Published online Apr 15, 2013. doi: 10.4239/wjd.v4.i2.40
Revised: March 6, 2013
Accepted: March 15, 2013
Published online: April 15, 2013
Processing time: 131 Days and 6.5 Hours
AIM: To determine the parental transmission of diabetes mellitus (DM) and evaluate its influence on the clinical characteristics.
METHODS: This was a cross sectional study. The survey was carried out in urban and semi-urban primary health care centers. Of the 2400 registered with diagnosed diabetes, 1980 agreed and gave their consent to take part in this study, thus giving a response rate of 82.5%. Face to face interviews were conducted using a structured questionnaire followed by laboratory tests. DM was defined according to the World Health Organization expert group. A trained nurse performed physical examinations and measurements.
RESULTS: Of the study population, 72.9% reported a family history of DM. Family history of DM was significantly higher in females (54.2%; P = 0.04) and in the age group below 30 years (24%; P < 0.001). The prevalence of diabetes was higher among patients with a diabetic mother (25.4% vs 22.1%) and maternal aunts/uncles (31.2% vs 22.2%) compared to patients with a diabetic father and paternal aunts/uncles. Family history of DM was higher in patients of consanguineous parents (38.5%) than those of non-consanguineous parents (30.2%). The development of type 2 diabetes mellitus (T2DM) complications was higher in patients with either a paternal or maternal history of DM than in those without. No significant difference was observed in the metabolic characteristics of patients with/without family history of DM except for hypertension. Complications were higher in diabetic patients with a family history of DM.
CONCLUSION: The present study found a significant maternal effect in transmission of T2DM. Family history is associated with the increased incidence of diabetes.
Core tip: Diabetes is a disease that has a strong clustering in families and has a genetic component. Family history is a well-known risk factor for developing of type 2 diabetes mellitus (T2DM). The present study found a significant maternal effect in transmission of T2DM. Family history is associated with the increased incidence of diabetes.
- Citation: Bener A, Yousafzai MT, Al-Hamaq AO, Mohammad AG, DeFronzo RA. Parental transmission of type 2 diabetes mellitus in a highly endogamous population. World J Diabetes 2013; 4(2): 40-46
- URL: https://www.wjgnet.com/1948-9358/full/v4/i2/40.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i2.40
Diabetes is a multifactorial disease that involves complex interactions between genes, environment and health behavior. Type 2 diabetes mellitus (T2DM) is a common metabolic disorder, characterized by hyperglycemia caused by impaired glucose homeostasis, and represents a serious public health problem in many developed countries[1]. Current studies have revealed a definite global increase in the incidence and prevalence of diabetes. In 2000, 171 million people were estimated to be diabetic worldwide, which is projected to rise to 366 million cases in the year 2030[2]. It is the fourth or fifth leading cause of death in most developed countries[1]. Given the growing rate of diabetes and its far reaching societal and economic consequences, prevention of diabetes among people at high risk is a public health issue of clinical importance.
Diabetes is a disease that has a strong clustering in families and has a genetic component. It has been widely reported that the occurrence of T2DM is triggered by a genetic susceptibility and familial aggregation in several populations[3,4]. Family history is a well-known risk factor for the developing of T2DM. It was estimated that risk for diagnosed T2DM increases approximately two to four fold when one or both parents are affected[5]. Almost 25% to 33% of all T2DM patients have family members with diabetes. Having a first degree relative with the disease poses a 40% risk of developing diabetes[6]. T2DM patients are more likely to have diabetic mothers than diabetic fathers. The existence of excess maternal transmission of T2DM in offspring of affected mothers than affected fathers is currently debated[7]. Family history reflects both inherited genetic susceptibilities and shared environments which include cultural factors[8]. Thus, family history of diabetes may be a useful tool to identify individuals at increased risk of the disease and target behavior modifications that could potentially delay disease onset and improve health outcomes.
It was reported that several genetic disorders, congenital malformations and reproductive wastage are more frequent in consanguineous marriages[9]. A previous study by Bener et al[10] showed significant increase in the prevalence of common adult diseases in a population with a high rate of consanguinity. The incidence of consanguinity (51%) is relatively high in the State of Qatar with first cousin marriage predominantly comprising 26.7% of all marriages.
In Qatar, it was reported that diabetes is on the rise and if proper intervention and preventive strategies were not adopted, the epidemic of diabetes will prove fatal. The upcoming epidemic and projected increase in the prevalence of diabetes over the next two decades emphasize the importance of early detection[11] of diabetes in the population. Few studies have documented the prevalence of T2DM and its complications in the population of Qatar[12,13]. To the best of our knowledge, the patterns of familial transmission of T2DM in Qatar have not been studied so far. The significance of maternal or paternal inheritance in diabetes has been a matter of controversy and difference in various populations and races. Hence, this is the first cross-sectional survey of the Arab population in Qatar to determine the influence of familial history of T2DM in the offspring and evaluate its influence on the clinical characteristics of this disease.
This is a cross-sectional study which was conducted among diabetic patients registered in diabetic clinics of primary health care (PHC) centers of the Supreme Council of Health. The diabetes care is organized in most of the PHC centers. During the study period from January 2010 to January 2011, the study included T2DM patients registered in these diabetic clinics who were taking oral hypoglycemic drugs. In this study, multistage stratified cluster sampling was employed using the administrative divisions of the PHC in Qatar. Target population of each PHC is approximately equal. Stratification was done to obtain a representative sample of target population, with equal proportions from both urban and semi urban areas. The sample size was statistically calculated based on 17% prevalence rate of diabetes in Qatar[13], with 1% level of significance and assuming 2% bound on error of estimation, giving a minimum sample size of 2400 subjects for this study. Of the 2400 patients approached from different PHC centers (10 centers from an urban area and 2 centers from a semi-urban area), 1980 agreed to participate and gave verbal consent to take part in this study (82.5%). Also, any patients with incomplete laboratory values in the medical records were excluded from the study. The study was approved by the Hamad Medical Corporation prior to commencing data collection.
We developed a structured questionnaire consisting of questions relating to socio-demographic data, family history of diabetes mellitus (DM), lab investigations and complications. The first part included information about socio-demographic characteristics, including age, sex, marital status, education level, occupation, height, weight, blood pressure and parental consanguinity. The second section collected information about family history of DM with family relations and complications after the onset of diabetes. The third section included items about laboratory investigations such as blood glucose, glycated hemoglobin, high-density lipoprotein/low-density lipoprotein cholesterol levels, triglyceride, urea, creatinine, bilirubin, albumin etc. Necessary corrections and modifications were made in the questionnaire after the pilot study. Content validity, face validity and reliability of the questionnaire were tested using 50 subjects. These tests demonstrated a high level of validity and high degree of repeatability (kappa = 0.84)[14]. Family physicians and research nurses reviewed the medical files of diabetic patients in PHC and recorded all lab investigation measurements from their files.
A trained nurse performed physical examinations and measurements. In order to measure height (m), participants were asked to stand in bare feet while maintaining a straight posture on a height scale (SECA, Germany). Similarly, weight (kg) was measured using the same scale with light clothing and bare feet. Body mass index was calculated as the ratio of weight (kg) to the square of height (m).
Hypertension was defined as per World Health Organization (WHO) standardized criteria, “systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg or using anti-hypertensive medication”[15]. In order to measure blood pressure, subjects were asked to sit and rest for at least 10-15 min. Two readings of SBP and DBP were taken from his/her left arm at heart level while using standard zero mercury sphygmomanometer. An average of both readings for SBP and DBP was obtained.
Study participants with a history of T2DM and currently taking oral anti-diabetic medications were considered to have DM. DM was defined as per the WHO expert group[16], i.e., fasting venous blood glucose (FBS) concentration ≥ 7.0 mmol/L and/or 2 h post-oral glucose tolerance test (OGTT) venous blood glucose concentration ≥ 11.1 mmol/L. FBS was measured by glucose meter among all the participants and those with FBS < 7 mmol/L were further tested by an OGTT. In order to conduct OGTT, participants were asked to drink 75 g anhydrous glucose dissolved in 250 mL water within the space of 5 min. Samples were processed within 30 min of collection and the above laboratory tests were measured. Subjects with impaired FBS (venous blood glucose concentration for 5.6-6.9 mmol/L) or impaired OGTT (2 h post-OGTT venous blood glucose level of 7.8-11.0 mmol/L) were labeled as pre-diabetes. Glycosylated hemoglobin was analyzed using a high-performance liquid chromatography method with a range > 6.5% defined as “unsatisfactory” metabolic control.
Data were analyzed using the Statistical Package for Social Sciences (SPSS, version 19) software. Standard descriptive statistical analysis was performed. Student t test was used to ascertain the significance of differences between mean values of two continuous variables and one way analysis of variance was used to find the differences between continuous variables among more than two groups. Differences between categorical variables were tested through Pearson χ2 or Fisher’s exact test when the assumptions for χ2 test were not fulfilled. Two sided P value of less than 5% was considered as significant.
Table 1 shows the socio-demographic characteristics of patients with/without a family history of DM. Family history of DM was significantly higher in female patients (54.2%; P = 0.041), Qatari nationals (58%; P = 0.020) and in the age group below 30 years (24%; P < 0.001). Consanguinity was significantly higher in diabetic patients with family history of DM (38.5% vs 30.2%; P = 0.001) compared to those without family history of DM.
| With family history of DM1(n = 1444) | Without family history of DM(n = 536) | P value | |
| Age (yr) | < 0.001 | ||
| 18-30 | 346 (24.0) | 72 (13.4) | |
| 30-39 | 280 (19.4) | 66 (12.3) | |
| 40-49 | 258 (17.9) | 132 (24.6) | |
| 50-59 | 400 (27.7) | 194 (36.2) | |
| ≥ 60 | 160 (11.1) | 72 (13.4) | |
| Gender | 0.041 | ||
| Male | 661 (45.8) | 273 (50.9) | |
| Female | 783 (54.2) | 263 (49.1) | |
| Nationality | 0.020 | ||
| Qatari | 838 (58.0) | 342 (63.8) | |
| Other Arabs | 606 (42.0) | 194 (36.2) | |
| Educational level | 0.154 | ||
| Illiterate | 240 (16.6) | 71 (13.2) | |
| Elementary | 267 (18.5) | 114 (21.3) | |
| Intermediate | 305 (21.1) | 99 (18.5) | |
| Secondary | 381 (26.4) | 152 (28.4) | |
| University | 251 (17.4) | 100 (18.7) | |
| Occupation | 0.134 | ||
| Housewife | 370 (25.6) | 128 (23.9) | |
| Sedentary | 356 (24.7) | 162 (30.2) | |
| Professional | 249 (17.2) | 97 (18.1) | |
| Manual | 153 (10.6) | 53 (9.9) | |
| Businessmen | 127 (8.8) | 39 (7.3) | |
| Army/police clerk | 189 (13.1) | 57 (10.6) | |
| Monthly household income (QR) | 0.589 | ||
| < 5000 | 93 (6.4) | 39 (7.3) | |
| 5000-10 000 | 470 (32.5) | 175 (32.6) | |
| 10 000-15 000 | 510 (35.3) | 200 (37.3) | |
| > 15 000 | 371 (25.8) | 122 (22.8) | |
| Consanguinity | 0.001 | ||
| Yes | 556 (38.5) | 162 (30.2) | |
| No | 888 (61.5) | 374 (69.8) | |
Table 2 reveals the familial history of diabetes mellitus among diabetic patients. Of the total study population, 72.9% reported a family history of DM. The prevalence of DM in father, mother, brother and sister was 22.1%, 25.4%, 14.2% and 9.3% respectively. In 2nd degree relatives for uncles and aunts, a positive history of T2DM was more common among maternal aunts/uncles than in paternal aunts/uncles (31.2% vs 22.2%). On the maternal side, 83.7% of the diabetic patients have an affected mother (25.4%) and at least one relative (58.3%), compared to only 67.3% of diabetic patients with an affected father (22.1%) and one family member (45.2%) on the paternal side.
| n = 1980 | |
| Family history of diabetes1 | |
| Negative | 536 (27.1) |
| Positive | 1444 (72.9) |
| Family relations | |
| Father | 437 (22.1) |
| Mother | 503 (25.4) |
| Brother | 281 (14.2) |
| Sister | 184 (9.3) |
| Paternal uncle | 244 (12.3) |
| Paternal aunt | 195 (9.8) |
| Maternal uncle | 325 (16.4) |
| Maternal aunt | 293 (14.8) |
| Paternal grand father | 235 (11.9) |
| Paternal grand mother | 221 (11.2) |
| Maternal grand father | 264 (13.3) |
| Maternal grand mother | 272 (13.7) |
Table 3 gives physical, metabolic characteristics and complications among diabetic patients according to the family history of DM. No significant difference was found in the metabolic characteristics of diabetic patients according to the family history of DM except for the SBP (P = 0.033) and DBP (P = 0.025). The development of T2DM complications was higher in patients with either a paternal or maternal history of DM than in those without; significantly higher for sleep loss (13.9% vs 6.7%, 12.2% vs 6.7%; P < 0.001), hypertension (29.6% vs 20.9%, 22% vs 20.9%; P = 0.001), retinopathy (17.6% vs 11.4%, 13.3% vs 11.4%; P = 0.006) and antipathy (8% vs 4.7%, 7.7% vs 4.7%; P = 0.047).
| Parameters | Family history1 | No family history of DM | P value | |
| Paternal history (n = 635) | Maternal history (n = 809) | (n = 536) | ||
| Age (yr) | 44.9 ± 14 | 45.1 ± 15 | 46.7 ± 13.3 | 0.073 |
| Duration of diagnosis (yr) | 6.9 ± 4.2 | 7.3 ± 4.3 | 6.4 ± 3.3 | 0.001 |
| BMI (kg/m2) | 27.4 ± 4.9 | 26.8 ± 4.8 | 27.7 ± 4.9 | 0.033 |
| Metabolic characteristics | ||||
| Systolic blood pressure (mmHg) | 129.9 ± 19.5 | 129.1 ± 18.1 | 127.1 ± 14.7 | 0.033 |
| Diastolic blood pressure (mmHg) | 81.3 ± 11 | 80.4 ± 10.5 | 79.4 ± 8.5 | 0.025 |
| Fasting glucose (mmol/L) | 9.9 ± 8.0 | 9.1 ± 5.0 | 9.1 ± 4.7 | 0.092 |
| HbA1c | 8.2 ± 2.2 | 7.9 ± 2.1 | 8.1 ± 2.2 | 0.188 |
| Serum urea level | 5.9 ± 1.5 | 6.3 ± 2.3 | 5.8 ± 1.7 | 0.135 |
| Serum creatinine (mmol/L) | 77.2 ± 9.5 | 78.1 ± 9.8 | 73.8 ± 8.6 | 0.371 |
| Total cholesterol (mmol/L) | 5.0 ± 1.1 | 4.8 ± 1.1 | 4.9 ± 1.2 | 0.095 |
| Serum alkaline phosphate | 94.6 ± 11.3 | 101.7 ± 13.8 | 100 ± 12.4 | 0.402 |
| T2DM complications, n (%) | ||||
| Sleep loss | 88 (13.9) | 99 (12.2) | 36 (6.7) | < 0.001 |
| Hypertension | 167 (29.6) | 156 (22.0) | 111 (20.9) | 0.001 |
| Neuropathy | 60 (9.4) | 82 (10.1) | 44 (8.2) | 0.494 |
| Retinopathy | 112 (17.6) | 108 (13.3) | 61 (11.4) | 0.006 |
| Nephropathy | 93 (14.6) | 105 (13.0) | 58 (10.8) | 0.151 |
| Antipathy | 51 (8.0) | 62 (7.7) | 25 (4.7) | 0.047 |
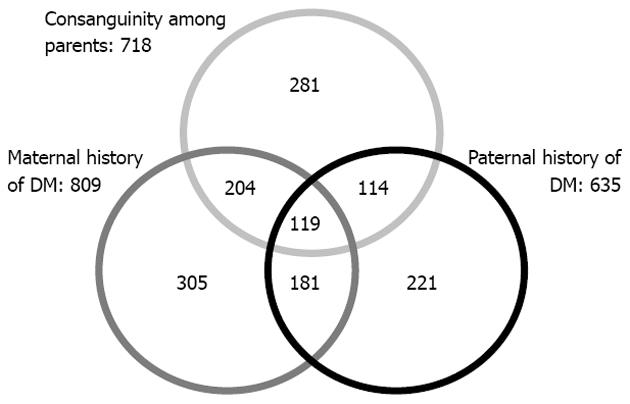
Table 4 shows physical and clinical characteristics and complications of T2DM according to family history of DM, while controlling for consanguinity. Out of 718, 556 (77.4%) of the diabetic patients of consanguineous parents had either a paternal (233/718; 32.5%) or maternal history (323/718; 45%) of DM; whereas family history of DM was lower in patients of non-consanguineous parents (888/1262; 70.4%). No significant difference was found in metabolic characteristics of patients according to the presence of DM in parents and relatives except for the SBP and DBP, found to be significantly higher among patients with maternal history of DM in the consanguineous group (P = 0.018, P = 0.007, respectively). In addition, hypertension, retinopathy and antipathy were significantly higher among patients with a paternal history of DM in the consanguineous group (P = 0.002, P = 0.007, P = 0.003, respectively). No significant difference was found in T2DM complications of patients according to paternal/paternal history of DM in the non-consanguineous group.
| Parameters | Consanguineous (n = 718) | Non-consanguineous (n = 1262) | ||||||
| Paternal history of DM | Maternal history of DM | Without familial history | P value | Paternal history of DM | Maternal history of DM | Without familial history | P value | |
| (n = 233) | (n = 323) | (n = 162) | (n = 402) | (n = 486) | (n = 374) | |||
| Age (yr) | 43.9 ± 14.7 | 45.1 ± 15.4 | 47.3 ± 13.4 | 0.058 | 45.5 ± 13.6 | 45.1 ± 14.8 | 46.3 ± 13.3 | 0.532 |
| BMI (kg/m2) | 27.4 ± 4.8 | 26.6 ± 4.9 | 28.1 ± 4.9 | 0.028 | 27.4 ± 5.0 | 26.9 ± 4.8 | 27.6 ± 4.8 | 0.428 |
| Duration of DM (yr) | 6.6 ± 3.9 | 7.2 ± 4.4 | 5.9 ± 2.9 | 0.004 | 7.2 ± 4.3 | 7.4 ± 4.2 | 6.7 ± 3.5 | 0.065 |
| Metabolic characteristics | ||||||||
| Systolic BP (mmHg) | 129.6 ± 20.4 | 130.7 ± 17.9 | 125.9 ± 11.2 | 0.018 | 130.1 ± 19.1 | 128.2 ± 18.1 | 127.8 ± 16.5 | 0.224 |
| Diastolic BP (mmHg) | 81.3 ± 11.4 | 81.1 ± 10.7 | 78.2 ± 8.0 | 0.007 | 81.2 ± 11.4 | 79.9 ± 10.4 | 80.0 ± 9.1 | 0.220 |
| Fasting glucose (mmol/L) | 10.3 ± 8.9 | 8.9 ± 5.0 | 8.9 ± 5.4 | 0.071 | 9.7 ± 9.6 | 9.3 ± 5.0 | 9.3 ± 4.3 | 0.689 |
| HbA1c (%) | 7.8 ± 2.1 | 7.7 ± 2.1 | 7.8 ± 2.3 | 0.900 | 8.3 ± 2.4 | 7.9 ± 2.2 | 8.3 ± 2.1 | 0.121 |
| Serum urea level | 5.8 ± 3.0 | 6.2 ± 3.2 | 5.9 ± 4.4 | 0.622 | 5.9 ± 2.7 | 6.3 ± 3.1 | 5.7 ± 2.8 | 0.170 |
| Creatinine (mmol/L) | 71.9 ± 8.5 | 78.0 ± 9.8 | 72.4 ± 9.4 | 0.313 | 75.5 ± 9.9 | 76.2 ± 10.2 | 70.9 ± 8.6 | 0.372 |
| Tot. cholesterol (mmol/L) | 4.9 ± 1.1 | 4.8 ± 1.1 | 4.9 ± 1.2 | 0.626 | 5.0 ± 1.2 | 4.8 ± 1.1 | 4.9 ± 1.3 | 0.173 |
| Serum alkaline phosphate | 92.2 ± 11.1 | 99.3 ± 12.8 | 93.5 ± 12.2 | 0.683 | 95.9 ± 12.5 | 102.9 ± 13.2 | 104.6 ± 13.5 | 0.460 |
| T2DM complications, n (%) | ||||||||
| Sleep loss | 44 (18.9) | 40 (12.4) | 12 (7.4) | 0.003 | 44 (10.9) | 59 (12.1) | 24 (6.4) | 0.017 |
| Hypertension | 67 (32.5) | 59 (20.6) | 31 (19.1) | 0.002 | 100 (27.9) | 97 (22.9) | 80 (21.7) | 0.114 |
| Neuropathy | 19 (8.2) | 34 (10.5) | 12 (7.4) | 0.446 | 41 (10.2) | 48 (9.9) | 32 (8.6) | 0.712 |
| Retinopathy | 44 (18.9) | 41 (12.7) | 13 (8.0) | 0.007 | 68 (16.9) | 67 (13.8) | 48 (12.8) | 0.231 |
| Nephropathy | 29 (12.4) | 40 (12.4) | 13 (8.0) | 0.303 | 64 (15.9) | 65 (13.4) | 45 (12) | 0.276 |
| Antipathy | 21 (9.0) | 21 (6.5) | 4 (2.5) | 0.003 | 30 (7.5) | 41 (8.4) | 21 (5.6) | 0.284 |
| Hypoglycemia | 62 (26.6) | 91 (28.2) | 46 (28.4) | 0.899 | 111 (27.6) | 113 (23.3) | 99 (26.5) | 0.299 |
| Impotence | 15 (6.4) | 17 (5.3) | 3 (1.9) | 0.104 | 33 (8.2) | 38 (7.8) | 19 (5.1) | 0.180 |
Figure 1 shows the association between consanguinity and family history of DM in an Arab diabetic population in Qatar. The Venn diagram clearly shows the overlapping of parental consanguinity with a paternal and maternal history of diabetes mellitus.
In the State of Qatar, as a result of changing lifestyle due to rapid urbanization, the prevalence of T2DM is increasing, as is observed worldwide. However, the role of genetic and environmental factors remains unclear. This is the first study to provide insight in the familial aggregation and transmission patterns of T2DM among an Arab population residing in Qatar. The study sample revealed that 72.9% of the subjects with DM had a positive family history of diabetes among at least one of their parents, siblings, uncles, aunts and grandparents. The degree of familial aggregation of diabetes among Tunisians[17] found that 70% of the diabetic patients had a positive family history of diabetes among at least one of their relatives from both sides, which is nearly identical to our study. A lower rate was observed in a French study[3] in which 66% of the diabetic patients had at least one relative with diabetes among their first and second degree relatives. Similar higher frequencies have also been reported among South Indians[18] (53.9%) and Pakistanis (70%)[19]. On the other hand, lower frequencies of positive family history have been reported by other studies in Asians[4] (36%), Europeans[20] (33%) and black South Africans (27%)[3]. In the study sample of 1980 diabetic patients, 71% reported at least one first degree familial member, which is similar to the study results of Crispim et al[21] (76.6%). These results support the strong familial aggregation of diabetes among an Arab population with a high prevalence among 1st degree relatives. Also, these study findings have proven that people with a family history of diabetes consider themselves to be at greater risk of developing diabetes in their offspring. These results are in agreement with a study by Hariri et al[22] that a family history of diabetes in a first-degree relative doubles a person’s risk of developing diabetes.
Another important study finding was that the investigation of parental transmission patterns of T2DM showed an excess of maternal transmission of T2DM as mothers were implicated more frequently than fathers[23]. In the study sample, 25.4% of the mothers of the diabetic patients were diabetic compared to 22.1% of the fathers. Consistent with our results, a higher frequency of positive family history among mothers than fathers was reported in studies conducted in Brazil[21] (48.4% vs 21.3%), Britain[23] (36% vs 15%), France[24] (33% vs 17%), Greece[25] (27.7% vs 11%) and Tunisia[17] (21% vs 10%).
The present study extends the scope of genetic influence on DM by including parents, siblings, uncles, aunts and grandparents in the familial history. It was observed that 83.7% of the diabetic patients have an affected mother and at least one relative on the maternal side, compared to only 67.3% of diabetic patients with an affected father and family member on the paternal side, suggesting a maternal transmission of T2DM in the Arab population. The excess maternal transmission of T2DM reported in this study is in line with studies from different populations with varying frequencies[3,4,18,21,25]. A positive family history of T2DM was more common among maternal aunts/uncles (31.2%) than in paternal aunts/uncles (22.2%), showing that this maternal effect likely extends to the previous generation in 2nd degree relatives, as reported in another study[21]. These study results support the existence of excess of maternal transmission of T2DM in their population. On the contrary, in the Framingham population study[26], maternal and paternal diabetes conferred equivalent risk for occurrence of T2DM in offspring. In contrast to these findings, McCarthy et al[27] found no difference in parental transmission of T2DM in a population with high prevalence of diabetes. Longer average life span in women could increase the likelihood that mothers develop T2DM. Fathers may have more undetected diabetes because of reduced screening rates and health care utilization or may develop diabetes at an older age than mothers.
In our study, there was an early onset of diabetes among patients with a family history of diabetes in the age group 18-30 years (24%) compared to other patients whose parents were non-diabetic (13.4%), which is similar to the results found in Greek diabetic patients[25]; this study reported that the presence of a family history of diabetes results in an early onset of the disease in the offspring. Younger age of the onset of diabetes had been noted, which implies that these subjects develop diabetes in the most productive years of their life and have a greater chances of developing complications[28]. Crispim et al[21] reported in their study that when the disease is diagnosed at an early age, the genetic component is more important to its development.
In the study sample, a positive family history of DM was more common among diabetic patients of consanguineous parents (77.4%) with high prevalence of maternal history (45%), whereas it was lower in patients of non-consanguineous parents (70.4%). It was reported in a recent study by Bener et al[10] that there was a significant increase in the prevalence of diabetes mellitus in consanguineous couples in Qatar. The current data showed that consanguinity increased the family history of DM in patients. This means that consanguinity is an important factor in the causation of diabetes mellitus in offspring.
The influence of various transmission patterns of T2DM on metabolic factors and diabetic complications have been examined. Results showed no significant difference in clinical parameters between patients with a parental or maternal history of diabetes in the study sample except for hypertension, which is similar to the study results by Bo et al[29]. A study in Tunisia[17] showed no significant difference in clinical parameters between patients with paternal or maternal history of diabetes in the studied sample. The development of sleep loss, hypertension, retinopathy and antipathy were significantly higher in the studied patients with a family history of DM than those without. Jali et al[28] found retinopathy and neuropathy less in patients with a family history of DM and risk was same in both the groups with respect to nephropathy.
Harrison et al[5] documented that family history information may serve as a useful tool for public health because it reflects both genetic and environmental factors. Examining family history of DM may be a valuable approach for identifying patients at risk for diabetes. In addition, this survey provides some indication that knowledge of family history of diabetes may lead to identifying people at increased risk of diabetes and perhaps motivate them to make preventive life style changes that could favorably affect both clinical practice and patient behavior.
In conclusion, the study findings showed an excess of maternal transmission of T2DM in a sample of an Arab diabetic population residing in Qatar. The data support the dominant maternal role in the development of diabetes mellitus in their offspring. No significant difference was observed between maternal and paternal diabetes in metabolic characteristics except for hypertension. Complications were higher in diabetic patients with a family history of DM. Family history of DM was higher in patients of consanguineous parents compared to non-consanguineous parents. The presence of a family history of diabetes resulted in an early onset of the disease of the offspring. Interventions to change life style habits among families might reduce the risk of diabetes in the offspring of diabetic patients.
The authors would like to thank the Hamad Medical Corporation for their support and ethical approval (ref: RP 10067/10).
Diabetes is a disease that has a strong clustering in families and has a genetic component. Family history is a well-known risk factor for developing type 2 diabetes mellitus (T2DM). The high incidence of consanguineous marriages in the State of Qatar highlighted the importance of determining the influence of familial history of T2DM in the offspring.
The study indicated that knowledge of family history of diabetes may lead to identifying people at increased risk of diabetes. The study highlighted the importance of identifying this high risk group and make preventive life style changes which might reduce the risk of diabetes in offspring.
The important study findings of this article are compared to studies conducted regionally and internationally which make the readers understand the high prevalence of diabetes mellitus in a consanguineous population.
This will encourage the researchers in this region to explore the paternal transmission of T2DM in their community and conduct intervention studies to change life style habits among families.
The authors recommended through this study that family history information may serve as a useful tool for public health because it reflects both genetic and environmental factors. Physicians should consider the family history of diabetes mellitus to identify the onset of DM in their offspring.
P- Reviewers Kumar KVS, Tayek J S- Editor Gou SX L- Editor Roemmele A E- Editor Li JY
| 1. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [PubMed] |
| 2. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 8947] [Article Influence: 426.0] [Reference Citation Analysis (1)] |
| 3. | Erasmus RT, Blanco Blanco E, Okesina AB, Mesa Arana J, Gqweta Z, Matsha T. Importance of family history in type 2 black South African diabetic patients. Postgrad Med J. 2001;77:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Lee SC, Pu YB, Chow CC, Yeung VT, Ko GT, So WY, Li JK, Chan WB, Ma RC, Critchley JA. Diabetes in Hong Kong Chinese: evidence for familial clustering and parental effects. Diabetes Care. 2000;23:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Harrison TA, Hindorff LA, Kim H, Wines RC, Bowen DJ, McGrath BB, Edwards KL. Family history of diabetes as a potential public health tool. Am J Prev Med. 2003;24:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | ADAM. Diabetes: Type 2. 2004 [cited 18/08/2011]. 2004; Available from: http: //adam.about.net/reports/000060_2.htm. |
| 7. | Groop LC, Tuomi T. Non-insulin-dependent diabetes mellitus--a collision between thrifty genes and an affluent society. Ann Med. 1997;29:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Keku TO, Millikan RC, Martin C, Rahkra-Burris TK, Sandler RS. Family history of colon cancer: what does it mean and how is it useful? Am J Prev Med. 2003;24:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Bittles AH, Mason WM, Greene J, Rao NA. Reproductive behavior and health in consanguineous marriages. Science. 1991;252:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 221] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Bener A, Hussain R, Teebi AS. Consanguineous marriages and their effects on common adult diseases: studies from an endogamous population. Med Princ Pract. 2007;16:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14 Suppl 5:S1-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Bener A, Zirie M, Al-Rikabi A. Genetics, obesity, and environmental risk factors associated with type 2 diabetes. Croat Med J. 2005;46:302-307. [PubMed] |
| 13. | Bener A, Zirie M, Janahi IM, Al-Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract. 2009;84:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological measurement. 1960;20:37-46. [DOI] [Full Text] |
| 15. | World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications, Report of a WHO Consultation 1, WHOINCDINCS/99.2. Geneva. 1999;. |
| 16. | World Health Organization. The prevention of diabetes and its complications. WHO report 2006 (accessed on 7/8/2010). 2006; Available from: http: //www.who.int/diabetes/preventionflyer/en/. |
| 17. | Arfa I, Abid A, Malouche D, Ben Alaya N, Azegue TR, Mannai I, Zorgati MM, Ben Rayana MC, Ben Ammar S, Blousa-Chabchoub S. Familial aggregation and excess maternal transmission of type 2 diabetes in Tunisia. Postgrad Med J. 2007;83:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Viswanathan M, McCarthy MI, Snehalatha C, Hitman GA, Ramachandran A. Familial aggregation of type 2 (non-insulin-dependent) diabetes mellitus in south India; absence of excess maternal transmission. Diabet Med. 1996;13:232-237. [PubMed] |
| 19. | Shera AS, Rafique G, Khawaja IA, Baqai S, King H. Pakistan National Diabetes Survey: prevalence of glucose intolerance and associated factors in Baluchistan province. Diabetes Res Clin Pract. 1999;44:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Cheţa D, Dumitrescu C, Georgescu M, Cocioabă G, Lichiardopol R, Stamoran M, Ionescu-Tîrgovişte C, Păunescu-Georgescu M, Mincu I. A study on the types of diabetes mellitus in first degree relatives of diabetic patients. Diabete Metab. 1990;16:11-15. [PubMed] |
| 21. | Crispim D, Canani LH, Gross JL, Tschiedel B, Souto KE, Roisenberg I. Familial history of type 2 diabetes in patients from Southern Brazil and its influence on the clinical characteristics of this disease. Arq Bras Endocrinol Metabol. 2006;50:862-868. [PubMed] |
| 22. | Hariri S, Yoon PW, Qureshi N, Valdez R, Scheuner MT, Khoury MJ. Family history of type 2 diabetes: a population-based screening tool for prevention? Genet Med. 2006;8:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Alcolado JC, Alcolado R. Importance of maternal history of non-insulin dependent diabetic patients. BMJ. 1991;302:1178-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Thomas F, Balkau B, Vauzelle-Kervroedan F, Papoz L. Maternal effect and familial aggregation in NIDDM. The CODIAB Study. CODIAB-INSERM-ZENECA Study Group. Diabetes. 1994;43:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Papazafiropoulou A, Sotiropoulos A, Skliros E, Kardara M, Kokolaki A, Apostolou O, Pappas S. Familial history of diabetes and clinical characteristics in Greek subjects with type 2 diabetes. BMC Endocr Disord. 2009;9:12. [PubMed] |
| 26. | Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 422] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 27. | McCarthy M, Cassell P, Tran T, Mathias L, ‘t Hart LM, Maassen JA, Snehalatha C, Ramachandran A, Viswanathan M, Hitman GA. Evaluation of the importance of maternal history of diabetes and of mitochondrial variation in the development of NIDDM. Diabet Med. 1996;13:420-428. [PubMed] |
| 28. | Jali MV, Kambar S, Jali SM, Gowda S. Familial early onset of type-2 diabetes mellitus and its complications. N Am J Med Sci. 2009;1:377-380. [PubMed] |
| 29. | Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Influence of a familial history of diabetes on the clinical characteristics of patients with Type 2 diabetes mellitus. Diabet Med. 2000;17:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |