increases when collagen 6 (COL6KOob/ob) is knocked-out in these animals.
Weakening of the extracellular matrix is associated with improved adipose tissue insulin response, reduced inflammation and TGF-beta signaling, and diminished adipocyte necrosis. Subsequently metabolic situation is improved.
The carboxy-terminal domain cleaved from COL6A3 promotes adipose tissue fibrosis, angiogenesis and inflammation. Increased production of this so called endotrophin in obesity contributes to metabolic disturbances. Blockage of endotrophin by a neutralizing antibody protects from adverse metabolic effects of high fat feeding[24]. Higher BMI is a risk factor for cancer and cancer-related deaths. Endotrophin enhances tumor growth and metastasis and thus may be one of the factors connecting obesity and malignant diseases[25,26].
IMMUNE CELLS IN ADIPOSE TISSUE FIBROSIS
Increased cell death of adipocytes in obesity may be partly caused by the rigid extracellular matrix. Crown-like structures representing macrophages surrounding dead adipocytes are characteristic for obese adipose tissue and correlate with the extent of interstitial fibrosis. Macrophage-inducible C-type lectin is expressed in these macrophages and enhances the formation of crown-like structures, activates myofibroblasts and expression of profibrotic genes[27]. Macrophage depletion and blockage of TLR4 signaling improve while infusion of lipopolysaccharide aggravates adipose tissue fibrosis further confirming a central role of immune cells in fat tissue dysfunction[28].
Mast cells are well known mediators of allergic reactions and it is known for a long time that their number is increased in obese adipose tissue[29]. Mast cells release inflammatory mediators and promote immune cell recruitment. Obese animals lose body weight after mast cell inactivation[30]. Progression of obesity is associated with mast cell maturation which induces COL5 shown to inhibit adipogenesis[31] (Figure 1). COL5 has also been found to be important for adipocyte maturation demonstrating that different experimental designs reveal discordant results[13]. Mast cells in human fat are activated and are mainly localized in fibrotic regions. Mast cell number is positively associated with fibrosis and macrophage accumulation[32].
LYSOPHOSPHATIDIC ACID AND FIBROSIS
Autotaxin is a secreted lysophospholipase D and hydrolyzes lysophosphatidylcholine to produce lysophosphatidic acid. Autotaxin is increased in obesity and liver fibrosis[33]. Lysophosphatidic acid inhibits adipogenesis and mice with an adipocyte-specific knockout of the lysophosphatidic acid receptor 1 (LPAR1) or treated with the receptor antagonist Ki16425 gain more weight and accumulate more adipose tissue. Despite being more obese animals show improved glucose tolerance[34]. Treatment of db/db mice with the LPAR antagonist Ki16425 reduces COL1 and COL4 mRNAs and collagen protein in inguinal and perigonadal adipose tissues. Human adipose tissue explants release autotaxin spontaneously and its levels increase over time. Lysophosphatidic acid in supernatants increases in parallel along with elevated expression of COL1 and COL3, TGF-β and alpha smooth muscle actin and higher level of collagen protein. In vitro fibrosis is blocked by the LPAR antagonist and interestingly by the HIF-1α inhibitor YC-1 while it is further increased by oleoyl-lysophosphatidic acid[35] (Figure 1). Upregulation of HIF-1α by lysophosphatidic acid has been shown in colon cancer cells[36]. Current data suggest that HIF-1 is involved in fibrotic processes even in the absence of hypoxia.
COLLAGEN EXPRESSION IN HUMAN OBESITY
In humans COL6A3 is mainly expressed by stromal vascular cells and is higher in subcutaneous than omental fat depot. In both adipose tissues its expression is reduced in obesity and increases upon weight loss in subcutaneous fat. Leptin dose dependently decreases COL6A3 demonstrating a role of this adipokine in adipose tissue extracellular matrix organization[37]. Animal studies have proven that leptin directly promotes liver fibrogenesis. Leptin induces COL1, TGF-βand CTGF in hepatic stellate cells and this effect is mediated via enhancing TGF-β release from Kupffer cells[38]. Whether leptin exerts opposing effects in the liver and adipose tissue or whether its activity may be affected by adipose tissue macrophages needs further studies.
In humans with a BMI between 35 and 55 expression of COL3A1, COL5A2 and COL6A3 is lower in omental and subcutaneous adipose tissue of those suffering from the metabolic syndrome compared to the healthy obese[39].
In contrast, positive correlations of COL6A3 expression in abdominal subcutaneous fat with body mass index (BMI) and fat mass have been described in a further study while an association with type 2 diabetes has not been identified. Elevated COL6A3 mRNA levels are found in patients with greater visceral fat mass and higher inflammation. Eight weeks overfeeding increases and pioglitazone reduces COL6A3 expression. Further, in abdominal subcutaneous adipose tissue COL5 is higher expressed in the obese than the lean[40].
In subcutaneous abdominal adipose tissue of patients with a wide range of BMI (19-40 kg/m2) there is a strong positive correlation of COL6 and CD68 mRNA expression. COL6 and CD68 expression are associated with BMI and inversely with insulin sensitivity. Fibrotic areas are increased in the obese fat tissue and are associated with macrophage number and negatively correlate with insulin sensitivity. Alternatively activated macrophages localize to fibrotic regions and express TGF-β[18]. COL5 is increased and elastin is reduced in obesity[19]. Collagens quantified by picrosirius red staining are found increased in subcutaneous and visceral adipose tissues of obese vs lean patients[32].
Picrosirius red has also been used to quantify subcutaneous and omental fat fibrosis in a further investigation. Positive associations with liver fibrosis and systemic IL-6 but not lipid and glucose parameters of the patients have been identified. The subcutaneous white adipose tissue stiffness measured by shear-wave velocity using a prototype vibration-controlled transient elastography method positively correlates with fasting glycemia and insulin, HbA1C and fat-free mass, and negatively with body fat and HDL cholesterol. Diabetes status is also significantly associated with increased shear-wave velocity. These data suggest that subcutaneous white adipose tissue stiffness is not solely defined by collagen content. Cross-linking of collagens and other extracellular matrix proteins such as elastin, laminin, and fibronectin most likely contribute to tissue rigidity[41].
In summary most data in humans find increased adipose tissue fibrosis in obesity. There is, however, no simple explanation for the discrepant results on the expression of single collagen species which are found reduced and induced in human obesity. Whether this is somehow related to differential composition of the extracellular matrix of the patients analyzed or to regional variations in adipose tissues needs further analysis. Techniques to directly measure adipose tissue stiffness may be more appropriate than determining mRNA expression of individual genes for the analysis of adipose tissue fibrosis.
ADIPOSE TISSUE FIBROSIS AND WEIGHT LOSS
Mild to modest liver fibrosis is reversible[42] and previous studies have shown that adipose tissue collagen levels are reduced by adiponectin and treatment with PPARgamma agonists in mice[23]. In patients, transcriptional and histological analysis of subcutaneous adipose tissue revealed persistence of fibrosis two years after bariatric surgery. As expected, adipocyte hypertrophy and inflammatory infiltration are improved[43]. Adipose tissue fibrosis is even found negatively associated with surgery-induced weight loss[44]. These findings have been confirmed in a second cohort and the association between collagen expression in white adipose tissue and gastric bypass induced weight loss persists even when age, diabetes and IL-6 have been considered[41]. It has also been shown that diet and surgery-induced weight loss increase COL6A3 expression in subcutaneous adipose tissue in accordance with low COL6A3 expression in the fat tissues of obese patients described in this study[37].
CONCLUSION
Adipose tissue fibrosis limits healthy growth of adipose tissue and is associated with metabolic complications in obesity. Hypoxia and subsequent activation of HIF-1 initiate profibrotic mechanisms in fat tissues. Fibrosis in obese fat tissues is not resolved upon weight loss and is even negatively associated with surgical-induced body weight reduction. More detailed analysis of the composition of extracellular matrix, biologic function of the individual constituents and non-invasive techniques to determine adipose tissue fibrosis will give further insights into the complex association of extracellular matrix proteins and metabolic health.
P- Reviewer: Lin GM, Lobo D S- Editor: Ji FF L- Editor: A E- Editor: Wu HL