INTRODUCTION
Glucose, amino acids and fatty acids are the substrates available for metabolic homeostasis and play important roles in insulin secretion. Pancreatic β-cells synthesise and secrete insulin in response to signals generated from glucose, amino acid and fatty acid metabolism but glucose is the prime stimulus for insulin secretion. Regulated insulin release requires tight coupling in the β-cell between glucose metabolism and insulin secretory response. As β-cells are continually exposed to a complex milieu of nutrients and other circulating factors (like incretins), it is important to understand the interplay between glucose metabolism and that of the two other primary nutrient classes, the amino acids and fatty acids. Specific amino acids are now known to acutely and chronically regulate insulin secretion from pancreatic β-cells in vivo and in vitro and lipid metabolism in the β-cell is critical for the regulation of insulin secretion[1].
The metabolism of glucose, amino acids and fatty acids results in the generation of metabolic coupling factors involved in regulating insulin exocytosis. These metabolic coupling factors generated from the metabolism of glucose, amino acids and fatty acids in the β-cell include ATP, NADPH, glutamate, long chain acyl-CoA and diacylglycerol[2]. Each of these coupling factors plays a key role in regulating insulin secretion. The exocytotic process is closely controlled by signals generated from nutrient metabolism as well as by neurotransmitters and circulating hormones.
Under normal physiological conditions the metabolism of glucose, amino acids and fatty acids is intricately controlled and will result in the regulated secretion of insulin. The secretion of insulin is precisely regulated to keep fasting blood glucose concentrations between 3.5-5.9 mmol/L. In some pathological states the signals generated from glucose, amino acid and fatty acid metabolism cause insulin hyper-secretion or dysregulation of insulin secretion. In these states insulin secretion becomes inappropriate for the level of blood glucose causing hyperinsulinaemic hypoglycaemia (HH).
HH is a major cause of persistent hypoglycaemia in the childhood period[3]. In the newborn and infancy periods HH can be either congenital or secondary to certain risk factors (such as intrauterine growth retardation). Congenital forms of HH are due to defects in key genes (ABCC8, KCNJ11, GLUD1, GCK, HADH, HNF4/HNF1A, SLC23A and UCP2) involved in regulating insulin secretion[4]. Loss of function mutations in the genes ABCC8 and KCNJ11 (which encode for the SUR 1 and KIR6.2, components of the β-cell potassium (KATP) channel subunits respectively) lead to the most severe forms of HH, which is usually medically unresponsive[5]. Clinically HH presents with fasting hypoglycaemia but in some patients the HH is typically triggered by the ingestion of protein (amino acids). Protein induced HH is observed in patients with gain of function mutations of GLUD1[6], loss-of-function mutations of ABCC8/KCJN11[7] and loss of function mutations in the HADH[8].
This state of the art review article will firstly discuss the molecular mechanisms of glucose, amino acid and fatty acid regulated insulin secretion and then focus on the current understanding of the molecular mechanisms involved in protein induced HH.
GLUCOSE MEDIATED INSULIN SECRETION BY THE PANCREATIC β-CELL
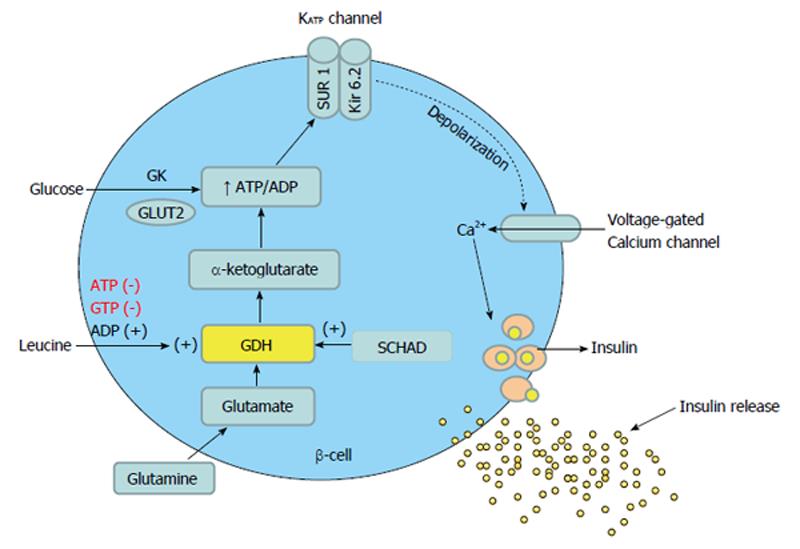
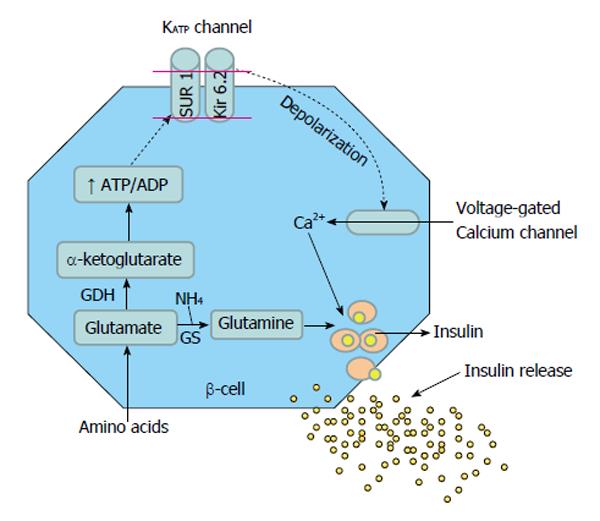
Glucose mediated secretion of insulin is initiated by the uptake of glucose by the β-cells via the glucose transporter. Glucose is then phosphorylated to glucose-6-phosphate by islet-specific glucokinase. Further metabolism of glucose increases the cellular ATP: ADP ratio, which closes ATP-dependent KATP channels in the β-cell membrane, causing membrane depolarization and influx of calcium. Intracellular free calcium then promotes margination of secretory granules, which fuse with the cell membrane before releasing their contents into the extracellular space by exocytosis (Figure 1)[9]. The functional integrity of both SUR 1 and KIR 6.2 proteins is necessary for KATP channel function and the genes encoding for these two proteins are localized very closely to each other on the short arm of chromosome 11 (11p14-15.1).
Figure 1 Glucose and protein mediated insulin secretion in the beta cell of pancreas.
GDH: Glutamate dehydrogenase; SCHAD: Short-chain 3-hydroxyacyl- CoA dehydrogenase; GK: Glucokinase; SUR: Sulfonylurea receptor; Kir 6.2: Potassium channel inwardly rectifying; GLUT2: Glucose transporter 2.
Although KATP channels have an essential role in linking the metabolism of glucose to the secretion of insulin, there is now evidence that there may well be other mechanisms of insulin secretion, the so-called KATP channel independent pathways of insulin secretion[10]. This pathway leads to augmented insulin release in the presence of raised cytosolic calcium (Ca2+) concentrations. Increases in the intracellular Ca2+ concentration in the pancreatic β-cell cause modest increases in insulin secretion, which can be dramatically increased by modulators of protein kinases and phosphatases. This suggests that steps distal to the elevation of cytosolic Ca2+ are of greater quantitative importance in controlling insulin secretion. It has also been shown that glucose can cause pronounced insulin secretion in Ca2+ depleted islets in the presence of activators of protein kinases A and C[11].
Given the key role of pancreatic β-cell KATP channels in regulating insulin secretion it is no surprise that genetic defects in the genes regulating the function of these channels lead to severe forms of HH. Recessive inactivating mutations in KATP channel subunits are the most common cause of HH[5,12]. So far, over 150 mutations have been identified in the ABCC8 and 25 in KCNJ11[13]. These include missense, frame shift, nonsense, insertions/deletions, splice site and regulatory mutations, either present in homozygous or compound heterozygous state. In the Ashkenazi Jewish population, two common (F1388del and c.3992-9G4A) mutations account for 90% of all cases of congenital HH[4].
The molecular basis of recessive inactivating ABCC8 and KCNJ11 mutations involves multiple defects in KATP channel biogenesis and turnover, in channel trafficking from the endoplasmic reticulum and Golgi apparatus to the plasma membrane and alterations of channels in response to both nucleotide regulation and open state frequency.
AMINO ACID MEDIATED INSULIN SECRETION
The observations that plasma levels of insulin increase consistently and significantly when healthy subjects ingest protein meals[14] or when intravenous mixtures of amino acids are administered[15], provide fundamental scientific evidence of the relationship between protein metabolism, amino acids and insulin secretion.
Protein metabolism begins when dietary proteins are broken down to amino acids by intestinal enzymes[16]. Large differences in capacity of individual amino acids to stimulate insulin release are noted in both animal and human studies[15,17]. For example, when 30 g each of 10 amino acids in a mixture was administered individually, arginine proved the most effective and histidine the least in stimulating insulin release[15]. Although leucine itself can stimulate insulin secretion, the phenomenon of protein meal or amino acid stimulated insulin secretion does not solely or largely depend on the presence of leucine[14,15].
Amino acids, alone[18] or in combination[19], act synergistically with glucose to potentiate the release of insulin. Synergism was also observed between amino acid pairs, where the synergistic effect was significantly greater with arginine-leucine than with arginine-phenylalanine and their combined effects greater than when amino acids were administered alone[20]. Indeed, the oral ingestion of amino acid mixtures in combination with carbohydrates produce stronger insulinotropic effects compared with carbohydrate-only preparations[21], a phenomenon mediated by the incretin hormones gastric inhibitory polypeptide and glucagon-like peptide-1 (GLP-1)[22]. Amino acids shown to have the highest insulinotropic effect include leucine, valine, lysine, and isoleucine[23]. Metabolism of amino acids can occur either by transamination or by oxidative deamination.
Transamination is an early step in the degradation of most amino acids and involves a chemical reaction between two molecules, an amino acid (with an amine NH2 group) and a keto acid (with a keto = O group), catalysed by a family of enzymes known as aminotransferases. Different aminotransferases are each specific for an amino acid or a group of chemically similar ones such as branch chain amino acids (BCAA). The keto acid that accepts the amino group is always alpha-ketoglutarate (α-KG), a metabolically important biological compound and key intermediate in the citric acid cycle. For example, alanine transaminase catalyses the transfer of an amino group from alanine to α-KG giving rise to pyruvate and glutamate.
On the other hand, oxidative deamination involves conversion of an amino acid into the corresponding keto acid by removing the amine group as ammonia, which goes into the urea cycle. As glutamate is the end product of many transamination reactions, oxidative deamination occurs primarily on glutamate, generating α-KG[16,24]. The main enzyme involved in oxidative deamination is glutamate dehydrogenase (GDH).
Glutamine and alanine are the most abundant amino acids in the blood and extracellular fluids. Whereas glutamine and alanine require the presence of glucose for insulin secretion, leucine is able to stimulate insulin secretion independently through the allosteric activation of GDH[25-28], generating α-KG. The further metabolism of α-KG is then involved in insulin production in two ways. First, by entering the TCA cycle, the ATP:ADP ratio is raised causing closure of the KATP channel and depolarisation of the β-cell. The voltage dependant calcium channel opens leading to an increase in cellular calcium concentration, triggering the release of insulin from storage granules (Figure 1)[29]. Second, α-KG inhibits isocitrate dehydrogenase resulting in increased cytosolic citrate needed for the synthesis of short and long chain acyl-CoA, which are coupling factors closely involved in insulin secretion[30].
LEUCINE
Leucine is one of the most potent insulin secretagogues among the BCAA that facilitates glucose-induced insulin release from pancreatic β-cells[31]. It does so via several mechanisms. First, in pancreatic β-cells, leucine and its non-metabolizable analogue 2-aminobicyclo (2.2.1) heptane-2-carboxylic acid, stimulate the secretion of insulin by acting indirectly as a positive allosteric activator of GDH to enhance glutaminolysis. Activated GDH facilitates the oxidation of glutamate to α-KG, which raises the ATP:ADP ratio resulting in closure of KATP channel, cellular depolarization, influx of calcium and exocytosis of insulin from the storage granules (Figure 1)[32]. Second, the transaminated product of leucine, α-ketoisocaproate (KIC) can cause insulin secretion through direct inhibition of the KATP channel[33]. Glucose completely blocks the effects of leucine but not of KIC on stimulation of insulin secretion by β-cells[34]. Third, leucine plays an important role in the regulation of the mammalian target of Rapamycin (mTOR) pathway, which was recently recognized as a critical regulator of metabolic response to nutrients and growth factors[35]. Recent data strongly suggest that leucine down-regulates the surface expression of α2 adrenergic receptors in pancreatic islets through activation of mTOR, leading to insulin secretion[36].
GLUTAMINE
As the most abundant amino acid found in the blood, glutamine has both nutritive and non-nutritive effects[37]. Glutamine is physiologically important for maintaining cellular function in tissues of the intestine, kidney, brain and liver[38]. It is an important precursor substrate for the synthesis of peptides, proteins and nucleotides[39], in particular ATP which is central in the β-cell signalling pathway. In SUR 1 knockout (KO) β-cells models, isolated pancreatic islets respond briskly to a physiological mixture of 20 amino acids even though these islets cannot be stimulated by glucose or by leucine. Glutamine played an important role in mediating amino acid stimulation of insulin release as 60% of the insulin response was attributable to glutamine even though it comprised 16% of the amino acid load[7].
Although glutamine itself functions as a key precursor for nucleic acids and nucleotides, in many physiological circumstances it acts to provide glutamate, which promotes a wider array of metabolic functions compared to glutamine. By oxidative deamination of glutamate, GDH liberates free ammonia and the α-KG is then oxidized in the tricarboxylic acid cycle (TCA) cycle, raising ATP levels that close KATP channels and depolarize the cell membrane to release insulin. Ammonia is added to glutamate by glutamine synthetase to form glutamine, the major inert-organ carrier for ammonia.
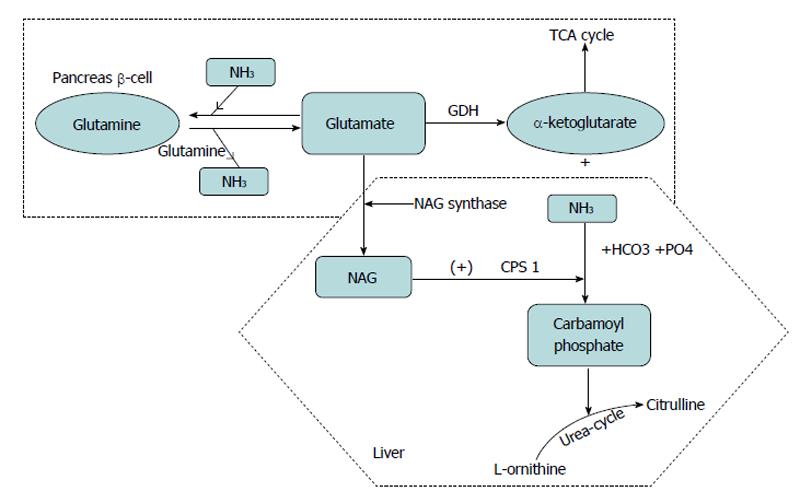
Glutamine can be cleaved by glutaminase to yield glutamate and NH3. The mitochondrial carbamoyl phosphate synthetase (CPS 1) then can catalyze the conversion of ammonia to carbamoyl phosphate. The CPS 1 enzyme is allosterically activated by N-Acetyl glutamate (NAG) produced from glutamate by NAG synthase and may thus be indirectly regulated by glutamate concentration. Carbamoyl phosphate thus formed combines with ornithine in the urea cycle. Thus glutamate also aids in ammonia detoxification and promotion of urea synthesis in the liver (Figure 2)[40,41]. However, the exact mechanism of glutamine linked hyperinsulinemia remains less well understood. Glutamine can also potentiate insulin secretion by stimulating enteroendocrine L-cells to synthesise and secrete the incretin GLP-1. This effect is attributable to a triggering pathway that elevates intracellular Ca2+ and an amplifying pathway mediated by elevated cAMP[42].
Figure 2 Glutamate metabolism.
Oxidation of glutamate by glutamate dehydrogenase liberates free ammonia (NH3) and alpha ketoglutarate, which enters tricarboxylic acid cycle cycle and generates ATP. In the liver glutamate also generates N-acetylglutamate (NAG), which in turn allosterically activates carbomyl phosphate synthetase (CPS) to regulate ammonia detoxification into urea. Glutamine provides a substrate for ammonia buffering, by adding ammonia to glutamate to form glutamine. TCA: Tricarboxylic acid cycle; GDH: Glutamate dehydrogenase.
ALANINE
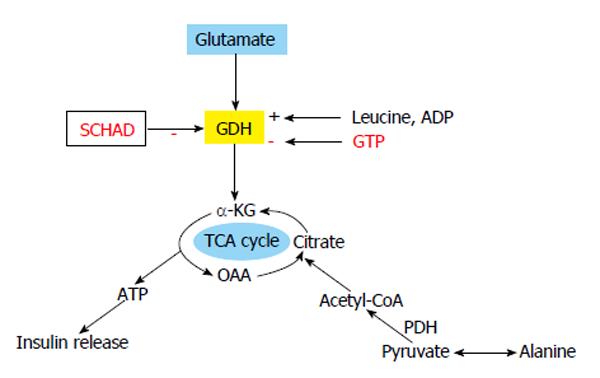
The mechanism of action of alanine as an insulin secretagogue is still unclear. Upon entry into the β-cell cytosol, alanine is deaminated and takes part in the TCA cycle through pyruvate and acetyl-CoA. This results in increase of the cellular content of ATP, closure of the KATP channel, depolarization of the plasma membrane, activation of voltage-gated calcium channel, increase in calcium influx and insulin exocytosis (Figure 3)[43]. Insulinotropic property of alanine has been reported by Dunne et al[44] and McClenaghan et al[45] to be the result of co-transport with Na+, leading to β-cell membrane depolarization and increase in cellular calcium. Current evidence suggests that the mode of action of alanine as an insulin secretagogue involves a combination of increased ATP generation, co-transport with Na+ and signal transduction[26,46].
Figure 3 Glutamate and alanine as insulin secretagogues.
Protein induced hyperinsulinaemic hypoglycaemia due to loss of function mutation in HADH gene (SCHAD). Alanine is deaminated to pyruvate and pyruvate dehydrogenase (PDH) converts it to acetyl CoA, which can enter TCA cycle to generate ATP for closing KATP channel. TCA: Tricarboxylic acid cycle; α-KG: Alpha ketoglutarate; GDH: Glutamate dehydrogenase; OAA: Oxaloacetic acid.
ARGININE
The mechanism of insulin release by arginine involves the mCAT2A amino acid transporter which electrogenically transports arginine into the β-cell, leading to increased intracellular calcium[47]. Accumulation of intracellular arginine leads to membrane depolarization, a further rise in intracellular calcium through opening of voltage-gated calcium channels, and insulin secretion[48]. Arginine can also influence insulin secretion by its conversion to glutamate, which allows the generation of metabolic coupling factors[49], however the detailed metabolism of arginine in the β-cell remains to be investigated.
FATTY ACID β-OXIDATION PATHWAY
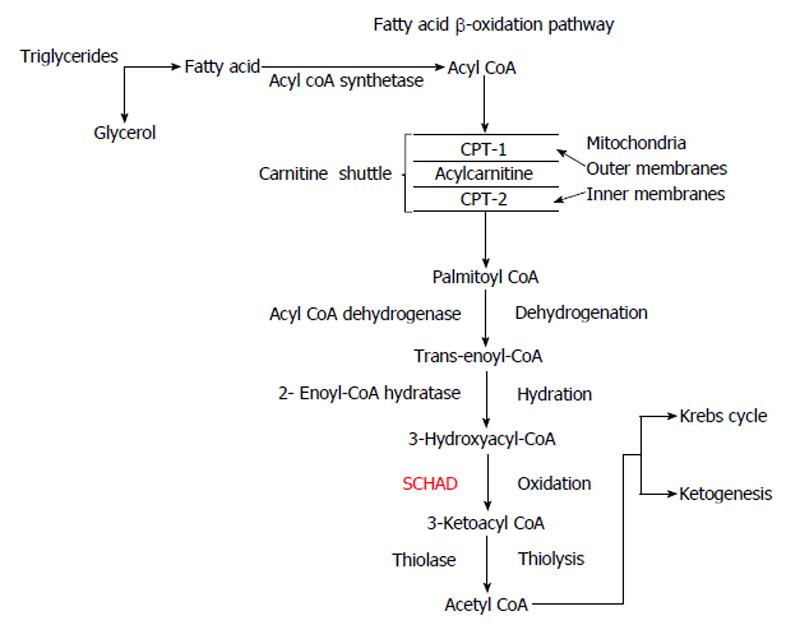
During the fasting state fatty acids (FA) are the most important substrates for ketogenesis to provide the brain with an “alternative fuel” source. Triglycerides are broken down to FA and glycerol in the process of lipolysis. β-oxidation of FA occurs in the peroxisomes and mitochondria. Short and medium chain FA can diffuse directly into the mitochondria and are then activated by acyl-CoA synthetase to acyl-CoA in the mitochondrial matrix, whereas long and very long chains FA are activated by acyl-CoA synthetase on the mitochondrial outer membrane. The “carnitine shuttle” allows acyl-CoA to penetrate the outer and inner mitochondrial membranes, catalysed by carnitine palmitoyltransferase-I and II (CPT- I and II) respectively, facilitated by the inner membrane exchange transporter, carnitine-acylcarnitine translocase[50].
In the mitochondrial matrix, acetyl-coA is generated by β-oxidation of acyl-CoA via a 4-step process involving dehydrogenation, hydration, oxidation and thiolysis (Figure 4)[50]. Acetyl-CoA finally enters the Krebs cycle. The short-chain 3-hydroxyacyl-CoA dehydrogenase (SCHAD), an intramitochondrial homodimer enzyme is essential for catalysing the penultimate reaction of 3-hydroxyacyl CoA to 3-ketoacyl-CoA. Possible molecular mechanisms involved in the pathogenesis of HH due to deficiency of SCHAD have been reported recently[8,51].
Figure 4 β-oxidation of fatty acids.
Acyl-CoA is converted to acetyl-CoA through dehydrogenation, hydration, oxidation and thiolysis. Acetyl-CoA can enter the Krebs cycle or can lead to ketogenesis. CPT-1: Carnitine palmitoyltransferase-1; SCHAD: 3-hydroxyacyl CoA dehydrogenase.
PROTEIN INDUCED INSULIN SECRETION-HISTORICAL PERSPECTIVE
The history of protein induced hypoglycaemia dates back to 1956 when Cochrane described three children with severe hypoglycaemia while on low carbohydrate and high protein diet. Even though amino acid-stimulated insulin secretion by pancreatic β-cells was known for long[52], the molecular mechanisms involved in the dysregulated islet cell function leading to HH due to genetic mutations remain poorly understood. In 1970 researchers reported that amino acids could induce insulin secretion only in the presence of glucose except in case of leucine where the insulin-stimulatory effect is abolished in presence of glucose[53]. Animal studies have suggested that amino acid oxidation and signalling effects are two vital steps in which the amino acid amplifies insulin release from the stored vesicles following β-cell depolarisation and influx of calcium. By early 80’s leucine’s property to induce insulin secretion by allosterically stimulating GDH was identified[29].
MOLECULAR MECHANISMS OF AMINO ACID INDUCED HYPERINSULINAEMIC HYPOGLYCAEMIA
Amino acids are known to enhance insulin secretion from primary islet β-cell lines under appropriate conditions. Leucine can stimulate insulin release on its own by allosterically activating GDH. In the β-cell mitochondria, GDH can stimulate insulin secretion by oxidative deamination of glutamate by raising α-KG, NADH/NAD and NADPH/NADP ratios. Protein sensitive hyperinsulinaemic hypoglycaemia occurs in three forms; gain-of-function mutations of GLUD1[6], loss of function mutations of ABCC8/KCNJ11[7] and loss of function mutations in HADH[8].
HYPERINSULINISM/HYPERAMMONAEMIA SYNDROME
Hyperinsulinism/hyperammonaemia syndrome (HI/ HA) syndrome is the second most common cause of congenital hyperinsulinism-(CHI), characterized by both fasting and protein sensitive hypoglycaemia together with persistently elevated plasma ammonia levels[6]. HI/HA is likely the disorder described by Cochrane et al[52] in 1955, with leucine sensitive hypoglycaemia in a child and her father. Zammarchi et al[54] first reported a case of hyperammonaemia with leucine sensitive hypoglycaemia. Activating mutations in the GLUD1 gene were reported to be the cause of HI/HA syndrome by Stanley et al[6,32] in 1998. Children usually present with recurrent symptomatic hypoglycaemic episodes (leucine sensitive) and persistent hyperammonaemia.
Molecular basis of HI/HA
The enzyme, GDH has a complex allosteric regulatory mechanism and is highly expressed in the pancreas, liver, kidney and brain. GDH catalyses the reversible oxidative deamination of glutamate to α-KG and ammonia, using NAD or NADP as co-factors. GDH is allosterically inhibited by GTP and activated by ADP and leucine[55].
In patients with HI/HA syndrome there is impairment of allosteric inhibition of GDH by GTP leading to gain-of GDH function. This causes increased leucine induced glutamate oxidation to α-KG, which explains the leucine sensitivity following a protein meal and postprandial hypoglycaemia. These patients on fasting develop hypoglycaemia following release of alanine and glutamine from skeletal muscle, which can stimulate insulin release mediated through GDH[56]. The mechanism of hyperammonaemia in HI/HA syndrome is still unclear. In liver, increased GDH activity may lead to hyperammonaemia through 2 possible mechanisms: elevated activity of GDH causing increased levels of ammonia from glutamate and excessive depletion of glutamate pool, reducing the availability of N-acetyl glutamate (NAG) via NAG synthase reaction. NAG is an allosteric activator of CPS 1 and deficiency of this can impair urea synthesis[32,57]. An alternative hypothesis for hyperammonaemia in HI/HA syndrome is that the excessive ammonia is due to abnormal muscle catabolism[58]. More recently the source of the hyperammonaemia in the HI/HA syndrome is thought to be the kidney[59].
Sirtuins and insulin secretion
Sirtuins are a family of NAD+ dependant enzymes having a critical role in metabolic adaptation to stress. Sirtuin4 (SIRT4), an intramitochondrial enzyme highly expressed in pancreatic β-cells, also regulates GDH. SIRT4 repress the activity of GDH by ADP-ribosylation in pancreatic β-cell mitochondria, down regulating insulin secretion mediated through amino acids. In normal glucose states, SIRT4 blunts amino acid-induced insulin secretion by repressing the activity of GDH[60,61]. In contrary GDH is released from the SIRT4-mediated inhibition via an undefined mechanism during fasting, thereby enhancing amino acid-induced insulin secretion[61]. In SIRT4 knockout mice, GDH activity is enhanced in β-cells, leading to the enhancement of glucose and amino acid-stimulated insulin secretion[61]. So loss of function mutation of SIRT4 can present with a phenotype similar to gain of function mutation of GLUD1. However no humans have yet been described with protein induced hyperinsulinism due to SIRT4 mutations[62].
Clinical presentation of HI/HA
The infants with HI/HA syndrome are usually born at term and not macrosomic. The major clinical feature is recurrent episodes of symptomatic HH after first few months of life. These may occur with fasting or can be provoked by protein feeding. Hypoglycaemia in HI/HA syndrome is not as severe as seen in HH due to KATP channel mutations. Hyperammonaemia, a characteristic biochemical marker of HI/HA syndrome, is typically mild to moderate (up to 3-5 times the upper limit of normal) and is not associated with lethargy, irritability, or coma. The plasma amino acid profile remains normal in HI/HA syndrome in contrast to abnormal profile observed in the other causes of hyperammonaemia[62,63].
Protein diet or blood glucose levels do not affect the plasma ammonia levels in patients with HI/HA syndrome[54,64]. Kapoor et al[62] reported some patients who have mutations in GLUD1 with HH but with normal serum ammonia levels and the authors proposed that this could be due to mosaicism for the mutation in the liver, where the mutation is absent or seen in < 50% in hepatocytes. Hyperammonaemia is resistant to detoxification compounds (sodium benzoate and N-carbamylglutamate) or protein-restricted diet[65].
Kapoor et al[62] have published the clinical characteristics of patients with HI/HA due to GLUD mutations. Of the twenty patients most of them were appropriate for the gestational age and presented at a mean age of 23.4 wk. Nineteen of them had hyperammonaemia. Thirteen of the 17-screened probands had 7 different heterozygous mutations and three novel mutations were identified (N410D, D451V, P436L). More than 90% cases responded to diazoxide. Seizure was the most common (94%) symptom, 43% of them developed generalized epilepsy with a higher preponderance in cases with mutations in exons 6 and 7 of GLUD1 gene[62,66].
Earlier in 2004, Stanley et al[32,57] has reported that over activity of GDH in the brain decreases the levels of glutamate and glutamine, protecting the central nervous system from the neurotoxicity of its accumulation.
GDH transgenic mice harbouring the human GDH-HI H454Y mutation develop a hypoglycaemia phenotype[67] and insulin secretion studies in these mice are associated with increased oxidative deamination of glutamate via GDH, this confirming the key role of GDH in amino acid stimulated insulin secretion.
Using a β-cell-specific GDH KO mouse model [βGlud1 (-/-)] islets isolated from these mice showed diminished of insulin release when stimulated by glutamine combined with 2-aminobicyclo (2.2.1) heptane-2-carboxylic acid or l-leucine[68]. Further studies in these mice showed that permissive levels of glutamate were required for the full development of glucose-stimulated insulin secretion and that GDH plays an indispensable role in this process.
Management of HI/HA
Treatment of HI/HA is aimed at correction of fasting and protein induced hypoglycaemia. Diazoxide remains the main stay of treatment and affected patients are well controlled with a dose of 5-15 mg/kg per day[69]. Being a KATP channel agonist, diazoxide prevents β-cell membrane depolarization and inhibits insulin secretion by keeping KATP channels open. Diazoxide is usually combined with hydrochlorothiazide in neonates to counteract its fluid retention side effects. Hypertrichosis seen in infants on diazoxide usually resolves on discontinuation[70]. Recent reports of large symptomatic pericardial effusion in infants on diazoxide, warrants meticulous cardiovascular monitoring while on treatment[71].
Green tea flavonoids and HI/HA
Naturally occurring compounds from green tea, discovered by the Chinese Emperor Shen-Nung in 2737 B.C. has been used as a remedy to treat a number of ailments, including diabetes mellitus[72]. Green tea is a significant source of a type of flavonoid called catechin, which includes epigallocatechin gallate (EGCG), epigallocatechin, epicatechin gallate (ECG) and epicatechin, of which EGCG and ECG have a strong inhibitory effect on GDH function[72,73].
Animal studies have shown that ECG binds to the same site as the allosteric regulator ADP and hijacks the ADP activation site. In pancreatic islet cells of transgenic mice expressing a human HI/HA form of GDH, a hyper-response to glutamine caused by dysregulated GDH is blocked by the addition of EGCG[73]. Above all EGCG has the property to inhibit GTP-insensitive GDH mutations, opening the window of therapeutic potential to treat GDH hyperinsulinism. EGCG also has been shown to block glutamine stimulated calcium influx and insulin secretion in GDH transgenic mice islets[74].
Several novel GDH inhibitors are identified and are under trial[75]. Current evidence support the pathological basis of hyperammonaemia to be due to gain in GDH activity and excessive oxidation of glutamate, reducing the level needed for the synthesis of NAG and thereby slowing the clearance of ammonia (Figure 2). In this context N-carbamylglutamate (Carglumic acid), a carbamoyl phosphate synthetase activator has a potential role in the treatment of hyperammonaemia in HI/HA syndrome[69,76,77]. De novo mutations in GLUD1 have been reported in 70% of GDH-HI cases with the remainder inherited in an autosomal dominant pattern[69].
PROTEIN INDUCED HYPOGLYCAEMIA DUE TO DEFECTS IN KATP CHANNEL GENES
Mutations in the ABCC8/KCNJ11 genes are the most common cause of CHI[5]. The observation that patients with KATP channel null mutations can develop HH following high protein meal in the absence of leucine sensitivity[78], demonstrates that amino acids can induce HH via GDH and KATP channel independent pathways. Patient with GLUD1 mutations show leucine sensitive hypoglycaemia whereas those with ABCC8/KCNJ11 mutations are not leucine sensitive. Thus, protein-induced HH is not necessarily synonymous with leucine-sensitive HH. The GDH and KATP channel independent mechanism of protein induced HH can be explained through the direct induction of insulin release by glutamine, formed by the ATP-dependent condensation of glutamate with ammonia, catalysed by glutamine synthetase (Figure 5).
Figure 5 Protein Induced Hypoglycaemia due to defects in KATP channel genes.
GDH: Glutamate dehydrogenase; GK: Glucokinase.
Role of glutamine in insulin secretion in patients with KATP channel defects
Glutamine plays a pivotal role in glucose and amino acid stimulated insulin secretion as a signalling molecule, which is followed by β-cell depolarization and influx of calcium and insulin release. Prerequisites for glutamine to function in β-cell include elevated ATP levels and increased cytosolic calcium[7]. Role of glutamine in stimulation of insulin release has been shown in patients with mutations of SUR 1[78]. Animal studies have shown that β-cells of SUR 1-/- mice are markedly sensitive to glutamine stimulation[7,67]. Li et al[7,67] has shown that β-cells lacking SUR 1 protein were hyper-responsive to glutamine and amino acid mixture but were refractory to glucose stimulation. This amino acid response was reduced by 60% when glutamine was omitted from the amino acid mixture[7]. Two possible mechanisms are considered but still remain unsettled: Metabolism of amino acids is enhanced while glucose is impaired in SUR 1 lacking β-cell which could be the result of persistent elevation of cytosolic calcium and secondly glutamine may be triggering insulin release by a hypothetical novel mechanisms like activation of protein kinase pathways[11,78,79].
PROTEIN INDUCED HYPERINSULINAEMIC HYPOGLYCAEMIA DUE TO LOSS OF FUNCTION MUTATION IN HADH GENE
Mutations causing genetic defects have been described in many of the enzymes involved in mitochondrial fatty acid oxidation. Recently, mutations in the penultimate enzyme in the fatty acid oxidation chain have been described that result in quite different symptoms from those normally seen. Patients with the mutations in HADH present with protein (leucine)-induced HH, suggesting a link between fatty acid oxidation, amino acid metabolism and insulin secretion[80].
Short-chain-3-hydroxyacyl-CoA dehydrogenase catalyses the penultimate reaction of the β-oxidation cycle for medium and short chain 3-hydroxy fatty-acyl-CoA’s. SCHAD deficiency impairs short chain fatty acid oxidation. First insights into the molecular mechanism involved in SCHAD deficiency came with the observation of Clayton et al[8] (2001) that fatty acid beta oxidation defect is associated with HH, supporting the concept of lipid signalling pathway in the control of insulin secretion[81].
Clinical aspects of patients with HADH mutations
Affected children with SCHAD deficiency on fasting as well as following a protein meal, either present with mild late onset hypoglycaemia or severe neonatal hypoglycaemia with raised levels of fatty acid metabolites including plasma hydroxybutyrylcarnitine and urinary 3-hydroxyglutaric acid[82,83]. Most often they present with hypoglycaemic seizures. Kapoor et al[84] in 2009 reported for the first time that human mutations of HADH gene cause severe dietary protein sensitivity leading to HH and they may have normal acylcarnitine and urinary organic acid profiles. These cases had novel HADH gene mutations. The enzymes GDH and SCHAD have a direct protein-protein interaction, which is lost in patients with HADH mutations causing leucine induced HH. Leucine sensitivity is evident in patients with HADH gene mutations (Figure 3). There is no associated loss of inhibitory effect of GTP on GDH, as seen with GLUD1 mutations[85].
The interaction between SCHAD and GLUD1
SCHAD has a vital role in insulin secretion, suggested by the high degree of expression of HADH gene in β-cells of pancreas[86]. Hardy et al[87], using RNA interference, identified HADH gene as one of the 4 essential genes required for normal insulin secretion. FOXA2, a transcription factor encoded by the gene FOXA2, is essential for β-cell differentiation and its function has been shown to regulate HADH expression[88]. Additionally, severe hyperinsulinism after leucine tolerance testing was reported in all patients having HADH gene mutations[85]. Further reports on the loss of protein-protein interaction in human cases between SCHAD and GDH were published[51,83,89]. Heslegrave et al[85] made a similar observation of the loss of interaction between SCHAD and GDH in lymphoblasts.
Sund et al[90] showed severe HH in FOXA2 β-cell KO mice. Islets from these mice were shown to have reduced expression of both SCHAD and Kir6.2 and had severe HH. Li et al[51] showed that HADH KO mice developed a hyperinsulinaemic response following leucine loading and an exacerbation of the same on addition of glutamine and alanine. When glutamine and leucine were removed from the amino acid mixture, KO mice islets failed to induce HH, suggesting the role of GDH activation for abnormal insulin secretion.
Recent studies on HADH KO mice showed an increased sensitivity to amino acid stimulated insulin secretion indicating activation of the glutaminolysis pathway via GDH to increase ATP production and thereby insulin. Binding of SCHAD to GDH was also shown in immunoprecipitation experiments. These research works indicate that hyperinsulinism in SCHAD-deficient states is caused by loss of “moonlighting function” (a protein having additional functions in other pathways) of SCHAD protein, which otherwise provides a direct inhibitory regulation of GDH in β-cells[51,91]. So in pancreatic β-cells, mutations resulting in the absence of SCHAD protein leads to abnormal activation of GDH, causing hyperinsulinism.
The activation of GDH in HADH gene mutant patients or mouse KO models is limited to pancreatic β-cells and hence deficiency of SCHAD enzyme does not lead to hyperammonemia unlike in HI/HA syndrome[51,92]. Further evidence for protein-protein interaction between enzymes came from Zhang et al[93]. They showed the co-precipitation of GDH with SCHAD when anti-SCHAD antibody was used as bait in wild type mouse liver mitochondria, confirming the previous observation that GDH activation in SCHAD deficiency is due to loss of protein-protein interaction.
Diazoxide remains the treatment of choice in HH due to HADH gene mutations. This also confirms the intactness of KATP channel in patients with SCHAD deficiency[8,82,84,92].
CONCLUSION
The interplay between glucose metabolism and that of the two other primary nutrient classes, amino acids and fatty acids is critical for regulated insulin secretion. Protein induced HH is observed in patients with mutations in GLUD1, HADH and ABCC8/KCNJ11. GDH and SCHAD play important roles in integrating amino acid and fatty acid signals for insulin secretion.
P- Reviewer: Gómez-Sáez J, Junghyo J, Kietzmann T, Lehtonen SH S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ