Published online Mar 15, 2015. doi: 10.4239/wjd.v6.i2.217
Peer-review started: November 2, 2014
First decision: November 27, 2014
Revised: December 10, 2014
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: March 15, 2015
Processing time: 137 Days and 16.8 Hours
The World Health Organization estimates that diabetes mellitus (DM) will become the seventh leading cause of death during the next two decades. DM affects approximately 350 million individuals worldwide and additional millions that remain undiagnosed are estimated to suffer from the complications of DM. Although the complications of DM can be seen throughout the body, the nervous, cardiac, and vascular systems can be significantly affected and lead to disorders that include cognitive loss, stroke, atherosclerosis, cardiac failure, and endothelial stem cell impairment. At the cellular level, oxidative stress is a significant determinant of cell fate during DM and leads to endoplasmic reticulum stress, mitochondrial dysfunction, apoptosis, and autophagy. Multiple strategies are being developed to combat the complications of DM, but it is the mechanistic target of rapamycin (mTOR) that is gaining interest in drug development circles especially for protective therapies that involve cytokines and growth factors such as erythropoietin. The pathways of mTOR linked to mTOR complex 1, mTOR complex 2, AMP activated protein kinase, and the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) complex can ultimately influence neuronal, cardiac, and vascular cell survival during oxidant stress in DM through a fine interplay between apoptosis and autophagy. Further understanding of these mTOR regulated pathways should foster novel strategies for the complications of DM that impact millions of individuals with death and disability.
Core tip: The pathways of mechanistic target of rapamycin (mTOR) linked to mTOR complex 1, mTOR complex 2, AMP activated protein kinase, and tuberous sclerosis 1/tuberous sclerosis 2 complex can offer novel strategies for the complications of diabetes mellitus to prevent death and disability for the millions of individuals afflicted with this disorder.
- Citation: Maiese K. mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes 2015; 6(2): 217-224
- URL: https://www.wjgnet.com/1948-9358/full/v6/i2/217.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i2.217
The incidence of diabetes mellitus (DM) throughout the world is increasing at an exponential rate such that the World Health Organization predicts that DM will be the seventh leading cause of death by the year 2030[1]. In 2013, greater than a million deaths were attributable to DM that is believed to affect 347 million individuals throughout the world. In the United States, 21 million individuals are diagnosed with DM and another 8 million individuals are estimated to suffer from DM but are currently undiagnosed[2]. Reduced activity, increased body weight, and poor nutritional intake are considered significant factors that can lead to adult onset DM[3,4]. Duration of obese-years rather than body mass index can become a significant risk for developing DM[5].
DM is defined as being either non-insulin dependent (type 1) or insulin dependent (type 2)[6,7]. Type 1 DM occurs in approximately 5%-10% of DM patients and is an autoimmune disorder with the presence of alleles of the human leukocyte antigen class II genes within the major histocompatibility complex. Destruction of pancreatic β-cells with inflammatory infiltration of the islets of Langerhans results in lost insulin production and regulation. About 90% of patients with type 1 DM have increased titers of autoantibodies (type 1A DM). The remaining 10% of type 1 DM individuals do not have serum autoantibodies and are considered to have maturity-onset diabetes of the young that can be a result of β-cell dysfunction with autosomal-dominant inheritance (type IB DM). Type 2 DM occurs in approximately 80%-90% of individuals with DM greater than the age of 40. Although approximately 10% of individuals with type 2 DM may have elevated serum autoantibodies similar to type 1 DM, type 2 DM represents a progressive deterioration of glucose tolerance with early β-cell compensation that has cell hyperplasia followed by a decrease in β-cell mass. Insulin resistance ensues as well as impairments in insulin secretion. Insulin resistance also may be a component of type 1 DM in some patients. Defective insulin secretion can result from impaired β-cell function, chronic exposure to free fatty acids and hyperglycemia, as well as the absence of inhibitory feedback through plasma glucagon levels.
As a disease that affects all systems of the body, DM can lead to multiple clinical impairments especially in the nervous, cardiac, and vascular systems. DM results in cognitive loss not only through vascular disease and stroke[8], but also during chronic neurodegenerative disorders such as Alzheimer’s disease[9,10]. Insulin resistance similar to its occurrence in DM also has been reported in patients with Alzheimer’s disease, suggesting that degenerative disorders such as Alzheimer’s disease could be mediated in some patient populations by impaired cellular metabolism[11]. DM also results in neuropsychiatric disorders[12,13], retinal disease[14-16], and peripheral nerve disorders[17]. In the cardiac system, DM can lead to sympathetic nerve dysfunction[18], cardiac fibrosis[19,20], ischemic reperfusion injury[21], cardiomyocyte injury[22], and cardiac hypertrophy[23]. DM also can significantly impact endothelial cells either in the brain or elsewhere in the body. Exposure to elevated glucose levels can result in endothelial cell senescence[24], dysfunctional mobilization of endothelial progenitor cells from the bone marrow[25], injury to the neuroglialvascular unit[14], loss of angiogenesis[26], and endothelial cell injury and loss[27-33].
During DM, oxidative stress is an important driver of cell injury[4,6,34-39]. In murine animal models of type 2 DM, oxidative stress can lead to elevated glutathione levels and increased lipid peroxidation[23]. “Highly-oxidized glycated” low density lipoproteins that can occur in DM lead to oxidative and endoplasmic reticulum stress in human retinal capillary pericytes. Subsequently, mitochondrial dysfunction and cell death with apoptosis and autophagy ensues[15]. Exposure of glucolipotoxicity caused by elevated plasma glucose and lipid levels to pancreatic β-cells promotes oxidative stress with cytochrome c release, caspase activation, and apoptosis[40]. Advanced glycation end products (AGEs), entities that promote complications in DM[41], lead to the release of reactive oxygen species (ROS) and caspase activation[37]. In addition, high fat diets[42] as well as free fatty acids have been shown to release ROS, lead to mitochondrial DNA damage, and impair pancreatic β-cell function[43]. In cardiomyocytes[20,22,44], neurons[8,15,30,45,46], and endothelial cells[14,25,27-29,47], exposure to elevated glucose levels foster oxidant stress mechanisms that can impair cellular function and lead to cell death. In clinical studies, patients with type 2 DM display serum markers of oxidative stress with ischemia-modified albumin[48]. Interestingly, elevations in serum glucose can increase antioxidant enzyme levels in human endothelial cells, suggesting that some cells may initiate a reparative process against oxidative stress injury[49]. Of note, chronic hyperglycemia is not necessary to lead to oxidative stress injury, since even brief periods of hyperglycemia generate ROS[50]. Clinical correlates support these experimental studies to show that both acute glucose swings as well as chronic hyperglycemia can trigger oxidative stress mechanisms during type 2 DM[51].
Numerous cellular pathways can lead to oxidative stress during DM. As a result, multiple therapeutic avenues are being pursued to develop therapy against the complications of DM. These strategies include the recent focus upon sirtuins[24,47,52-56], protein tyrosine phosphatases[57,58], broad antioxidant therapies[3,7,17,31,34,38,59], forkhead transcription factors[56,60-63], and growth factors[32,64-68].
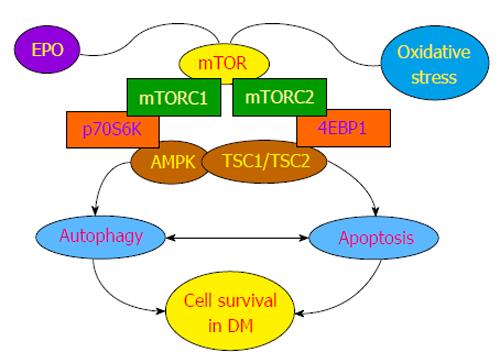
In reference to growth factors, the cytokine and growth factor erythropoietin (EPO) serves as a provocative model for potential treatments for the complications of DM (Figure 1). EPO blocks cell injury in studies of diabetic retinal degeneration[14], maintains endothelial cell integrity during experimental models of DM[27,28], facilitates wound healing during DM[65], reduces high glucose-induced oxidative stress in renal tubular cells[69], maintains cellular mitochondrial function and energy metabolism[32], and regulates the detrimental effects of obesity in animal models[33]. Although EPO affects multiple cellular signal transduction pathways in the body[70,71], of particular interest are the signal transduction pathways of the mechanistic target of rapamycin (mTOR) controlled by EPO that are intimately linked to cellular metabolism and DM[72-76]. mTOR can influence neuronal, glial, and cell to cell activity[77,78]. EPO uses mTOR to protect cells against oxygen-glucose deprivation[79,80], limit cell injury during β-amyloid exposure[81], control bone homeostasis[82], improve cognitive function in models of sepsis-associated encephalopathy[83], foster retinal progenitor cell survival during oxidant stress[84], and promote the neuronal phenotype of adult neuronal precursor cells[85].
mTOR, also known as the mammalian target of rapamycin and FK506-binding protein 12-rapamycin complex-associated protein 1, is a 289-ku serine/threonine protein kinase. mTOR is encoded by a single gene FRAP1[86-88] and is a component of the protein complexes mTOR complex 1 (mTORC1) and mTORC2 (Figure 1). Rapamycin, an agent that inhibits mTOR activity, blocks mTORC1 by preventing the phosphorylation of mTOR. In some cases with chronic administration, rapamycin also can inhibit mTORC2. mTORC1 is composed of raptor (regulatory-associated protein of mTOR), the proline rich Akt substrate 40 ku, deptor (DEP domain-containing mTOR interacting protein), and mLST8/G L (mammalian lethal with Sec13 protein 8, termed mLST8). Two important targets of mTORC1 through mLST8 that promote mTOR kinase activity are p70 ribosomal S6 kinase and the eukaryotic initiation factor 4E-binding protein 1[89,90]. mTORC2 is composed of rictor (rapamycin-insensitive companion of mTOR), deptor, mLST8, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with rictor-1 (Protor-1)[75,91].
In addition to phosphoinositide 3-kinase and protein kinase B (Akt)[6,92], mTOR signaling also is governed by AMP activated protein kinase (AMPK)[75,91]. AMPK can control the activity of the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that is an inhibitor of mTORC1. AMPK phosphorylates TSC2 as well as Raptor to block the activity of mTORC1 during energy stress[93]. AMPK also controls TSC1/2 activity through RTP801 (REDD1/ product of the Ddit4 gene). AMPK activity can increase REDD1 expression, such as in the presence of hypoxic environments, to suppress mTORC1 activity by releasing TSC2 from its inhibitory binding to protein 14-3-3[94].
AMPK can have dual roles in cell survival (Figure 1). AMPK activation can suppress β-amyloid (Aβ) production[95], regulate tau phosphorylation[96], limit oxidative stress that can lead to hypertension[97], increase cell survival during hypoxia[98], and promote autophagy that may resolve memory impairment[99]. However, in other experimental models, AMPK activity has been suggested to influence neuroinflammation[100], lead to aberrant Aβ stress[96] and Aβ toxicity[101], result in cardiac dysfunction[102], and result in the hypertrophy of cardiac tissues[103]. In regards to cellular metabolism with AMPK[104], AMPK can reduce insulin resistance and diminish oxidative stress mediated through the programmed cell death pathway of autophagy[105], reduce myocardial ischemia in experimental models of diabetes[21], be necessary for proper metabolic function of cells[106], and block adipocyte differentiation, lipid accumulation, and obesity[107]. Loss of AMPK may lead to insulin resistance[108].
For the development of new strategies against DM with mTOR, a careful balance in the activity of the programmed cell death pathways of apoptosis and autophagy must be considered. Both apoptosis[4,7,17,32,38,109] and autophagy[6,74,110,111] can influence cell survival during oxidative stress[112]. In regards to cellular metabolic pathways, activation of mTOR that blocks apoptotic pathways may limit insulin resistance and vascular thrombosis in patients with metabolic syndrome[113]. Increased activity of mTOR also may prevent the development of atherosclerosis[114]. Furthermore, mTOR activation through glucagon-like peptide-1 agonists has recently been reported to protect pancreatic β-cells from cholesterol mediated apoptotic cell injury[115], promote pancreatic β-cell proliferation[116], and prevent neural apoptotic cell loss during DM through the epidermal growth factor receptor[117].
In other studies with DM, it is the induction of autophagy with requisite mTOR inhibition that is suggested to foster cellular protection. For example, metformin, an agent used to control hyperglycemia in DM, inhibits mTOR activity and promotes autophagy. Metformin can offer protection against endothelial cell senescence[24], limit androgen up-regulation during prostate cancer through mTOR inhibition[118], prevent cell loss during hypoxia through increased AMPK activity[98], and protect against neuronal cell apoptosis[119]. Metformin through pathways that activate AMPK also prevents cardiomyopathy in experimental models of DM[120], fosters cardiomyocyte cell survival[121], and reduces cortical infarction in stroke models[122].
Additional work suggests that autophagy irrespective of the contribution of mTOR may be protective during DM. Autophagy haploinsufficiency in murine animal models of obesity leads to increased insulin resistance with elevated lipids and inflammation[123], suggesting that loss of autophagy may foster the progression from obesity to DM. Autophagy also may be required to remove misfolded proteins and eliminate non-functioning mitochondria to prevent β-cell dysfunction and the onset of DM[124]. In addition, exercise in mice has been shown to initiate autophagy and regulate glucose homeostasis[125]. These results may be associated with observations that autophagy has been reported to improve insulin sensitivity during high fat diets in mice[105].
Yet, in other experimental models, autophagy may not be beneficial even though it can be less of a prominent modulator of cell survival than apoptosis in some experimental models[126]. Autophagy during high glucose exposure has been shown to impair endothelial progenitor cells, lead to mitochondrial oxidative stress, and prevent the formation of new blood vessels[127]. Increased autophagy also has been associated with significant loss of cardiac and liver tissue in diabetic rats during attempts to achieve glycemic control through diet modification[128]. During periods of elevated glucose that occur in DM, AGEs have been shown to lead to the induction of autophagy and vascular smooth muscle proliferation that can result in atherosclerosis[129] as well as cardiomyopathy[44].
DM is a significant and growing disorder throughout the world that leads to increased disability and death through multiple complications in the nervous, cardiac, and vascular systems. Current therapies for these complications are limited. As a result, novel therapeutic strategies are required to address the cellular mechanisms of oxidant stress and cell injury that can mediate complications of DM. Given the recent discovery that cytoprotective strategies against oxidative stress, i.e., EPO, employ mTOR, the mTOR signaling pathways that include AMPK and TSC1/TSC2 have become increasingly recognized as a potential targets for the treatment of the complications of DM. However, future work will need to concentrate upon the complex relationship that the programmed cell death pathways of apoptosis and autophagy hold over cellular survival and longevity to attain both efficacy and safety for mTOR targeted strategies.
P- Reviewer: Donato R, Teng RJ, Wang M S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: World Health Organization 2011; . |
| 2. | Centers for Disease Control and Prevention. National diabetes statistics report: Estimates of diabetes and its burden in the United States. Atlanta, GA: US Department of Health and Human Services 2014; . |
| 3. | Maiese K, Chong ZZ, Shang YC, Hou J. Novel avenues of drug discovery and biomarkers for diabetes mellitus. J Clin Pharmacol. 2011;51:128-152. [PubMed] |
| 4. | Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert Opin Drug Discov. 2013;8:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Abdullah A, Wolfe R, Mannan H, Stoelwinder JU, Stevenson C, Peeters A. Epidemiologic merit of obese-years, the combination of degree and duration of obesity. Am J Epidemiol. 2012;176:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant stress and signal transduction in the nervous system with the PI 3-K, Akt, and mTOR cascade. Int J Mol Sci. 2012;13:13830-13866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Maiese K, Shang YC, Chong ZZ, Hou J. Diabetes mellitus: channeling care through cellular discovery. Curr Neurovasc Res. 2010;7:59-64. [PubMed] |
| 8. | Zhao Z, Huang G, Wang B, Zhong Y. Inhibition of NF-kappaB activation by Pyrrolidine dithiocarbamate partially attenuates hippocampal MMP-9 activation and improves cognitive deficits in streptozotocin-induced diabetic rats. Behav Brain Res. 2013;238:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Sonnen JA, Larson EB, Brickell K, Crane PK, Woltjer R, Montine TJ, Craft S. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 12. | Aksu I, Ates M, Baykara B, Kiray M, Sisman AR, Buyuk E, Baykara B, Cetinkaya C, Gumus H, Uysal N. Anxiety correlates to decreased blood and prefrontal cortex IGF-1 levels in streptozotocin induced diabetes. Neurosci Lett. 2012;531:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Reagan LP. Diabetes as a chronic metabolic stressor: causes, consequences and clinical complications. Exp Neurol. 2012;233:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Busch S, Kannt A, Kolibabka M, Schlotterer A, Wang Q, Lin J, Feng Y, Hoffmann S, Gretz N, Hammes HP. Systemic treatment with erythropoietin protects the neurovascular unit in a rat model of retinal neurodegeneration. PLoS One. 2014;9:e102013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Fu D, Wu M, Zhang J, Du M, Yang S, Hammad SM, Wilson K, Chen J, Lyons TJ. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012;55:3128-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, Medeiros D, Lin D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood). 2011;236:1051-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014;6:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, Dasilva JN. Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc Diabetol. 2011;10:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Puthanveetil P, Wan A, Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc Res. 2013;97:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Zhang C, Zhang L, Chen S, Feng B, Lu X, Bai Y, Liang G, Tan Y, Shao M, Skibba M. The prevention of diabetic cardiomyopathy by non-mitogenic acidic fibroblast growth factor is probably mediated by the suppression of oxidative stress and damage. PLoS One. 2013;8:e82287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Paiva MA, Rutter-Locher Z, Gonçalves LM, Providência LA, Davidson SM, Yellon DM, Mocanu MM. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123-H2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Li Y, Li Y, Ye Y. Dickkopf-1 (DKK1) phosphatase and tensin homolog on chromosome 10 (PTEN) crosstalk via microRNA interference in the diabetic heart. Basic Res Cardiol. 2013;108:352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289:4145-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171:523-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 25. | Barthelmes D, Zhu L, Shen W, Gillies MC, Irhimeh MR. Differential gene expression in Lin-/VEGF-R2+ bone marrow-derived endothelial progenitor cells isolated from diabetic mice. Cardiovasc Diabetol. 2014;13:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Chen JX, Tuo Q, Liao DF, Zeng H. Inhibition of protein tyrosine phosphatase improves angiogenesis via enhancing Ang-1/Tie-2 signaling in diabetes. Exp Diabetes Res. 2012;2012:836759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3β, and β-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Hou J, Chong ZZ, Shang YC, Maiese K. FOXO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010;321:194-206. [PubMed] |
| 30. | Liu Q, Li J, Cheng R, Chen Y, Lee K, Hu Y, Yi J, Liu Z, Ma JX. Nitrosative stress plays an important role in Wnt pathway activation in diabetic retinopathy. Antioxid Redox Signal. 2013;18:1141-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Schaffer SW, Jong CJ, Mozaffari M. Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascul Pharmacol. 2012;57:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci. 2014;10:921-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, Teng R, Noguchi CT. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014;15:10296-10333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Bagul PK, Banerjee SK. Insulin resistance, oxidative stress and cardiovascular complications: role of sirtuins. Curr Pharm Des. 2013;19:5663-5677. [PubMed] |
| 35. | Liu J, Li J, Li WJ, Wang CM. The role of uncoupling proteins in diabetes mellitus. J Diabetes Res. 2013;2013:585897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Peng Y, Huang S, Cheng B, Nie X, Enhe J, Feng C, Fu X. Mesenchymal stem cells: a revolution in therapeutic strategies of age-related diseases. Ageing Res Rev. 2013;12:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Weinberg E, Maymon T, Weinreb M. AGEs induce caspase-mediated apoptosis of rat BMSCs via TNFα production and oxidative stress. J Mol Endocrinol. 2014;52:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Xu YJ, Tappia PS, Neki NS, Dhalla NS. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail Rev. 2014;19:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Yang H, Jin X, Kei Lam CW, Yan SK. Oxidative stress and diabetes mellitus. Clin Chem Lab Med. 2011;49:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 40. | Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP1-derived nonapeptide GLP1(28-36)amide protects pancreatic β-cells from glucolipotoxicity. J Endocrinol. 2012;213:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63-71. [PubMed] |
| 42. | Ribeiro MC, Barbosa NB, de Almeida TM, Parcianello LM, Perottoni J, de Avila DS, Rocha JB. High-fat diet and hydrochlorothiazide increase oxidative stress in brain of rats. Cell Biochem Funct. 2009;27:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes. 2006;55:1022-1028. [PubMed] |
| 44. | Lee Y, Hong Y, Lee SR, Chang KT, Hong Y. Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab Anim Res. 2012;28:99-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Das F, Dey N, Venkatesan B, Kasinath BS, Ghosh-Choudhury N, Choudhury GG. High glucose upregulation of early-onset Parkinson’s disease protein DJ-1 integrates the PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal. 2011;23:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Mao XY, Cao DF, Li X, Yin JY, Wang ZB, Zhang Y, Mao CX, Zhou HH, Liu ZQ. Huperzine A ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Int J Mol Sci. 2014;15:7667-7683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7:95-112. [PubMed] |
| 48. | Kurban S, Mehmetoglu I, Yerlikaya HF, Gönen S, Erdem S. Effect of chronic regular exercise on serum ischemia-modified albumin levels and oxidative stress in type 2 diabetes mellitus. Endocr Res. 2011;36:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996;45:471-477. [PubMed] |
| 50. | Yano M, Hasegawa G, Ishii M, Yamasaki M, Fukui M, Nakamura N, Yoshikawa T. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1769] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 52. | Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr Neurovasc Res. 2008;5:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Halperin-Sheinfeld M, Gertler A, Okun E, Sredni B, Cohen HY. The Tellurium compound, AS101, increases SIRT1 level and activity and prevents type 2 diabetes. Aging (Albany NY). 2012;4:436-447. [PubMed] |
| 54. | Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52:1173-1185. [PubMed] |
| 55. | Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD(+)-dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13:1075-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Shao S, Yang Y, Yuan G, Zhang M, Yu X. Signaling molecules involved in lipid-induced pancreatic beta-cell dysfunction. DNA Cell Biol. 2013;32:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251-1267. [PubMed] |
| 58. | Xu E, Schwab M, Marette A. Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev Endocr Metab Disord. 2014;15:79-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446-3485. [PubMed] |
| 60. | Kibbe C, Chen J, Xu G, Jing G, Shalev A. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. J Biol Chem. 2013;288:23194-23202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012;50:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 62. | Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond). 2009;116:191-203. [PubMed] |
| 63. | Zhao Y, Yu Y, Tian X, Yang X, Li X, Jiang F, Chen Y, Shi M. Association study to evaluate FoxO1 and FoxO3 gene in CHD in Han Chinese. PLoS One. 2014;9:e86252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmoulière A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen. 2014;22:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: new directions for the nervous system. Int J Mol Sci. 2012;13:11102-11129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90-95. [PubMed] |
| 68. | White MF. IRS2 integrates insulin/IGF1 signalling with metabolism, neurodegeneration and longevity. Diabetes Obes Metab. 2014;16 Suppl 1:4-15. [PubMed] |
| 69. | Dang J, Jia R, Tu Y, Xiao S, Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother. 2010;64:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194-213. [PubMed] |
| 71. | Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. ScientificWorldJournal. 2009;9:1072-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Chong ZZ, Maiese K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol. 2012;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 73. | Hao J, Zhu L, Li F, Liu Q, Zhao X, Liu S, Xing L, Feng X, Duan H. Phospho-mTOR: a novel target in regulation of renal lipid metabolism abnormality of diabetes. Exp Cell Res. 2013;319:2296-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1198-R1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 76. | Wang H, Zhang Q, Wen Q, Zheng Y, Lazarovici P, Jiang H, Lin J, Zheng W. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 77. | Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int J Mol Sci. 2014;15:16848-16884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 78. | Liu J, Reeves C, Michalak Z, Coppola A, Diehl B, Sisodiya SM, Thom M. Evidence for mTOR pathway activation in a spectrum of epilepsy-associated pathologies. Acta Neuropathol Commun. 2014;2:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 79. | Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS One. 2012;7:e45456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8:270-285. [PubMed] |
| 81. | Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY). 2012;4:187-201. [PubMed] |
| 82. | Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, Wang J, Krebsbach PH, Taichman RS. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Wang GB, Ni YL, Zhou XP, Zhang WF. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014;385:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, Hsu H, Wang H, Tropepe V, Ward M. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci. 2011;47:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Marfia G, Madaschi L, Marra F, Menarini M, Bottai D, Formenti A, Bellardita C, Di Giulio AM, Carelli S, Gorio A. Adult neural precursors isolated from post mortem brain yield mostly neurons: an erythropoietin-dependent process. Neurobiol Dis. 2011;43:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res. 2014;11:177-186. [PubMed] |
| 87. | Neasta J, Barak S, Hamida SB, Ron D. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem. 2014;130:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 88. | Tang Z, Baykal AT, Gao H, Quezada HC, Zhang H, Bereczki E, Serhatli M, Baykal B, Acioglu C, Wang S. mTor is a signaling hub in cell survival: a mass-spectrometry-based proteomics investigation. J Proteome Res. 2014;13:2433-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Maiese K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res. 2014;9:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99:128-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299-315. [PubMed] |
| 93. | Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 2948] [Article Influence: 173.4] [Reference Citation Analysis (0)] |
| 94. | DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 95. | Cai Z, Li B, Li K, Zhao B. Down-regulation of amyloid-β through AMPK activation by inhibitors of GSK-3β in SH-SY5Y and SH-SY5Y-AβPP695 cells. J Alzheimers Dis. 2012;29:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem. 2011;118:460-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 97. | Cheng PW, Ho WY, Su YT, Lu PJ, Chen BZ, Cheng WH, Lu WH, Sun GC, Yeh TC, Hsiao M. Resveratrol decreases fructose-induced oxidative stress, mediated by NADPH oxidase via an AMPK-dependent mechanism. Br J Pharmacol. 2014;171:2739-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 98. | Sheng B, Liu J, Li GH. Metformin preconditioning protects Daphnia pulex from lethal hypoxic insult involving AMPK, HIF and mTOR signaling. Comp Biochem Physiol B Biochem Mol Biol. 2012;163:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, Li C, Hu L, Jiang H, Shen X. Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both β-amyloid production and clearance. J Neurosci. 2013;33:13138-13149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 100. | Russo E, Andreozzi F, Iuliano R, Dattilo V, Procopio T, Fiume G, Mimmi S, Perrotti N, Citraro R, Sesti G. Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav Immun. 2014;42:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 101. | Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10:29-38. [PubMed] |
| 102. | Aragno M, Mastrocola R, Ghé C, Arnoletti E, Bassino E, Alloatti G, Muccioli G. Obestatin induced recovery of myocardial dysfunction in type 1 diabetic rats: underlying mechanisms. Cardiovasc Diabetol. 2012;11:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem. 2011;286:18465-18473. [PubMed] |
| 104. | Martínez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, Dieguez C, Lopez M. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. 2014;14:3-21. [PubMed] |
| 105. | Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu A, Sweeney G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 106. | Jessen N, Koh HJ, Folmes CD, Wagg C, Fujii N, Løfgren B, Wolf CM, Berul CI, Hirshman MF, Lopaschuk GD. Ablation of LKB1 in the heart leads to energy deprivation and impaired cardiac function. Biochim Biophys Acta. 2010;1802:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARγ and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J Agric Food Chem. 2012;60:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426-2434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 109. | Damasceno DC, Sinzato YK, Bueno A, Netto AO, Dallaqua B, Gallego FQ, Iessi IL, Corvino SB, Serrano RG, Marini G. Mild diabetes models and their maternal-fetal repercussions. J Diabetes Res. 2013;2013:473575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 111. | Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012;61:272-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 112. | Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217-234. [PubMed] |
| 113. | Pasini E, Flati V, Paiardi S, Rizzoni D, Porteri E, Aquilani R, Assanelli D, Corsetti G, Speca S, Rezzani R. Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol. 2010;9:46. [PubMed] |
| 114. | Peng N, Meng N, Wang S, Zhao F, Zhao J, Su L, Zhang S, Zhang Y, Zhao B, Miao J. An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E-/- mice. Sci Rep. 2014;4:5519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 115. | Zhou J, Wu J, Zheng F, Jin M, Li H. Glucagon-like peptide-1 analog-mediated protection against cholesterol-induced apoptosis via mammalian target of rapamycin activation in pancreatic βTC-6 cells -1mTORβTC-6. J Diabetes. 2015;7:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, Shu H, Liu Y, Li CL. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 117. | Kimura R, Okouchi M, Kato T, Imaeda K, Okayama N, Asai K, Joh T. Epidermal growth factor receptor transactivation is necessary for glucagon-like peptide-1 to protect PC12 cells from apoptosis. Neuroendocrinology. 2013;97:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Malaguarnera R, Sacco A, Morcavallo A, Squatrito S, Migliaccio A, Morrione A, Maggiolini M, Belfiore A. Metformin inhibits androgen-induced IGF-IR up-regulation in prostate cancer cells by disrupting membrane-initiated androgen signaling. Endocrinology. 2014;155:1207-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012;13:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 120. | Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 402] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 121. | He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 122. | Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171:3146-3157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 123. | Lim YM, Lim H, Hur KY, Quan W, Lee HY, Cheon H, Ryu D, Koo SH, Kim HL, Kim J. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat Commun. 2014;5:4934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 124. | Liu Y, Shi S, Gu Z, Du Y, Liu M, Yan S, Gao J, Li J, Shao Y, Zhong W. Impaired autophagic function in rat islets with aging. Age (Dordr). 2013;35:1531-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 896] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 126. | Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of β-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012;9:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, Lee CH, Bae ON. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull. 2014;37:1248-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 128. | Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, Kim S, Kim SY, Choi SB, Noh YH. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 129. | Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med. 2012;29:613-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |