Copyright
©The Author(s) 2025.
World J Diabetes. Jun 15, 2025; 16(6): 102727
Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.102727
Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.102727
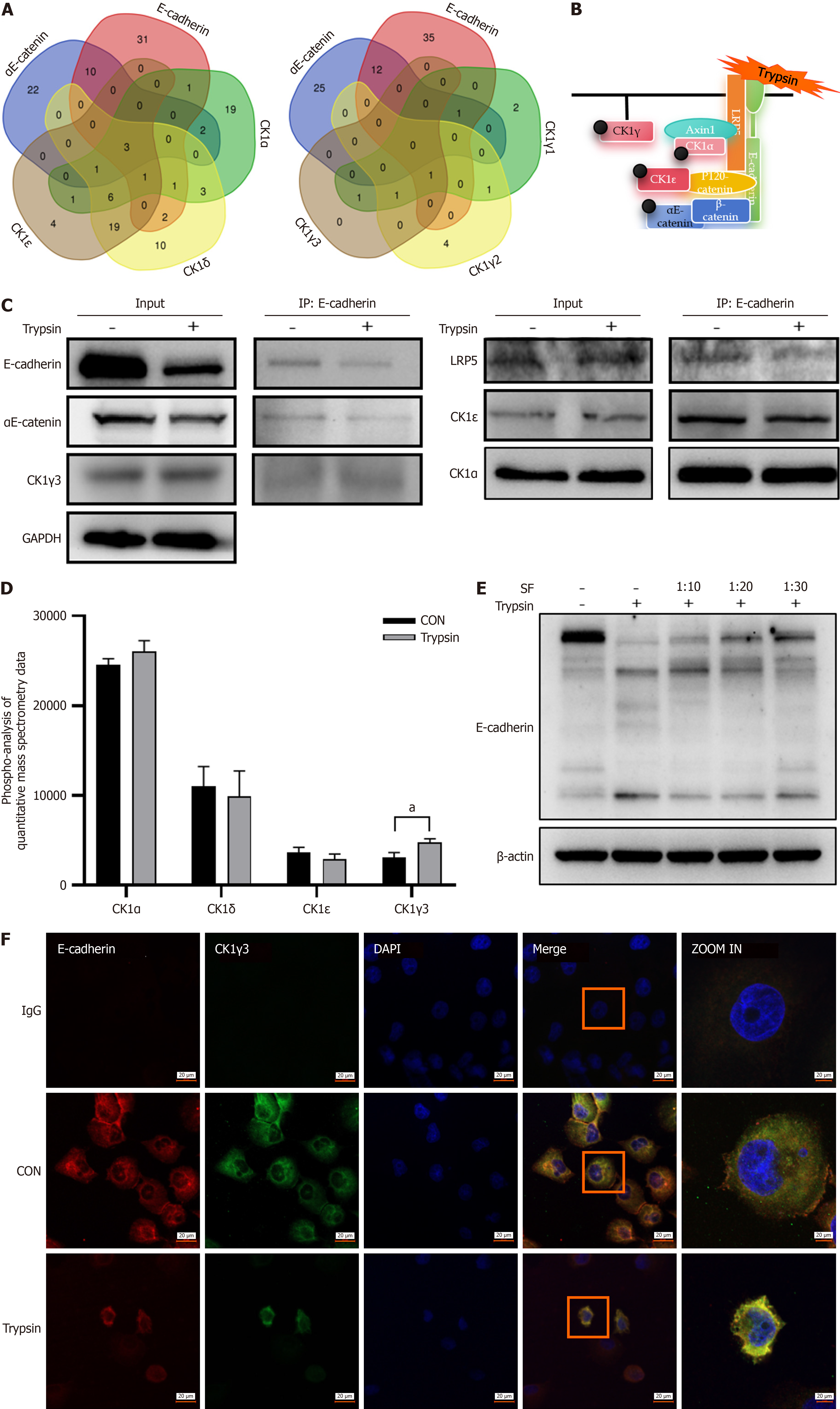
Figure 5 Trypsin promotes binding of casein kinase-1γ3 to the E-cadherin complex.
A: Wayne diagram, showing intersection of E-cadherin, αE-catenin, and casein kinase-1 (CK1) subtypes interacting proteins; B: Hypothetical model of the interaction among CK1, E-cadherin, and αE-catenin; C: Immunoprecipitation (IP) reflecting the protein composition of the E-cadherin complex; D: Quantitative mass spectrometric data analysis of phosphorylated protein, control group and trypsin representing the samples before and after trypsin treatment, and the ordinate reflects the level of phosphorylation; E: Western blot images reflect the hydrolysis of E-cadherin by trypsin and the blocking effect of different concentrations of trypsin inhibitor soybean flour (SF); F: Representative confocal images show colocalization changes of E-cadherin and CK1γ3 before and after trypsin treatment, Cy3 labeling E-cadherin (red), fluorescein isothiocyanate isomer labeling CK1γ3 (green), 4’,6-diamidino-2-phenylindole (DAPI) labeling nucleus (blue), magnification 20 ×, increased to 63 ×. aP < 0.05. P calculated vs control (CON). GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
- Citation: Gao L, Lai JS, Chen H, Qian LX, Hong WJ, Li LC. Mechanism of trypsin-mediated differentiation of pancreatic progenitor cells into functional islet-like clusters. World J Diabetes 2025; 16(6): 102727
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/102727.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.102727