Published online Oct 15, 2014. doi: 10.4239/wjd.v5.i5.651
Revised: July 9, 2014
Accepted: July 18, 2014
Published online: October 15, 2014
Diabetes is the most important risk factors for chronic kidney disease (CKD). The risk of CKD attributable to diabetes continues to rise worldwide. Diabetic patients with CKD need complicated treatment for their metabolic disorders as well as for related comorbidities. They have to treat, often intensively, hypertension, dyslipidaemia, bone disease, anaemia, and frequently established cardiovascular disease. The treatment of hypoglycaemia in diabetic persons with CKD must tie their individual goals of glycaemia (usually less tight glycaemic control) and knowledge on the pharmacokinetics and pharmacodynamics of drugs available to a person with kidney disease. The problem is complicated from the fact that in many efficacy studies patients with CKD are excluded so data of safety and efficacy for these patients are missing. This results in fear of use by lack of evidence. Metformin is globally accepted as the first choice in practically all therapeutic algorithms for diabetic subjects. The advantages of metformin are low risk of hypoglycaemia, modest weight loss, effectiveness and low cost. Data of UKPDS indicate that treatment based on metformin results in less total as well cardiovascular mortality. Metformin remains the drug of choice for patients with diabetes and CKD provided that their estimate Glomerular Filtration Rate (eGFR) remains above 30 mL/min per square meter. For diabetic patients with eGFR between 30-60 mL/min per square meter more frequent monitoring of renal function and dose reduction of metformin is needed. The use of sulfonylureas, glinides and insulin carry a higher risk of hypoglycemia in these patients and must be very careful. Lower doses and slower titration of the dose is needed. Is better to avoid sulfonylureas with active hepatic metabolites, which are renally excreted. Very useful drugs for this group of patients emerge dipeptidyl peptidase 4 inhibitors. These drugs do not cause hypoglycemia and most of them (linagliptin is an exception) require dose reduction in various stages of renal disease.
Core tip: Chronic kidney disease (CKD) is very often among diabetic persons. In every day clinical practice doctors worldwide have to deal with these patients and help them to achieve their metabolic goals. Despite this, many studies of antidiabetic drugs have excluded people with CKD. So, we lack solid evidence on the effectiveness and safety of these drugs. In this review I propose therapeutic algorithms for diabetic patients in different stages of CKD and clarify some questions about the use of popular antidiabetic drugs as metformin and sulfonylureas.
- Citation: Ioannidis I. Diabetes treatment in patients with renal disease: Is the landscape clear enough? World J Diabetes 2014; 5(5): 651-658
- URL: https://www.wjgnet.com/1948-9358/full/v5/i5/651.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i5.651
Chronic kidney disease (CKD) affects million of people worldwide. CKD is becoming a major problem for public health as it leads to increased morbidity and mortality. Patients with end stage chronic kidney disease often need kidney transplantation[1].
The prevalence of chronic kidney disease to the estimated 11% of the United States population. Patients with chronic kidney disease have an increased risk of cardiovascular disease and progression of renal disease in end-stage renal failure. End stage renal failure leads to dialysis or transplantation[2,3].
Diabetes is the most important risk factors for CKD. The risk of CKD attributable to diabetes continues to rise worldwide.
The National Kidney Foundation and the American Heart Association have recently issued guidelines for the management of cardiovascular risk in people with kidney disease by stating emphatically that these individuals are at very high cardiovascular risk.
For diabetic patients with chronic kidney disease, the risk of cardiovascular disease is even higher classifying these individuals in the highest risk group for cardiovascular disease. Diabetic subjects with microalbuminuria have increased risk (2x) of cardiovascular disease than those with normoalbuminuria. Proteinuria and decreased Glomerular Filtration Rate (GFR) contribute synergistically to increase cardiovascular risk. Most diabetic patients with CKD stage 3 will suffer a serious cardiovascular event, possibly fatal before their chronic kidney disease progress to end stage kidney failure.
Diabetic patients need complicated treatment for their metabolic problems as well as for related comorbidities. They have to treat, often intensively, hypertension, dyslipidaemia, bone disease, anaemia, and frequently established cardiovascular disease (CVD). Thus, the problem for the appropriate selection of antidiabetic treatment for patients with diabetes and CKD is usual in every day clinical practice[4,5].
Recent guidelines for the treatment of diabetes (ADA, EASD 2012) propose personalization of glycaemic goals. For the majority of diabetic patients the appropriate goal is a haemoglobin A1c (HbA1c) < 7% but for patients with severe comorbidities a goal between 7% and 8% is acceptable. Diabetic subjects with CKD usually belong to this group.
The glycated HbA1c is the most popular and well-accepted biological marker for the assessment of long-term glycaemic control. This also applies to patients with diabetes and renal disease. However, the method has significant limitations in these patients. The measurement is influenced by both renal function and complications of chronic kidney disease such as haemolysis, iron deficiency and metabolic acidosis.
In most cases diabetic subjects with chronic kidney disease must rely more on self-monitoring of blood glucose with usual glucose meters. Patients with diabetes and CKD have usually already established CVD. These patients are also in greater risk of hypoglycaemia. We know from physiology that normal renal function conveys a 30% of neoglycogenesis, which is necessary to avoid hypoglycaemia especially in prolonged fasting periods[6].
Many diabetics with uraemia have also nutritional problems and some times cachexia. The use of insulin as well as of sulfonylureas or glinides (short acting secretagogues) leads to increased rate of hypoglycaemia in this group of patients[7,8].
On the other hand, many drugs have renal metabolism and their metabolites are usually active prolonging their time of action. The use of antidiabetic drugs, especially the new classes, is conflicted. The major problem is that in many efficacy studies patients with CKD are excluded so data of safety and efficacy for these patients are missing. This results in fear of use by lack of evidence[9].
Nevertheless, pharmacokinetics and pharmacodynamics data for many new drugs help us to understand the potential risks and benefits for these subjects. Even if these basic data are reassuring the clinical point remains critical: We cannot use new drugs based only on these evidence! We need results form efficacy studies and then approval from FDA and EMEA[10].
Finally, the use of antidiabetic drugs is more complicated in these patients because many people with kidney disease are often elderly, and have long lasting disease and significant co-morbidities. These people take many drugs and they have high risk of drug interactions.
For all diabetic subjects we have to estimate their renal function. 1st step: Serum creatinine/annually (or every 3-4 mo in selected patients); 2nd step: Based on serum creatinine we estimate GFR (eGFR). eGFR is usually based on patient characteristics (as age, sex and race) as well as serum creatinine levels. The most popular method of assessment of renal
MDRD: GFR = 175 × SerumCr-1.154× age-0.203 [× 1.212 (if patient is black) × 0.742 (if female)]
function with the greater precision is the Modification of Diet in Renal Disease (MDRD) equation. This equation is based on data of MDRD Study. This equation (MDRD) is especially accurate in GFR < 60 mL/min.
For higher GFR another equation can also be used: Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) method based on data of CKD-EPI.
CKD-EPI: GFR = 141 × min (Scr/κ, 1)α× max (Scr/κ, 1) - 1.209 × 0.993Age× 1.018 (if female) × 1.159 (if black)
Where Scr is serum creatinine (mg/dL), κ is 0.7 for females and 0.9 for males, α is -0.329 for females and -0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.
Usually we use friendly calculators to estimate GFR. Many programs are also free available for smartphones.
The classical formula of Cockcroft-Gault is not used anymore because it overestimates GFR. (Body weight in the formula must be lean weight and not total weight).
Metformin is globally accepted as the first choice in practically all therapeutic algorithms for diabetic subjects. The advantages of metformin are low risk of hypoglycaemia, modest weight loss, effectiveness and low cost. Data of UKPDS indicate that treatment based on metformin results in less total as well cardiovascular mortality.
Many diabetologists as well as practitioners are fear to use metformin in patients with renal problems even if they have only albuminuria. There is a lot of confusion about the real restriction of its use in patients with CKD[11].
Metformin is slowly absorbed when administered orally. The bioavailability of the drug is low (50%-60%).
Metformin achieves a maximum plasma concentration one to three hours after ingestion, if taken in the form of immediate release or in 4-8 h with the extended release form. Metformin is not connected with albumin or any other protein in plasma. This results in a high volume of distribution up to 1000 even after the first dose[12].
In patients with moderate and severe CKD Cmax is increased 173% and 390%, respectively, compared with patients with normal renal function.
In normal pH metformin remains as hydrophilic cation. Less than 0.01% of the drug is unionized in blood. Lipid solubility of metformin is low. So, metformin can not diffuses through cell membranes. Phenformin, another member of antidiabetic drug class biguanides, which is no longer in the market, is more lipophilic than metformin due to different side chain. Metformin is not metabolized in the liver. Metformin is actively excreted by the urinary tube and found unchanged in the urine. After 24 h, if renal function is normal, metformin is not detected in the blood after administration of a single dose. The half-life of metformin is plasma is about 6 h[13].
The absorption of metformin in the intestine is mediated by a transporter known as plasma membrane monoamine transporter. Several metformin transporters are implicated in its intestinal absorption as well as in its hepatic uptake and renal excretion. These transporters are either Organic Cation Transporters (OCTs) or multidrug and toxin extrusion proteins (MATEs).
The kidneys also actively excrete metformin. Metformin enters renal cells of the renal tubule from circulation. This procedure takes place on the basolateral membrane of the cells and is mediated by OCT2.
Then, metformin is excreted into the lumen. This excretion is facilitated by MATE (1 and 2-K). These extrusion proteins are located in the apical membrane of renal proximal tubule cells.
Metformin is also reabsorbed in renal tubules and this action is mediated by OCT1, which is located also in proximal and distal tubules.
The molecular mechanisms underlying metformin action appear to be complex. Metformin entries into the hepatic cell and facilitate phosphorylation and activation of AMP-activated protein kinase (AMPK). Activation of this key-kinase (energy status sensor) lead to many effects related to metabolism of glucose and lipids. Metformin inhibits hepatic neoglycogenesis also in a direct manner. Metformin inhibits complex I of the mitochondrial respiratory chain. This inhibition, leads to an incretion of AMP:ATP ratio, which activate AMPK. This inhibition leads also to increased anaerobic metabolism of glucose in cytoplasm and the production of lactic acid. Thus, metformin is related with increased risk of lactic acidosis when renal elimination of lactic acid is decreased (renal disease, reduced GFR) or hepatic function is severely damaged (lactic acid is used in hepatocytes to produce glucose-neoglycogenesis-). The risk of lactic acidosis is also increased in patients with tissue hypoxia (shock, severe heart failure, sepsis, surgery related hypotension, etc.)[14].
Risk of lactic acidosis was greater with phenformin because it’s a more potent inhibitor of mitochondrial respiration. Phenformin has hepatic metabolism with an inactive metabolite. The enzyme CYP2D6 metabolizes phenformin into an inactive metabolite. A small ratio of patients (about 2.8%) has a polymorphism of the enzyme that makes them poor metabolizers. In these patients the risk of lactic acid is even greater (due to higher levels of phenformin).
Nevertheless, analysis of data from may trials (347 comparative trials and cohort studies) from Cochrane Database systematic review in 2010, showed no cases of lactic acidosis in 70490 patient-years of metformin.
Statistical analysis of these data suggested that the upper limit for the incidence of lactic acidosis per 100000 patient-years was 4.3 cases (lower than 5.4 cases in the non-metformin group).
In this analysis also, levels of lactic acid seems to be no different in the two groups.
In most studies however lactic acidosis was not a pre specified end point and there were no data about lactic acid levels.
In the Table 1 we summarize the current recommendations about the use of metformin in CKD.
| eGFR (mL/min per 1.73 m2) | Use of metformin |
| > 60 (CKD 1 and 2) | No contraindication |
| Check of renal function annually | |
| 45-60 (CKD 3a) | Use of metformin-reduce dose (no more than 1.5-2 g daily) |
| Frequent check of renal function (every 3-6 mo) | |
| 30-45 (CKD 3b) | Reduce dose (no more than 1-1.5 g daily) |
| No new cases | |
| Frequent check of renal function (every 3-6 mo) | |
| < 30 (CKD 4 and 5) | Stop metformin |
All diabetic subjects at risk of acute renal failure must discontinue at least temporally metformin. Clinical situations related to increase of acute renal failure includes hepatic insufficiency and use of radiocontrast agents and antimicrobial drugs. Fluid substitution as well as support of cardiac output is useful in certain clinical conditions. Monitoring of urine output and serum creatinine lack sensitivity and specificity in acute renal failure, they remain the most used parameters in clinical practice.
At last, when we change the dose of drugs affecting blood pressure and potentially renal perfusion we have to monitor renal function closely and to reduce the dose of metformin (use of diuretics or increase of their dose, start of use of ACEIs and ARBs, unstable heart failure with frequent hospitalizations, etc.).
Pioglitazone has only and exclusively hepatic metabolism. It does not cause hypoglycemia and it can be given theoretically without dose adjustment at all stages of CKD. Pioglitazone is related with fluid retention, anemia and osteoporosis. These side effects complicate the existing problems with anemia and bone disease in subjects with diabetes and CKD[15,16].
The use of pioglitazone is generally limited in these patients and in decreased dose (usually 15 mg once daily).
Sulfonylureas are old drugs widely used worldwide. These drugs ease the secretion of insulin and are related with increased risk of hypoglycemia, which is a major issue for CKD patients.
Glibenclamide (glyburide) is metabolized in the liver and excreted by the kidneys equally and intestine. Some metabolites are active and can accumulate in CKD despite the fact that biliary removal partially counteracts the limited renal excretion.
Hypoglycemia may be serious and lasting more than 24 h in CKD.
The use of glibenclamide in subjects with moderate CKD (eGFR 60-90 mL/min) should be limited (reduced dose, frequent monitoring due to increased risk of hypoglycemia). The drug and is contraindicated in stage ≥ 3 CKD (eGFR < 60 mL/min)[17].
Glimepiride is metabolized by the liver to two major metabolites each of which has hypoglycemic activity. In renal disease these metabolites summed. Although the half-life is 5-7 h, the drug can cause severe hypoglycemia that lasts more than 24 h. Its use is safe in GFR > 60 mL/min and with a reduced dose of up to 30 mL/min. In CKD stage 4 or 5 the use of glimepiride is dangerous[18].
Gliclazide is metabolized by the liver to inactive metabolites that are eliminated in the urine. Thus, gliclazide causes less hypoglycemia than other sulfonylureas. In CKD sage 1, 2, 3 (eGFR > 30 mL/min) gliclazide can be used. There are no data in patients with severe CKD but according to its metabolism the use (in reduced dose) of gliclazide is also permitted in these subjects[19].
Glipizide also does not need dose adjustment in severe and moderate renal disease and can be used safely. (The only caution remains the risk of hypoglycemia).
Glinides, repaglinide and nateglinide, are short acting secretagogues. The short duration of their action means reduced risk of hypoglycemia compared to sulfonylureas. This is an advantage for diabetic subjects with CKD because they belong in the high risk for hypoglycemia group as already mentioned.
Repaglinide is absorbed from the gastrointestinal tract and metabolized in the liver by oxidation and conjugation with glucuronic acid. The major metabolites of repaglinide are M1, M2 and M4. These metabolites are excreted via the bile into the feces and have no hypoglycemic activity[20].
Repaglinide can be used even in CKD stages 4 and 5 without dose reduction.
Nateglinide is also rapidly absorbed from the gastrointestinal tract and metabolized in liver to 9 main metabolites (M1-M9). These metabolites have much weaker hypoglycemic activity than the parent compound. The only metabolite that retains high activity is the metabolite M7. The concentration of this metabolite however is low (< 7%), resulting in a hypoglycemic effect, which is attributed mainly to intact nateglinide. The excretion of the drug in urine is unchanged form at 16% and by 84% in the form of metabolites.
In CKD stage 5 we avoid nateglinide, and in stage 4 we adjust the dose (60 mg × 3)[21].
Dipeptidyl peptidase 4 (DPP-4) inhibitors (gliptins) constitute a new class of antidiabetic drugs with a very favorable profile: safety, efficacy, and low risk of hypoglycemia and weight neutrality[22].
Gliptins are inhibitors of the enzyme DPP-4. This enzyme degrades and inactivates many active peptides. Among them are incretin hormones. These hormones, namely glucagon like peptide 1 (GLP-1) and glucose dependent insulinotropic polypeptide stimulates glucose dependent insulin secretion by b cells in pancreatic islets. At the same time they suppress glucagon production by a cells in the same islets. Their role in glucose homeostasis seems to be important. These hormones are secreted in low levels when we are fasting but their secretion is rapidly increased after meal consumption. Their action results also in reduced glucagon secretion, which in turns reduces hepatic glucose production.
Vildagliptin, Sitagliptin, Saxagliptin, Linagliptin and Alogliptin belong to this class and are already available in the market. Their place in algorithms for patients with diabetes and CKD is important. We can use them all in CKD but with dose adjustment for the majority of the members of this class. (Only linagliptin does not need dose adjustment in any stage of CKD)[23].
In Table 2 we summarize the dose adjustments for all gliptins in diabetic subjects with CKD.
| CKD | ||||
| CKD 1, 2 and 3a (Clcr > 50 mL/min) | CKD 3b (Clcr 30-50 mL/min) | CKD stage 4 (Clcr 15-30 mL/min) | CKD stage 5 (ESRD) | |
| Sitagliptin (Januvia) | √ (100 mg × 1) | 1/2 dose (50 mg × 1) | 1/4 dose (25 mg × 1) | 1/4 dose (25 mg × 1) |
| Vildagliptin (Galvus) | √ (50 mg × 2) | 50 mg × 1 | 50 mg (no experience) | |
| Saxagliptin (Onglyza) | √ (5 mg × 1) | 1/2 dose (2.5 mg × 1) | 1/2 dose (2.5 mg × 1) | 1/2 dose (2.5 mg × 1) |
| Linagliptin (Trajenta) | √ (5 mg × 1) | √ (5 mg × 1) | √ (5 mg × 1) | P (5 mg × 1) |
| Alogliptin (Nesina) | √ (25 mg × 1) | 1/2 dose (12.5 mg × 1) | 1/4 dose (6.25 mg × 1) | 1/4 dose (6.25 mg × 1) |
Sitagliptin does not undergo extensive metabolism. In the liver sitagliptin partially metabolized by oxidation in a limited rate by the enzyme CYP3A4. Nevertheless, most of the drug is excreted in the intact form in the urine (more than 80%). Sitagliptin is filtered in renal glomerulus but also is actively excreted by active tubular secretion[24].
Six metabolites are detected in amounts of < 1% to 7%. These metabolites M1 to M6 are products of hepatic metabolism.
Chemically the changes in these metabolites are: Μ1: N-sulfation, M4: N-carbamyl glucuronidation, M6: hydroxylation followed by either glucuronidation (M3), and oxidative desaturation followed by cyclization (M2 and M5). These metabolites are practically inactive.
In renal disease the elimination of the drug is reduced resulting in 2- or 4-fold increase of the concentration of the drug (for CIcr 30-50 mL/min and < 30 mL/min respectively). The dose adjustment is based on these properties.
In Phase I studies of sitagliptin dosing up to 600 mg daily doe not results to dose-related side effects, at least in the short term (up to 28 d). These data indicates that if we don’t adjust the dose in CKD practically it might be safe at least for a short period[25,26].
Vildagliptin is absorbed quickly (85.4% of the drug). The maximum plasma level is detected at 1.1-h post dose.
Plasma radioactivity (after the administration of radioactive labeled drug) due to vildagliptin is 25.7% and to its major metabolite M20.7 is 55%. The half- life of vildagliptin is 2.8 h. Eighty-five percent of the drug is excreted in the urine (22.6% as vildagliptin the rest as inactive metabolites) and the remaining 15% in feces (4.54% as vildagliptin). In humans, the main pathway of metabolism of the drug is carboxylation, which results in the form of the active metabolite M20.7. DPP-4 contributes to formation of this metabolite. Other minor metabolites are: M15.3, which results from hydrolysis of amide bonds, M20.2 from glucuronidation of the pyrrolidine ring and M20.9, M21.6 from oxidation of the pyrrolidine ring. All these metabolites are inactive[27].
Hydrolysis takes place in multiple tissues or organs. Exposure to vildagliptin in subjects with type 2 diabetes and renal disease of various stages cannot be accurately predicted because the kidneys play a small role in the removal of the drug while participating in metabolism via hydrolysis[28].
In diabetic subjects with chronic kidney disease stage 1 or 2 (eGFR > 50 mL/min per 1.73 m2), dose adjustment of vildagliptin is not required.
In patients with chronic kidney disease stage ≥ 3, both vildagliptin and its active metabolite M20.7 are less excreted via the kidneys. In these patients a dose adjustment is required. (When eGFR is < 50 mg/mL per 1.73 m2 the dose is 50 mg × 1).
Saxagliptin is primarily hepatic metabolized by the cytochrome P450 3A4/5 (CYP3A4/5). The major metabolite of this drug is also active as also a DPP-4 inhibitor, and retains half of the potency of parent drug.
All the drugs, which are also metabolized in this cytochrome CYP3A4/5, may alter the pharmacokinetics of the drug and its active metabolite. Twenty-four percent of the drug is excreted in the urine as saxagliptin and 36% as its active metabolite. There is also some active renal excretion of the drug. A significant part (more than 20%) can be found in the feces as a sum of excreted in bile drug and unabsorbed drug[29].
In diabetic patients with chronic kidney disease stages 1 and 2 increased concentration of saxagliptin and its active metabolite remains clinically irrelevant and no dose adjustment is needed.
In diabetic subjects with chronic kidney disease stages ≥ 3 half dose is recommended (2.5 mg × 1 daily) to achieve the same plasma concentrations compared to subjects with normal renal function. The same dose is recommended in patients with end-stage renal disease (requiring hemodialysis).
This DPP-4 inhibitor is not practically metabolized and is excreted unchanged in the urine. (More than 70% of the parent drug). One minor metabolite named M1 is active but its concentration remains quite low (< 1%)[30]. Alogliptin is excreted by glomerulus filtration as well as by active tubular secretion.
In patients with CKD stage 1 and 2 no dose adjustment is needed (25 mg × 1 daily). In patients with CKD stage 3 (CrCl≥ 30 to < 60 mL/min), the recommended dose is 12.5 mg once daily and in patients with CKD stage ≥ 4 the recommended dose is 6.25 mg once daily. The same dose is required in patients with end-stage renal disease requiring dialysis.
Linagliptin is primarily nonrenally excreted: 80% of the drug is eliminated via the bile and gut and only 5% is eliminated via the kidney[31]. The drug is not practically metabolized and is excreted unchanged. There is no need of dose adjustment in any stage of CKD (5 mg × 1 for all diabetic subjects).
These drugs are injectable and are potent without risk of hypoglycemia. They have to be used with caution in patients with CKD because their gastrointestinal side effects can induce deterioration of renal disease. (Dehydration due to vomiting or diarrhea).
Exenatide is excreted only by the kidneys and undergoes fragmentation in the renal tubule. It does not metabolized by DPP-4 nor the neutral endopeptidase (NEP). There is no hepatic metabolism of exenatide[32].
In CKD stage 3 dose reduction is needed (5 μg × 2 and close monitoring). In CKD stage 4 and 5 (clearance < 30 mL/min) is not allowed.
Liraglutide is cleaved in vivo by the enzyme DPP-4 that elicits two amino acids at the N terminus of the peptide. NEP also metabolizes liraglutide into several metabolites[33].
Of the administered drug (radioactive labeled) only 26.3% appears in the urine and feces, while breathing excretes 15%. Twenty point one percent of radioactivity is excreted in the urine mainly as water and only 6.3% in substances other than water.
Liraglutide is degraded entirely in the body and is not excreted in urine and feces. These characteristics indicate that we can use in all stages of CKD. Nevertheless we have not yet clinical studies in patients with eGFR < 60 mL/min[34] (there is ongoing studies with preliminary, not yet published, positive results of safety and effectiveness in patients with CKD stage ≥ 3).
The kidneys carry out one third of exogenous insulin degradation. It is filtered at the glomerulus and is absorbed by the proximal tubule. Sixty percent of the renal clearance is due to glomerular filtration and 40% in the secretion by uptake from peritubular vessels. Reduction in renal filtration is partially counterbalanced by secretion[35]. The dose of exogenous insulin is reduced 25% when eGFR is 10-50 mL/min and 50% when eGFR is < 10 mL/min[36].
The landscape is not clear enough in diabetes treatment in CKD. The risk of hypoglycaemia, which is higher in subjects with both diabetes and CKD, leads to selection of appropriate drugs with low risk of hypoglycaemia such as metformin (reduced dose) and DPP-4 inhibitors. When insulin treatment is appropriate, dose adjustment is usually required especially in CKD stages 4 and 5. Finally, many people with diabetes have a less strict target of glycaemia.
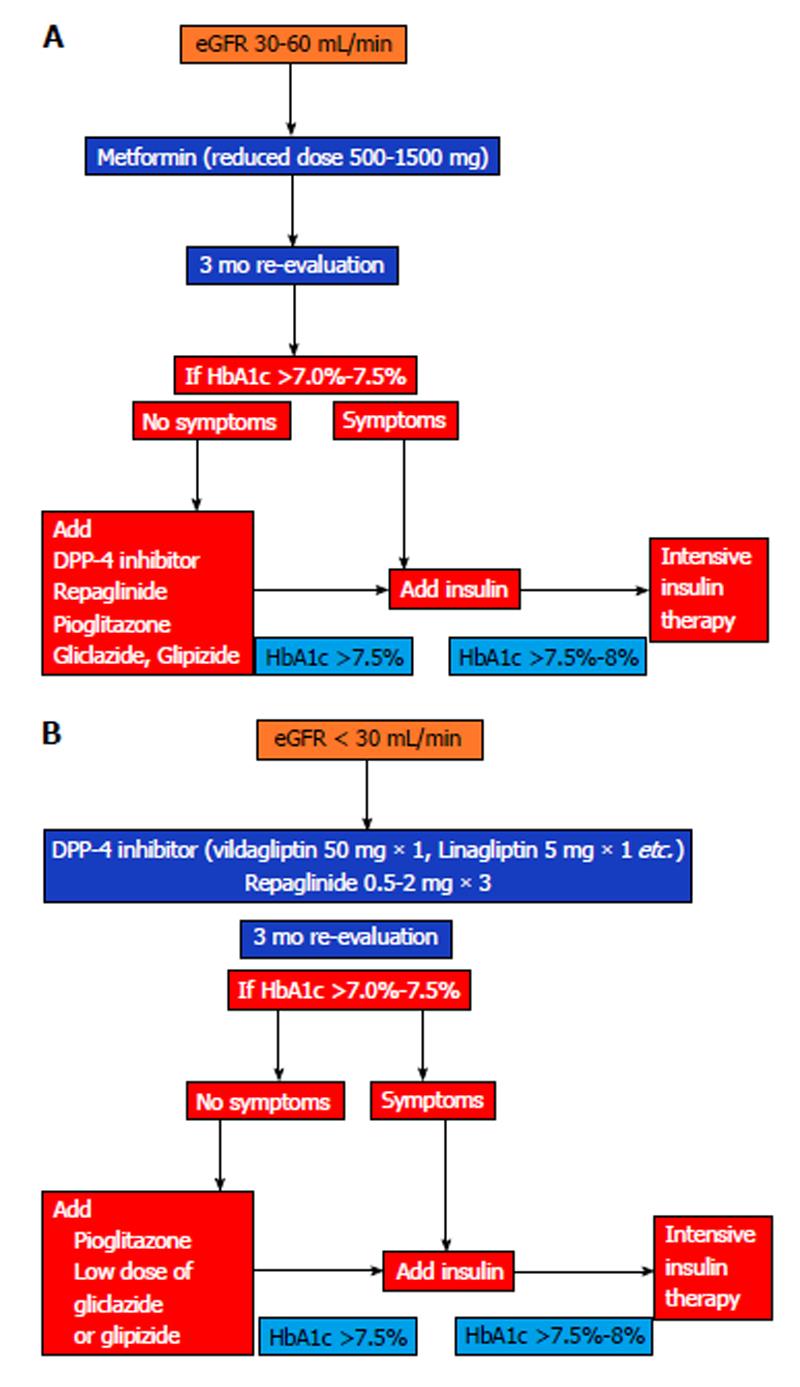
Based on all these data I propose the algorithms as shown in Figure 1.
P- Reviewer: Mitra A, Tamemoto H, Zhang Q S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 686] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 2. | Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W. ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1-420. [PubMed] [Cited in This Article: ] |
| 3. | National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 829] [Cited by in F6Publishing: 873] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 4. | Kerri L, Cavanaugh . Diabetes Management Issues for Patients With Chronic Kidney Disease. Clin Diabetes. 2007;25:90-97. [DOI] [Cited in This Article: ] |
| 5. | Lubowsky ND, Siegel R, Pittas AG. Management of glycemia in patients with diabetes mellitus and CKD. Am J Kidney Dis. 2007;50:865-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 389] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Schernthaner G, Ritz E, Schernthaner GH. Strict glycaemic control in diabetic patients with CKD or ESRD: beneficial or deadly? Nephrol Dial Transplant. 2010;25:2044-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17:365-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Haneda M, Morikawa A. Which hypoglycaemic agents to use in type 2 diabetic subjects with CKD and how? Nephrol Dial Transplant. 2009;24:338-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Zanchi A, Lehmann R, Philippe J. Antidiabetic drugs and kidney disease--recommendations of the Swiss Society for Endocrinology and Diabetology. Swiss Med Wkly. 2012;142:w13629. [PubMed] [Cited in This Article: ] |
| 11. | Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820-827. [PubMed] [Cited in This Article: ] |
| 12. | Nye HJ, Herrington WG. Metformin: the safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract. 2011;118:c380-c383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059-4067. [PubMed] [Cited in This Article: ] |
| 15. | Budde K, Neumayer HH, Fritsche L, Sulowicz W, Stompôr T, Eckland D. The pharmacokinetics of pioglitazone in patients with impaired renal function. Br J Clin Pharmacol. 2003;55:368-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Abe M, Kikuchi F, Okada K, Matsumoto K. Plasma concentration of pioglitazone in patients with type 2 diabetes on hemodialysis. Ther Apher Dial. 2009;13:238-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Jönsson A, Rydberg T, Sterner G, Melander A. Pharmacokinetics of glibenclamide and its metabolites in diabetic patients with impaired renal function. Eur J Clin Pharmacol. 1998;53:429-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Rosenkranz B, Profozic V, Metelko Z, Mrzljak V, Lange C, Malerczyk V. Pharmacokinetics and safety of glimepiride at clinically effective doses in diabetic patients with renal impairment. Diabetologia. 1996;39:1617-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Sarkar A, Tiwari A, Bhasin PS, Mitra M. Pharmacological and Pharmaceutical Profile of Gliclazide: A ReviewJournal of Applied Pharmaceutical Science 01 (09).. 2011;11-19. [Cited in This Article: ] |
| 20. | Marbury TC, Ruckle JL, Hatorp V, Andersen MP, Nielsen KK, Huang WC, Strange P. Pharmacokinetics of repaglinide in subjects with renal impairment. Clin Pharmacol Ther. 2000;67:7-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Weaver ML, Orwig BA, Rodriguez LC, Graham ED, Chin JA, Shapiro MJ, McLeod JF, Mangold JB. Pharmacokinetics and metabolism of nateglinide in humans. Drug Metab Dispos. 2001;29:415-421. [PubMed] [Cited in This Article: ] |
| 22. | Idris I, Donnelly R. Dipeptidyl peptidase-IV inhibitors: a major new class of oral antidiabetic drug. Diabetes Obes Metab. 2007;9:153-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab. 2010;12:648-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Chan JC, Scott R, Arjona Ferreira JC, Sheng D, Gonzalez E, Davies MJ, Stein PP, Kaufman KD, Amatruda JM, Williams-Herman D. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab. 2008;10:545-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Vincent SH, Reed JR, Bergman AJ, Elmore CS, Zhu B, Xu S, Ebel D, Larson P, Zeng W, Chen L. Metabolism and excretion of the dipeptidyl peptidase 4 inhibitor [14C]sitagliptin in humans. Drug Metab Dispos. 2007;35:533-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Bergman AJ, Cote J, Yi B, Marbury T, Swan SK, Smith W, Gottesdiener K, Wagner J, Herman GA. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007;30:1862-1864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Kothny W, Shao Q, Groop PH, Lukashevich V. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obes Metab. 2012;14:1032-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab. 2011;13:947-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Nowicki M, Rychlik I, Haller H, Warren M, Suchower L, Gause-Nilsson I, Schützer KM. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract. 2011;65:1230-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Christopher R, Covington P, Davenport M, Fleck P, Mekki QA, Wann ER, Karim A. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin Ther. 2008;30:513-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, Halabi A, Woerle HJ. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*). Diabetes Obes Metab. 2011;13:939-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Linnebjerg H, Kothare PA, Park S, Mace K, Reddy S, Mitchell M, Lins R. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64:317-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Malm-Erjefält M, Bjørnsdottir I, Vanggaard J, Helleberg H, Larsen U, Oosterhuis B, van Lier JJ, Zdravkovic M, Olsen AK. Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab Dispos. 2010;38:1944-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 2009;68:898-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 178] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Reilly JB, Berns JS. Selection and dosing of medications for management of diabetes in patients with advanced kidney disease. Semin Dial. 2010;23:163-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |