Copyright
©The Author(s) 2015.
World J Diabetes. Feb 15, 2015; 6(1): 125-135
Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.125
Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.125
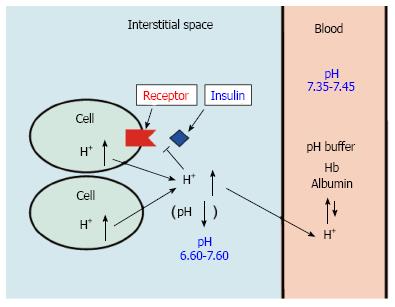
Figure 1 pH of interstitial fluid and blood, and binding affinity of insulin to its receptor.
Interstitial fluids have little pH-buffering molecules, while blood has very strong, powerful pH buffering molecules such as hemoglobin and albumin. Thus, even under mild but not severe metabolic disorder conditions, blood pH is kept constant within a normal range (7.35-7.45), but interstitial fluid pH would be lower than a normal level.
- Citation: Marunaka Y. Roles of interstitial fluid pH in diabetes mellitus: Glycolysis and mitochondrial function. World J Diabetes 2015; 6(1): 125-135
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/125.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.125