INTRODUCTION
Diabetes mellitus (DM) causes diabetic retinopathy (DR) and diabetic macular edema (DME) as a consequence of diabetic microangiopathy. Nowadays, DM might be considered as a pandemic disease with its incidence and prevalence increasing exponentially, even becoming epidemic. The prevalence of DR increases with the time of evolution of the systemic disease, with nearly 100% of patients showing some degree of diabetic retinopathy after 20 years of evolution. On the other hand, DME develops in 14%-25% of patients after 10 years of DM[1-3]. DR is a major cause of visual loss and a leading cause of blindness, whereas DME is the most common cause of visual loss in people under 50 years of age in developed countries[4,5].
Retinal neovascularization is a significant risk factor for severe visual loss in patients with DM, with optic disk new vessels as the maximum expression of such risk[6]. Laser photocoagulation has been the mainstay of treatment for DME and DR, but only 60% of patients with proliferative diabetic retinopathy (PDR) respond to panretinal photocoagulation, with regression of the neovascularization within 3 mo[2]. Laser photocoagulation mainly preserves vision rather than restoring it in cases of DME and PDR[7-9].
More recently, new therapeutic approaches have been developed for the management of both PDR and DME, including intravitreal injections of steroids (triamcinolone, sustained release intravitreal corticosteroid implant) or vascular endothelial factor inhibitors (VEGF), such as pegaptanib (Macugen; OSI pharmaceuticals, Melville, NY), bevacizumab (Avastin; Genentech, San Francisco, CA), ranibizumab (Lucentis; Genentech, San Francisco, CA) and aflibercept (Eylea, Regeneron Pharmaceutical, Inc, Tarrytown, NY), achieving vision improvement in a significant number of patients[10-12].
The incidence of DME and the progression rate of PDR are significantly lower in patients with spontaneous or surgical posterior vitreous detachment (PVD). It has been demonstrated that the adherence of the posterior hyaloid to the inner limiting membrane plays an important role in the development of DME and also in the growth of new vessels and its consequences, vitreous hemorrhage and tractional retinal detachment[13-17].
Enzymatic vitrectomy or pharmacological vitreolysis by intravitreal injection of autologous plasmin enzyme (APE) has been proposed as an effective neoadjuvant treatment for vitreous surgery by facilitating the surgical detachment of the posterior hyaloid and vitreoretinal membranes[18-20].
Plasmin, a serine protease, is active against laminin and fibronectin, located in the interface between the posterior vitreous cortex and the internal limiting membrane, and is responsible for the attachment of the vitreous to the retinal surface[21].
The aim of the present review was to analyze the clinical efficacy of the intravitreal injection of APE in the treatment of DR and DME and to determine the role of enzymatic vitrectomy as a therapeutic approach in such cases.
RESEARCH
The primary outcome was considered as the proportion of patients with a complete PVD following intravitreal injection of APE. The secondary outcomes were: number of patients with improvement in visual acuity and/or central macular thickness measured by optical coherence tomography (OCT); regression of new vessels in cases of PDR; and safety assessment of intravitreal injection of APE.
Before the injection of APE, all patients underwent a comprehensive ocular examination, including best corrected visual acuity (BCVA), slit-lamp examination, tonometry, indirect ophthalmoscopy, macular thickness measurement and assessment of posterior hyaloid status by OCT, color photographs and fluorescein angiography.
APE injection
A total volume of 0.2 mL of APE was obtained by a simplified method[21] and prepared 45 min prior to injection, sterilized through a 0.22 μm pore filter. Patients received unilateral intravitreal injections of APE through pars plana using a 30-gauge needle according to the routine procedure of intravitreal injections.
TREATMENT
A total of 63 eyes of 52 patients were included in the present study. Of them, 10 eyes showed PDR with optic disk new vessels and 53 eyes showed DME (33 refractory DME cases and 20 treatment-naïve patients).
Primary outcome: posterior vitreous detachment
A complete PVD appeared in 38% of cases (24 eyes) after one injection of plasmin and the total increased to 51% (32 eyes) after the second injection, separated at least by one month.
Secondary outcomes
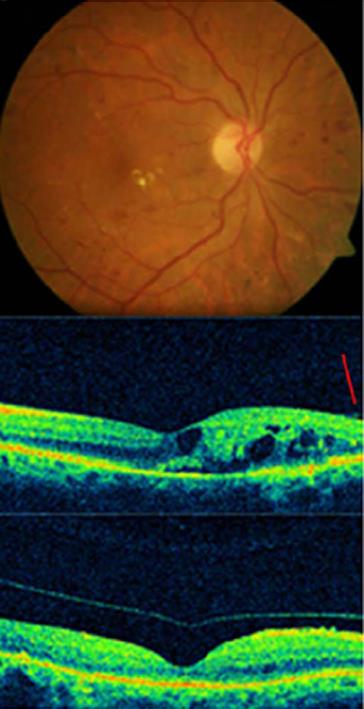
Central macular thickness decreased in all cases with DME, even in cases without PVD following intravitreal injections of APE. BCVA improved in 89% of the treated eyes (56) (Figures 1, 2).
Figure 1 Complete posterior vitreous detachment and complete resolution of macular edema in a diabetic patient treated with autologous plasmin.
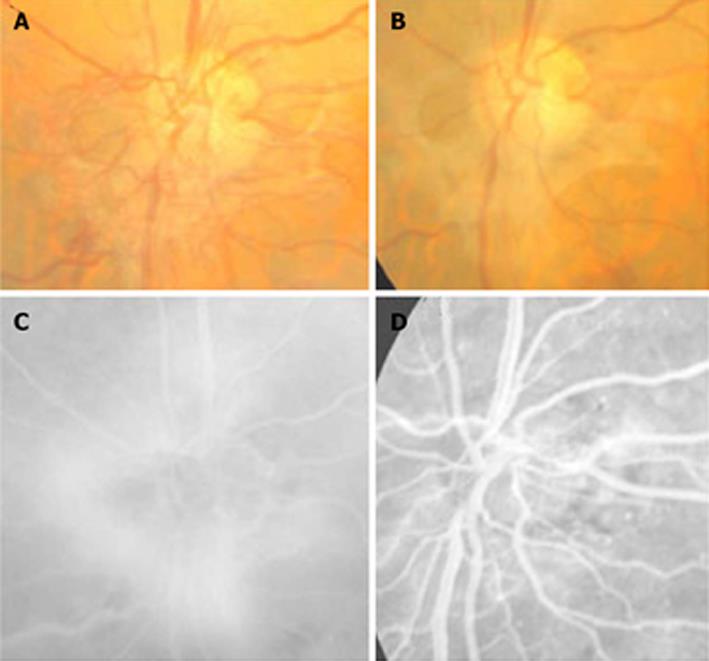
In cases with PDR, a highly significant regression in the new vessel area was observed in 5 cases (50%) (Figure 3); in 2 cases a partial regression was evidenced (20%), whereas the 3 remaining cases did not experience any change (30%).
Figure 3 Regression of the new vessels of the optic disc in a treated eye with plasmin.
A, B: Color images before and after treatment; C: Fluorescein angiogram shows the active vessels; D: The important but not complete resolution of leakage.
Safety profile
The treatment was well tolerated in all cases. No side effects were evidenced through the follow-up (uveitis, vitreous hemorrhage, cataract, ocular hypertension or retinal detachment).
DISCUSSION
The analyzed data suggested a possible role for enzymatic vitreolysis by intravitreal injection of APE for the management of PDR and DME. The efficacy of this procedure is related to the biochemical modification of the vitreous composition induced by the enzymatic separation or weakening of the union between the posterior vitreous cortex and the internal limiting membrane, leading to the development of PVD, and the liquefaction of the vitreous gel[22]. This procedure is dynamic with an important impact on the vitreous cavity. PVD not only has a protective role in a variety of retinal disorders, but also induces changes in the intraocular concentration of molecules, including VEGF, and increases intravitreal molecular diffusion coefficients and intravitreal oxygen levels[23-26]; this could also contribute to the good results obtained with this therapy.
APE employed in the present study was obtained by a simplified method and can be prepared in the operating room 45 min prior to the intravitreal injection. This constitutes a significant advantage compared to previously described techniques that required a longer and more expensive procedure. On the other hand, the method we propose has a wide variability and we cannot exactly recognize the concentration of APE that is injected and it depends on the plasminogen levels in each patient[27-30].
We achieved an overall efficacy of 50% in terms of inducing PVD in cases of DME and PDR, without any significant ocular side effects.
In conclusion, enzymatic vitrectomy performed by the intravitreal injection of APE might be effective and could be considered as an alternative for diabetic patients before performing other treatments, such as intravitreal injections of anti-VEGF or steroids, surgical vitrectomy or laser. Further studies are necessary to assess the efficacy and safety for the longer term and most convenient therapeutic strategy, including association of treatments, in diabetic patients to get better and more permanent results.