Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.392
Peer-review started: November 18, 2023
First decision: December 8, 2023
Revised: December 16, 2023
Accepted: January 25, 2024
Article in press: January 25, 2024
Published online: March 15, 2024
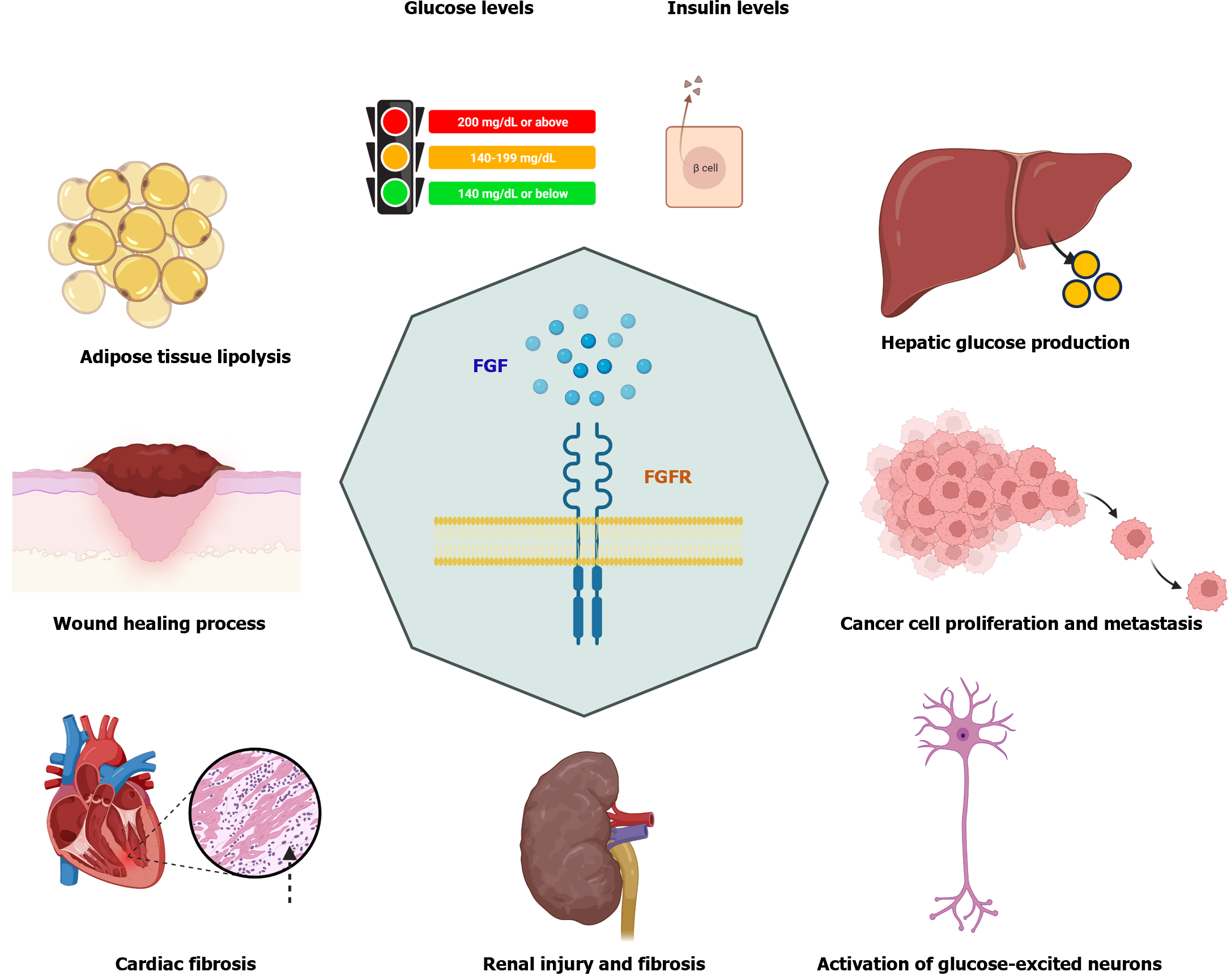
Diabetes affects about 422 million people worldwide, causing 1.5 million deaths each year. However, the incidence of diabetes is increasing, including several types of diabetes. Type 1 diabetes (5%-10% of diabetic cases) and type 2 diabetes (90%-95% of diabetic cases) are the main types of diabetes in the clinic. Accumulating evidence shows that the fibroblast growth factor (FGF) family plays important roles in many metabolic disorders, including type 1 and type 2 diabetes. FGF consists of 23 family members (FGF-1-23) in humans. Here, we review current findings of FGFs in the treatment of diabetes and management of diabetic complications. Some FGFs (e.g., FGF-15, FGF-19, and FGF-21) have been broadly investigated in preclinical studies for the diagnosis and treatment of diabetes, and their therapeutic roles in diabetes are currently under investigation in clinical trials. Overall, the roles of FGFs in diabetes and diabetic complications are involved in numerous processes. First, FGF intervention can prevent high-fat diet-induced obesity and insulin resistance and reduce the levels of fasting blood glucose and triglycerides by regulating lipolysis in adipose tissues and hepatic glucose production. Second, modulation of FGF expression can inhibit renal and cardiac fibrosis by regulating the expression of extracellular matrix components, promote diabetic wound healing process and bone repair, and inhibit cancer cell proliferation and migration. Finally, FGFs can regulate the activation of glucose-excited neurons and the expression of thermogenic genes.
Core Tip: Diabetes affects about 422 million people worldwide, causing 1.5 million deaths each year. However, the incidence of diabetes is increasing, including both type 1 and type 2 diabetes. New therapies are needed to treat diabetes and manage its complications. The fibroblast growth factor (FGF) family members play important roles in many metabolic disorders, including diabetes. To date, a total of 23 family members (FGF1-23) have been found in humans. Some FGFs, such as FGF-15, FGF-19, and FGF-21, have antidiabetic functions in preclinical studies, and they are under investigation in clinical trials for examining the therapeutic effects in patients.
- Citation: Zhang CY, Yang M. Roles of fibroblast growth factors in the treatment of diabetes. World J Diabetes 2024; 15(3): 392-402
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/392.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.392
Diabetes mellitus (DM) is a metabolic disorder that affects different ages of people by inducing abnormal levels of blood sugar in the body. According to the report on the official website of the World Health Organization (WHO, https://www.who.int/, accessed on October 26, 2023), there are about 422 million people with diabetes worldwide, and 1.5 million deaths are directly caused by diabetes each year. The incidence of diabetes is increasing[1,2]. There are several types of diabetes. Type 1 DM (T1DM, 5%-10% of diabetic cases) and type 2 DM (T2DM, 90%-95% of diabetic cases) are the main types of diabetes in the clinic[3]. T1DM occurs when the insulin-producing pancreatic beta cells are damaged by factors such as autoimmune attack[4], while T2DM is characterized by both insulin resistance and beta cell dysfunction that cause persistent hyperglycemia[5]. As reported by the WHO, diabetes and diabetes-related kidney disease caused about 2 million deaths in 2019. Therefore, new therapies are urgently needed to treat diabetes and diabetes-related complications.
Fibroblast growth factors (FGFs), consisting of 23 family members (FGF-1-23) in humans[6,7], play important roles in metabolic homeostasis and cell biological processes since alteration of the expression of FGFs is implicated in many chronic diseases. These diseases include obesity[8,9], metabolic-associated fatty liver disease[10-13], diabetes[14,15], and diabetic complications such as hyperthyroidism[16], chronic kidney disease (CKD)[17,18], cardiovascular disease[19,20], and cancer[21,22]. Investigations have shown that FGFs can function as molecular targets for the treatment of diabetes and diabetes-associated metabolic disorders.
In this mini-review, we first review the roles of FGFs in the pathogenesis of diabetes and diabetic complications, and then we briefly summarize the findings of clinical trials regarding the functions of FGFs in the treatment of diabetes and metabolic disorders.
Of the recognized 23 FGFs, some have been extensively investigated such as FGF-21 in diabetes, and others have not been well studied such as FGF-8. Although the same family of FGFs has similar principle functions, the functions of each member remain distinct in diabetes. Therefore, the following section will briefly introduce the function of each member and mainly focus on the function related to metabolic syndrome, diabetes, and diabetic complications.
FGF-1 can be produced by adipose tissue to regulate glucose uptake by modulating the glucose transporters (GLUTs), GLUT1 and GLUT4[23]. FGF-1 also inhibits lipolysis in adipose tissues to suppress the production of free fatty acids (FFAs) that transport into the liver to produce hepatic glucose. Mechanistically, FGF-1 binds to its FGF receptor 1 (FGFR1) to activate the phosphorylation of phosphodiesterase 4D to inhibit lipolysis in adipocytes by inhibiting cyclic adenosine monophosphate-protein kinase A axis[24]. A single parenteral treatment of recombinant FGF-1 can reduce glucose levels in diabetic ob/ob mice and diet-induced obese (DIO) mice that mimic human T2DM[25]. In summary, FGF-1 displays anti-obesity and antidiabetic function by regulating glucose transport, FFA production in obese tissues, and glucose production in the liver.
The binding of FGF-2 to its receptor FGFR can activate intracellular mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 to increase intramuscular adipogenesis in the aged human skeletal muscle, by increasing the phosphorylation of Fos-related antigen and microRNA-29a (miR-29a) expression levels[26]. In mice with diabetic nephropathy, Klotho (a co-receptor for FGF-23) can inhibit renal injury and fibrosis by suppressing FGF-2 expression that is negatively associated with E-cadherin expression[27]. However, FGF-2 isoforms may play different roles in diabetic nephropathy in genetically diabetic db/db mice, with the upregulation of low molecular weight FGF-2 expression and downregulation of high molecular weight FGF-2 expression in the kidney[28]. Thus, FGF-2 may have an unfavorable role in diabetes and relative diseases.
One study showed that there is insulin-dependent diabetes mellitus locus on chromosome 11q13 (IDDM4), which is located near the FGF-3 locus[29]. In addition, FGF-3 and its receptor are downregulated in diabetic retinopathy[30,31]. However, the specific role of FGF-3 in diabetes remains unknown and needs to be further investigated.
The expression levels of FGF-4 and FGFR-2 are increased in the embryo of female BALB/c mice with diabetes compared to their expression levels in the embryo of non-diabetic control mice[32], suggesting their roles in embryo development in maternal diabetes. Administration of FGF-4 via intracerebroventricular injection shows an antidiabetic function in male db/db mice and DIO mice by activating glucose-excited neurons via FGFR1 and deactivating glucose-inhibited neurons[33]. These studies suggest that the roles of FGF-4 in diabetes may be different in embryo development and postnatal.
FGF-5 can regulate the apoptosis and proinflammation of retinal ganglion cells in diabetic retinopathy by upregulating the expression of cytokines such as tumor necrosis factor-α and interleukin-6[34]. This study also showed that the expression of FGF-5 can be regulated by miR-145-5p, functioning as a potential treatment option. Long non-coding RNA (lncRNA) taurine up-regulated gene 1 (TUG1) expression was downregulated in the islets of mice with a high-fat diet (HFD) compared to that in mice fed a normal diet. Knockdown of lncRNA TUG1 can inhibit glucose-induced proliferation of islet cell line MIN6 cells and promote cell apoptosis by increasing the expression of miR-188-3p to suppress the expression of FGF-5[35]. Overall, FGF-5 shows anti-apoptotic function in obesity and diabetic retinopathy, which can be regulated by non-coding RNAs.
The expression levels of FGF-6 and FGF-9 in adipose tissues can be induced by thermogenic factors such as exposure to cold and exercise, and these two FGFs can upregulate the expression of uncoupling protein-1 (UCP1) in brown and white preadipocytes by activating FGFR3[36]. Overexpression of FGF-6 in inguinal white adipose tissues can inhibit HFD–induced obesity and insulin resistance in lean mice. Mechanistically, FGF-6 functions as an autocrine or paracrine factor to promote platelet-derived growth factor receptor alpha-expressing adipocyte progenitor cell proliferation by regulating the extracellular signal–regulated kinase signaling pathway[37]. Another study showed that overexpression of FGF-6 in mouse skeletal muscle tissues can suppress HFD-induced insulin resistance and body weight increase[38]. In summary, overexpression of FGF-6 can inhibit HFD-induced insulin resistance in obese subjects.
Treatment with FGF-7-loaded galactosylated poly (DL-lactide-co-glycolic acid) particles can improve the islet engraftment into the liver and normalize blood glucose levels in mice with diabetes[39]. In addition, FGFs play a key role in the diabetic wound healing process[40]. For example, one study reveals that inhibition of miR-155 can restore FGF-7 expression to improve diabetic wound healing and reduce wound inflammation[41]. In summary, FGF-7 has diverse roles in diabetic subjects by reducing glucose levels and improving wound healing.
FGF-8 plays a key role in brain development and neuron differentiation by interacting with its receptors such as FGFR1[42]. However, the specific role of FGF-8 in T2DM and its relative metabolic disorders remains to be studied.
The expression of FGF-9 is increased in the subcutaneous white adipose tissues in obese humans and mice, which can inhibit thermogenic gene expression to activate the hypoxia-inducible factor (HIF) pathway to regulate the adipose browning process[43]. Like FGF-6, FGF-9 can induce the expression of UCP1 in adipocytes and preadipocytes via binding with FGFR3 to regulate systemic energy metabolism[36]. Another study demonstrated that the expression of FGF-9 is increased in patients with nonalcoholic steatohepatitis-associated hepatocellular carcinoma (HCC), which promotes the expression of extracellular matrix components by regulating the β-catenin signaling pathway[44]. Therefore, the function of FGF-9 is tissue-dependent.
FGF-10 and its receptor FGFR2b are involved in the development of the digestive system, including the pancreas[45]. FGF-10 is required for the development of the pancreas during early organogenesis[46,47]. As an angiogenic factor, FGF-10 expression is upregulated in epididymal white adipose tissue, endothelial cells, and preadipocytes in HIF-1α deficient mice[48].
FGF-11 functions differently in adipocytes and other cells. FGF-11, a master mediator of adipogenesis, can inhibit adipocyte differentiation by regulating the expression of peroxisome proliferator-activated receptor gamma (PPARγ). By contrast, the PPARγ agonist rosiglitazone can restore adipogenesis, which is suppressed by knockdown of the gene FGF11[49]. Knockdown of FGF11 can significantly reduce mesangial cell proliferation and fibrosis in the progression of diabetic nephropathy[50]. Silencing FGF11 in the mouse hypothalamus can reduce HFD-induced body weight gain and fat accumulation by increasing brown adipose tissue thermogenesis and insulin intolerance[51]. In addition, FGF-11 regulates the differentiation and thermogenesis of brown adipocytes in goats[52].
The role of FGF-12 is mainly investigated in cardiovascular disease. FGF-12 upregulation can improve cardiac dysfunction in mice with myocardial infarction by reducing the production of extracellular matrix components in cardiac fibroblasts induced by angiotensin II, including fibronectin and collagens I and III[53]. It also plays an important role in vascular remodeling by regulating the phenotypic change of vascular smooth muscle cells[54].
The serum level of FGF-13 was decreased in patients with impaired glucose tolerance and T2DM compared to that in the healthy controls, suggesting that it could serve as a diagnostic marker for T2DM[55]. In addition, FGF-13 plays an important role in diabetic nephropathy[56] and obesity[57]. However, the function of FGF-13 in glucose regulation and T2DM remains to be studied.
Currently, the effects of FGF-14 are broadly investigated in tumors. FGF-14 is downregulated in lung adenocarcinomas[58], playing a pivotal role in cancer cell proliferation and migration. Overexpression of FGF-14 is associated with a better overall survival of pancreatic ductal adenocarcinoma patients[59].
Mouse FGF-15 is the homolog of human FGF-19. Overexpression of mouse FGF-15 or administration of recombinant human FGF-19 can decrease the levels of fasting blood glucose, FFAs, and triglycerides, and homeostasis model assessment of insulin resistance cores in pregnant mice with HFD compared to corresponding control mice[60]. The antidiabetic effects of total flavonoids extracted from tea are mediated by activation of the farnesoid X receptor/FGF-15 axis[61]. Another study also showed that FGF-15/FGF-19 treatment can inhibit hepatic lipogenesis in mice by activating small heterodimer partner and DNA methyltransferase-3a[62]. Overall, FGF-15 displays antidiabetic function by reducing the levels of fasting blood glucose, FFAs, insulin resistance, and hepatic lipogenesis.
FGF-16 is a target of microRNAs, such as miR-372-3p and miR-144-3p, which can regulate high glucose-induced glomerular endothelial cell dysfunction in patients with diabetic retinopathy[63] and suppress high-glucose-induced proliferation of human umbilical vein endothelial cells and human retinal endothelial cells to potentially suppress diabetic retinopathy[64]. Another study also showed that FGF-16 can be regulated by miR-520b to regulate lung cancer cell proliferation[65]. In summary, FGF-16 regulates cell dysfunction and proliferation in diabetes and cancers.
The function of FGF-17 has been investigated in cancers. FGF-17 has been shown to function as a potent diagnostic marker for acute myeloid leukemia[66]. As a subfamily member of FGF-8, it has been detected to be upregulated in 59% of human HCC samples to contribute to angiogenesis and cancer cell survival[67]. The roles of FGF-17 in diabetes are less studied.
FGF-18 plays multiple roles in many diseases including bone repair[68], diabetic wound healing[69], and cancer[70-72]. A recent study showed that the expression of FGF-18 is associated with liver fibrosis in human liver tissues, which can promote liver fibrosis in mouse models[73]. However, the specific role of FGF-18 in diabetes remains to be studied.
Intracerebroventricular injection of recombinant FGF-1 or FGF-19 can induce a 60% reduction of glucose production in the livers of mice with T1DM, as well as lipolysis in the body[74]. A clinical trial study finds that circulating serum levels of FGF-19 are significantly decreased in obese patients independent of insulin resistance[75]. Another study also reveals that serum levels of FGF-19 are significantly decreased in patients with T2DM and metabolic syndrome compared to healthy controls[76]. Low serum level of FGF-19 is positively associated with T1DM as a contributing factor, which is negatively associated with the levels of fasting blood glucose[77]. These results suggest that FGF-19 can regulate the levels of glucose to ameliorate insulin resistance and diabetes.
FGF-20 has favorable roles in several chronic diseases. For example, FGF-20 plays a protective role in cardiac hypertrophy by activating silent information regulator 1 to inhibit oxidative stress-induced myocardial injury[78]. Increased plasma FGF-20 protein can delay the progression of diabetic renal diseases at the end stage[79]. In addition, rs12720208 polymorphism in the gene FGF20 has been found to be associated with the susceptibility of Parkinson’s disease[80]. The function of FGF-20 in diabetes remains unclear.
The expression level of FGF-21 has been found to be positively associated with the risk of T2DM in a cross-sectional study in the southern part of China, serving as a potential diagnostic marker[81]. Treatment with recombinant human FGF-21 can ameliorate insulin resistance, hyperglycemia, and endothelial dysfunction in T2DM mice induced by HFD-streptozotocin treatment by activating the calcium/calmodulin-dependent protein kinase kinase 2/AMP-activated protein kinase alpha signaling pathway[82]. FGF-21 as a peptide hormone plays beneficial effects on weight loss, glucose and fatty acid metabolism, and inflammation[83].
FGF-22 plays an essential role in the recovery process of spinal cord injury, which can inhibit endoplasmic reticulum stress-induced apoptosis[84,85]. The rs8109113 polymorphism of the gene FGF22 has been shown to be associated with hypertension and height[86]. Currently, the function of FGF-22 remains under further investigation.
FGF-23 plays an important role in maintaining serum phosphate concentration in CKD. Patients with diabetic kidney disease received a high-phosphate diet at a daily dose of 1800 mg for 6 d had an increased serum FGF-23 at the first 3 d from baseline, but had a trend to decrease after day 3, whereas this diet steadily increased the level of FGF-23 in non-diabetic patients[87]. Ramipril, an angiotensin-converting enzyme inhibitor, is commonly applied to treat hypertension, heart failure, and diabetic kidney disease. Ramipril treatment significantly decreases serum FGF-23 levels, resulting in improvement in proteinuria and an endothelium-dependent flow-mediated response to ischemia in patients with T2DM and stage 1 CKD[88]. Overall, FGFs exhibit diverse and different roles in diabetes and the associated diseases (Table 1), and targeting some FGFs (e.g., FGF-15, FGF-19, and FGF-21) may facilitate the treatment of diabetes.
| Diabetes | FGFs | Functions | Ref. |
| Type 2 diabetes | FGF-1 | A single parenteral treatment of recombinant FGF-1 can decrease glucose levels in diabetic ob/ob mice and DIO mice that mimic human type 2 diabetes | Suh et al[25] |
| Diabetic nephropathy | FGF-2 | In mice with diabetic nephropathy, Klotho (a co-receptor for FGF-23) can inhibit renal injury and fibrosis by suppressing FGF-2 expression that is negatively associated with E-cadherin expression | Dong et al[27] |
| Diabetic retinopathy | FGF-3 | FGF-3 and its receptor have been found to be downregulated in diabetic retinopathy | Ljubimov et al[30], Saghizadeh et al[31] |
| Type 2 diabetes | FGF-4 | Intracerebroventricular administration of FGF-4 shows an anti-diabetic function in male db/db mice and DIO mice by activating glucose-excited neurons via FGFR1, while it can also deactivate glucose-inhibited neurons | Sun et al[33] |
| Type 2 diabetes | FGF-5 | Knockdown of lncRNA TUG1 can inhibit glucose-induced proliferation of islet cell line MIN6 cells and promote cell apoptosis by increasing expression miR-188-3p to suppress the expression of FGF-5 | Zhang et al[35] |
| Obesity and insulin resistance | FGF-6 | Overexpression of FGF-6 in inguinal white adipose tissue can inhibit HFD–induced obesity and insulin resistance in lean mice, while overexpression of FGF-6 in mouse skeletal muscle tissues can also suppress HFD-induced insulin resistance and bodyweight increase | Liu et al[37], Xu et al[38] |
| Type 1 diabetes | FGF-7 | Treatment with FGF-7-loaded galactosylated poly (DL-lactide-co-glycolic acid) particles can improve the islet engraftment into the liver and normalize blood glucose levels in mice with diabetes | Alwahsh et al[39] |
| Neuron differentiation | FGF-8 | FGF-8 plays a key role in brain development and neuron differentiation by interacting with its receptors such as FGFR1 | Yellapragada et al[42] |
| Non-alcoholic steatohepatitis (NASH)-associated hepatocellular carcinoma (HCC) | FGF-9 | A study also shows that the expression of FGF-9 was increased in patients with NASH-HCC, which regulated the expression of extracellular matrix components by regulating the β-catenin signaling pathway | Zhang et al[44] |
| Pancreas organogenesis | FGF-10 | FGF-10 is required for the development of the pancreas during early organogenesis | Bhushan et al[46], Norgaard et al[47] |
| Diabetic nephropathy | FGF-11 | FGF-11 knockdown can significantly reduce mesangial cell proliferation and fibrosis in the progression of diabetic nephropathy | Liu et al[50] |
| Cardiac dysfunction | FGF-12 | FGF-12 upregulation can improve cardiac dysfunction in mice with myocardial infarction by reducing the production of extracellular matrix components in cardiac fibroblasts induced by angiotensin II, including fibronectin and collagen I and III | Liu et al[53] |
| Type 2 diabetes | FGF-13 | The serum level of FGF-13 was decreased in patients with impaired glucose tolerance and T2DM compared to that in the healthy controls, suggesting that it could serve as a diagnostic marker for T2DM | Che et al[55] |
| Cancers | FGF-14 | FGF-14 plays a pivotal role in cancer progression and prognosis | Turkowski et al[58], Raja et al[59] |
| Diabetes, obesity, insulin resistance, and non-alcoholic fatty liver disease | FGF-15 | Mouse FGF-15 is the homolog of human FGF-19. Overexpression or activation of FGF-15 or FGF-19 can decrease the levels of fasting blood glucose, free fatty acids, triglycerides, and insulin resistance, which also displays anti-diabetic effects and inhibits hepatic lipogenesis | Zhao et al[60], Hu et al[61], Kim et al[62] |
| Diabetic nephropathy and diabetic retinopathy | FGF-16 | FGF-16 is a target of microRNAs such as miR-372-3p and miR-144-3p, which can regulate high glucose-induced glomerular endothelial cell dysfunction in patients with diabetic nephropathy and suppress high-glucose-induced proliferation of human umbilical vein endothelial cells and human retinal endothelial cells to potentially suppress diabetic retinopathy | Meng et al[63], Chen et al[64] |
| Cancers | FGF-17 | FGF-17 plays a key role in cancer diagnosis (e.g., acute myeloid leukemia) and cancer cell survival (e.g., hepatocellular carcinoma) | Ling and Du[66], Gauglhofer et al[67] |
| Liver fibrosis | FGF-18 | Overexpression of FGF-18 in mouse liver can promote liver fibrosis development | Tsuchiya et al[73] |
| Type 1 and type 2 diabetes | FGF-19 | Serum levels of FGF-19 were significantly decreased in patients with T1DM and T2DM | Barutcuoglu et al[76], Hu et al[77] |
| Diabetic renal diseases | FGF-20 | Increased plasma FGF-20 protein could delay the progression of diabetic renal diseases at the end stage | Md Dom et al[79] |
| Type 2 diabetes | FGF-21 | Treatment with recombinant human FGF-21 can ameliorate insulin resistance, hyperglycemia, and endothelial dysfunction in T2DM mice induced by HFD-STZ treatment by activating the CaMKK2/AMP-AMPKα signaling pathway | Ying et al[82] |
| Spinal cord injury | FGF-22 | FGF-22 plays an essential role in the recovery process of spinal cord injury, which can inhibit endoplasmic reticulum stress-induced apoptosis | Aljović et al[84], Zhu et al[85] |
| Diabetic nephropathy | FGF-23 | FGF-23 could be implicated in proteinuria and endothelial dysfunction in patients with diabetic nephropathy | Yilmaz et al[88] |
In this section, we briefly introduce several clinical trials about the roles of FGF in diabetes and diabetic complications. Several trials (https://clinicaltrials.gov, numbers including NCT02667964, NCT01858597 or NCT03816605, NCT00491322, NCT04012983, and NCT05937737) have been performed to investigate the roles of FGFs in insulin secretion, insulin resistance, regulation in the expression of insulin receptor substrate 1 and glucose transporter 1 in gestational diabetes mellitus (GDM), and function as biomarkers for periodontal disease in patients with diabetes, as well as the association of FGF expression levels with the intake of phytochemicals in diet and dietary total antioxidant capacity in patients with T2DM.
The impact of physical activity and diet intake on FGF expression in DM patients has been investigated. For example, the relationship between FGF-21 expression and physical activity in regulation of insulin secretion in patients with T1DM or T2DM, and healthy volunteers was investigated in a trial (NCT02667964). Another trial (NCT05937737) investigated the impact of phytochemical intake from the diet and total dietary antioxidant capacity measured by different methods on the expression of serum FGF-21 in patients with T2DM. Given the regulatory effect of vitamin D on insulin secretion in the pancreas, ergocalciferol (vitamin D2) was applied to treat vitamin D deficiency-related insulin resistance and regulate FGF-23 expression in patients (NCT00491322). In addition, the functions of FGFs have been investigated in diabetic complications. GDM is the most common complication in pregnant women. The roles of FGF-19 and FGF-21 in regulating insulin resistance, dyslipidemia, and glucose intolerance in GDM (NCT01858597 and NCT03816605), due to their effects on the expression of insulin receptor substrate-1 and glucose transporter-1 in placenta. Moreover, an observational study (NCT04012983) was conducted to investigate the diagnostic role of FGF-21 from gingival crevicular fluid in periodontal disease in diabetic and nondiabetic patients, in combination with an adipokine chemerin. However, the therapeutic roles of FGF in diabetes remain unknown. More clinical trials are expected to validate pre-clinical findings of FGFs such as FGF-19 and FGF-21 in diabetes.
In this minireview, the roles of FGFs in diabetes and other related diseases, such as metabolic syndrome, wound healing, and cancers in current studies are reviewed. The beneficial functions of FGFs in diabetes and diabetic complications comprise suppression of hepatic glucose production and lipolysis in adipose tissues, reduction of levels of fasting blood glucose and triglycerides, inhibition of renal injury and fibrosis, inhibition of HFD-induced obesity and insulin resistance, inhibition of cancer cell proliferation and migration, and promotion of diabetic wound healing process and bone repair (Figure 1). In addition, FGFs can regulate the activation of glucose-excited neurons, the expression of thermogenic genes, and the production of extracellular matrix components in cardiac fibroblasts. Although there are 23 FGF family members, only some FGFs such as FGF-15, FGF-19, and FGF-21 have been broadly investigated in cell and animal models for diabetic disease treatments. The functions of most FGFs in diabetes remain less studied. Moreover, only some clinical trials have been performed to investigate the roles of FGF in insulin secretion, insulin resistance, regulation in the expression of insulin receptor substrate 1 and glucose transporter 1 in gestational diabetes mellitus, function as biomarkers for periodontal disease in patients with diabetes, as well as their expression levels with the association of dietary total antioxidant capacity in patients with T2DM. Therefore, more clinical trials are waited to validate preclinical findings of the roles of FGF in diabetes and investigate new drugs or small molecules targeting FGFs to treat diabetes and diabetes-related metabolic disorders.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cai L, United States; Emran TB, Bangladesh S-Editor: Lin C L-Editor: Filipodia P-Editor: Chen YX
| 1. | Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66:241-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 338] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 2. | Cloete L. Diabetes mellitus: an overview of the types, symptoms, complications and management. Nurs Stand. 2022;37:61-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 3. | Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 896] [Cited by in F6Publishing: 842] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 4. | Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021;17:150-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 214] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 5. | Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013;4:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 448] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 6. | Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1222] [Cited by in F6Publishing: 1295] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 7. | She QY, Bao JF, Wang HZ, Liang H, Huang W, Wu J, Zhong Y, Ling H, Li A, Qin SL. Fibroblast growth factor 21: A "rheostat" for metabolic regulation? Metabolism. 2022;130:155166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Ziomber-Lisiak A, Piana K, Ostachowicz B, Wróbel P, Kasprzyk P, Kaszuba-Zwoińska J, Baranowska-Chowaniec A, Juszczak K, Szczerbowska-Boruchowska M. The New Markers of Early Obesity-Related Organ and Metabolic Abnormalities. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Chaiyasoot K, Khumkhana N, Deekum W, Chaichana C, Taweerutchana V, Srisuworanan N, Pramyothin P. Alteration of BDNF, SPARC, FGF-21, and GDF-15 circulating levels after 1 year of anti-obesity treatments and their association with 1-year weight loss. Endocrine. 2023;82:57-68. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 10. | Wang J, Zhang F, Yang W, Gao D, Yang L, Yu C, Chen C, Li X, Zhang JS. FGF1 ameliorates obesity-associated hepatic steatosis by reversing IGFBP2 hypermethylation. FASEB J. 2023;37:e22881. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Song L, Wang L, Hou Y, Zhou J, Chen C, Ye X, Dong W, Gao H, Liu Y, Qiao G, Pan T, Chen Q, Cao Y, Hu F, Rao Z, Chen Y, Han Y, Zheng M, Luo Y, Li X, Huang Z. FGF4 protects the liver from nonalcoholic fatty liver disease by activating the AMP-activated protein kinase-Caspase 6 signal axis. Hepatology. 2022;76:1105-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Kinne AS, Tillman EJ, Abdeen SJ, Johnson DE, Parmer ES, Hurst JP, de Temple B, Rinker S, Rolph TP, Bowsher RR. Noncompetitive immunoassay optimized for pharmacokinetic assessments of biologically active efruxifermin. J Pharm Biomed Anal. 2023;232:115402. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Mantovani A, Tilg H, Targher G. FGF-21 analogues for treatment of non-alcoholic steatohepatitis and fibrosis: a meta-analysis with fragility index of phase 2 randomised placebo-controlled trials. Gut. 2023;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 14. | Rosenstock M, Tseng L, Pierce A, Offman E, Chen CY, Charlton RW, Margalit M, Mansbach H. The Novel GlycoPEGylated FGF21 Analog Pegozafermin Activates Human FGF Receptors and Improves Metabolic and Liver Outcomes in Diabetic Monkeys and Healthy Human Volunteers. J Pharmacol Exp Ther. 2023;387:204-213. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 15. | Pedersen AKN, Gormsen LC, Nielsen S, Jessen N, Bjerre M. Metformin Improves the Prerequisites for FGF21 Signaling in Patients With Type 2 Diabetes. J Clin Endocrinol Metab. 2024;109:e552-e561. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 16. | Bonde Y, Breuer O, Lütjohann D, Sjöberg S, Angelin B, Rudling M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55:2408-2415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Tan X, Tao Q, Yin S, Fu G, Wang C, Xiang F, Hu H, Zhang S, Wang Z, Li D. A single administration of FGF2 after renal ischemia-reperfusion injury alleviates post-injury interstitial fibrosis. Nephrol Dial Transplant. 2023;38:2537-2549. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 18. | McKnight Q, Jenkins S, Li X, Nelson T, Marlier A, Cantley LG, Finberg KE, Fretz JA. IL-1β Drives Production of FGF-23 at the Onset of Chronic Kidney Disease in Mice. J Bone Miner Res. 2020;35:1352-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Hofer F, Hammer A, Pailer U, Koller L, Kazem N, Steinacher E, Steinlechner B, Andreas M, Laufer G, Wojta J, Zelniker TA, Hengstenberg C, Niessner A, Sulzgruber P. Relationship of Fibroblast Growth Factor 23 With Hospitalization for Heart Failure and Cardiovascular Outcomes in Patients Undergoing Cardiac Surgery. J Am Heart Assoc. 2023;12:e027875. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 20. | Donovan K, Herrington WG, Paré G, Pigeyre M, Haynes R, Sardell R, Butterworth AS, Folkersen L, Gustafsson S, Wang Q, Baigent C, Mälarstig A, Holmes MV, Staplin N; on behalf of the SCALLOP Consortium. Fibroblast Growth Factor-23 and Risk of Cardiovascular Diseases: A Mendelian Randomization Study. Clin J Am Soc Nephrol. 2023;18:17-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Roussot N, Lecuelle J, Dalens L, Truntzer C, Ghiringhelli F. FGF/FGFR genomic amplification as a predictive biomarker for immune checkpoint blockade resistance: a short report. J Immunother Cancer. 2023;11. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 22. | Castillo-Castrejon M, Sankofi BM, Murguia SJ, Udeme AA, Cen HH, Xia YH, Thomas NS, Berry WL, Jones KL, Richard VR, Zahedi RP, Borchers CH, Johnson JD, Wellberg EA. FGF1 supports glycolytic metabolism through the estrogen receptor in endocrine-resistant and obesity-associated breast cancer. Breast Cancer Res. 2023;25:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 23. | Nies VJM, Struik D, Liu S, Liu W, Kruit JK, Downes M, van Zutphen T, Verkade HJ, Evans RM, Jonker JW. Autocrine FGF1 signaling promotes glucose uptake in adipocytes. Proc Natl Acad Sci U S A. 2022;119:e2122382119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Sancar G, Liu S, Gasser E, Alvarez JG, Moutos C, Kim K, van Zutphen T, Wang Y, Huddy TF, Ross B, Dai Y, Zepeda D, Collins B, Tilley E, Kolar MJ, Yu RT, Atkins AR, van Dijk TH, Saghatelian A, Jonker JW, Downes M, Evans RM. FGF1 and insulin control lipolysis by convergent pathways. Cell Metab. 2022;34:171-183.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O, Huang Z, Liu W, Yoshihara E, van Dijk TH, Havinga R, Fan W, Yin YQ, Yu RT, Liddle C, Atkins AR, Olefsky JM, Mohammadi M, Downes M, Evans RM. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513:436-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 26. | Mathes S, Fahrner A, Ghoshdastider U, Rüdiger HA, Leunig M, Wolfrum C, Krützfeldt J. FGF-2-dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc Natl Acad Sci U S A. 2021;118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Dong QL, Zhao XH, Wang Q, Zhang LP, Yan XH, Wang XM, Li ZJ, Sun Y. Anti-aging gene Klotho ameliorates diabetic nephropathy in mice by inhibiting FGF2 signaling pathway. J Biol Regul Homeost Agents. 2020;34:1369-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 28. | Luo Y, Deng D, Lin L, Zhou Y, Wang L, Zou X, Wang X. FGF2 isoforms play distinct roles in tubular epithelial-to-mesenchymal transition in diabetic nephropathy. Exp Cell Res. 2022;420:113355. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 29. | Field LL, Tobias R, Magnus T. A locus on chromosome 15q26 (IDDM3) produces susceptibility to insulin-dependent diabetes mellitus. Nat Genet. 1994;8:189-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Ljubimov AV, Tajbakhsh J, Aoki A, Chai NN, Wang C, Ljubimova JY, Nelson SF, Saghizadeh M. Overexpression Of Cathepsins And Growth Factors Abnormalities Identified In Diabetic Corneas By Gene Microarray Analysis. Invest Ophthalmol Vis Sci. 2004;45:1043. [Cited in This Article: ] |
| 31. | Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne G, Carlson MR, Nelson SF, Ljubimov AV. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci. 2005;46:3604-3615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Yilmaz F, Micili SC, Erbil G. The role of FGF-4 and FGFR-2 on preimplantation embryo development in experimental maternal diabetes. Gynecol Endocrinol. 2022;38:248-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Sun H, Lin W, Tang Y, Tu H, Chen T, Zhou J, Wang D, Xu Q, Niu J, Dong W, Liu S, Ni X, Yang W, Zhao Y, Ying L, Zhang J, Li X, Mohammadi M, Shen WL, Huang Z. Sustained remission of type 2 diabetes in rodents by centrally administered fibroblast growth factor 4. Cell Metab. 2023;35:1022-1037.e6. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Cui C, Xu H. Downregulation of miR-145-5p elevates retinal ganglion cell survival to delay diabetic retinopathy progress by targeting FGF5. Biosci Biotechnol Biochem. 2019;83:1655-1662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Zhang P, Li YN, Tu S, Cheng XB. SP1-induced lncRNA TUG1 regulates proliferation and apoptosis in islet cells of type 2 diabetes mellitus via the miR-188-3p/FGF5 axis. Eur Rev Med Pharmacol Sci. 2021;25:1959-1966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 36. | Shamsi F, Xue R, Huang TL, Lundh M, Liu Y, Leiria LO, Lynes MD, Kempf E, Wang CH, Sugimoto S, Nigro P, Landgraf K, Schulz T, Li Y, Emanuelli B, Kothakota S, Williams LT, Jessen N, Pedersen SB, Böttcher Y, Blüher M, Körner A, Goodyear LJ, Mohammadi M, Kahn CR, Tseng YH. FGF6 and FGF9 regulate UCP1 expression independent of brown adipogenesis. Nat Commun. 2020;11:1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 37. | Liu C, Meng M, Xu B, Xu Y, Li G, Cao Y, Wang D, Qiu J, Yu J, Xu L, Ma X, Hu C. Fibroblast Growth Factor 6 Promotes Adipocyte Progenitor Cell Proliferation for Adipose Tissue Homeostasis. Diabetes. 2023;72:467-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 38. | Xu B, Liu C, Zhang H, Zhang R, Tang M, Huang Y, Jin L, Xu L, Hu C, Jia W. Skeletal muscle-targeted delivery of Fgf6 protects mice from diet-induced obesity and insulin resistance. JCI Insight. 2021;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Alwahsh SM, Qutachi O, Starkey Lewis PJ, Bond A, Noble J, Burgoyne P, Morton N, Carter R, Mann J, Ferreira-Gonzalez S, Alvarez-Paino M, Forbes SJ, Shakesheff KM, Forbes S. Fibroblast growth factor 7 releasing particles enhance islet engraftment and improve metabolic control following islet transplantation in mice with diabetes. Am J Transplant. 2021;21:2950-2963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Wang NQ, Jia WH, Yin L, Li N, Liang MD, Shang JM, Hou BY, Zhang L, Qiang GF, Du GH, Yang XY. Sex difference on fibroblast growth factors (FGFs) expression in skin and wound of streptozotocin(STZ)-induced type 1 diabetic mice. Mol Biol Rep. 2023;50:1981-1991. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 41. | Moura J, Sørensen A, Leal EC, Svendsen R, Carvalho L, Willemoes RJ, Jørgensen PT, Jenssen H, Wengel J, Dalgaard LT, Carvalho E. microRNA-155 inhibition restores Fibroblast Growth Factor 7 expression in diabetic skin and decreases wound inflammation. Sci Rep. 2019;9:5836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 42. | Yellapragada V, Eskici N, Wang Y, Madhusudan S, Vaaralahti K, Tuuri T, Raivio T. FGF8-FGFR1 signaling regulates human GnRH neuron differentiation in a time- and dose-dependent manner. Dis Model Mech. 2022;15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Sun Y, Wang R, Zhao S, Li W, Liu W, Tang L, Wang Z, Wang W, Liu R, Ning G, Wang J, Hong J. FGF9 inhibits browning program of white adipocytes and associates with human obesity. J Mol Endocrinol. 2019;62:79-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Zhang L, Zhang Q, Teng D, Guo M, Tang K, Wang Z, Wei X, Lin L, Zhang X, Wang X, Huang D, Ren C, Yang Q, Zhang W, Gao Y, Chen W, Chang Y, Zhang H. FGF9 Recruits β-Catenin to Increase Hepatic ECM Synthesis and Promote NASH-Driven HCC. Adv Sci (Weinh). 2023;10:e2301166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 45. | Lv YQ, Wu J, Li XK, Zhang JS, Bellusci S. Role of FGF10/FGFR2b Signaling in Mouse Digestive Tract Development, Repair and Regeneration Following Injury. Front Cell Dev Biol. 2019;7:326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109-5117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 326] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 47. | Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Fujisaka S. The role of adipose tissue M1/M2 macrophages in type 2 diabetes mellitus. Diabetol Int. 2021;12:74-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Lee KW, Jeong JY, An YJ, Lee JH, Yim HS. FGF11 influences 3T3-L1 preadipocyte differentiation by modulating the expression of PPARγ regulators. FEBS Open Bio. 2019;9:769-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Liu H, Wang X, Wang ZY, Li L. Circ_0080425 inhibits cell proliferation and fibrosis in diabetic nephropathy via sponging miR-24-3p and targeting fibroblast growth factor 11. J Cell Physiol. 2020;235:4520-4529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Cho JH, Kim K, Cho HC, Lee J, Kim EK. Silencing of hypothalamic FGF11 prevents diet-induced obesity. Mol Brain. 2022;15:75. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 52. | Jiang T, Su D, Liu X, Wang Y, Wang L. Transcriptomic Analysis Reveals Fibroblast Growth Factor 11 (FGF11) Role in Brown Adipocytes in Thermogenic Regulation of Goats. Int J Mol Sci. 2023;24. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 53. | Liu A, Zhang Y, Xun S, Zhou G, Lin L, Mei Y. Fibroblast growth factor 12 attenuated cardiac remodeling via suppressing oxidative stress. Peptides. 2022;153:170786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 54. | Xiong Y, Wang Y, Yang T, Luo Y, Xu S, Li L. Receptor Tyrosine Kinase: Still an Interesting Target to Inhibit the Proliferation of Vascular Smooth Muscle Cells. Am J Cardiovasc Drugs. 2023;23:497-518. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 55. | Chen Q, Li F, Gao Y, Yang F, Yuan L. Identification of FGF13 as a Potential Biomarker and Target for Diagnosis of Impaired Glucose Tolerance. Int J Mol Sci. 2023;24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 56. | Sun J, Guan X, Niu C, Chen P, Li Y, Wang X, Luo L, Liu M, Shou Y, Huang X, Cai Y, Zhu J, Fan J, Li X, Jin L, Cong W. FGF13-Sensitive Alteration of Parkin Safeguards Mitochondrial Homeostasis in Endothelium of Diabetic Nephropathy. Diabetes. 2023;72:97-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 57. | Sinden DS, Holman CD, Bare CJ, Sun X, Gade AR, Cohen DE, Pitt GS. Knockout of the X-linked Fgf13 in the hypothalamic paraventricular nucleus impairs sympathetic output to brown fat and causes obesity. FASEB J. 2019;33:11579-11594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Turkowski K, Herzberg F, Günther S, Brunn D, Weigert A, Meister M, Muley T, Kriegsmann M, Schneider MA, Winter H, Thomas M, Grimminger F, Seeger W, Savai Pullamsetti S, Savai R. Fibroblast Growth Factor-14 Acts as Tumor Suppressor in Lung Adenocarcinomas. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Raja A, Malik MFA, Haq F. Genomic relevance of FGF14 and associated genes on the prognosis of pancreatic cancer. PLoS One. 2021;16:e0252344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Zhao S, Wang D, Li Z, Xu S, Chen H, Ding W, Yang J, Zhao W, Sun B, Wang Z. FGF15/FGF19 alleviates insulin resistance and upregulates placental IRS1/GLUT expression in pregnant mice fed a high-fat diet. Placenta. 2021;112:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Hu YL, Li M, Ding L, Peng C, Wu Y, Liu W, Zhao D, Qin LL, Guo XY, Wu LL. Ampelopsis grossedentata improves type 2 diabetes mellitus through modulating the gut microbiota and bile acid metabolism. J Funct Foods. 2023;107:105622. [DOI] [Cited in This Article: ] |
| 62. | Kim YC, Seok S, Zhang Y, Ma J, Kong B, Guo G, Kemper B, Kemper JK. Intestinal FGF15/19 physiologically repress hepatic lipogenesis in the late fed-state by activating SHP and DNMT3A. Nat Commun. 2020;11:5969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Meng Z, Li F, Wang B. miR-372-3p is a potential diagnostic factor for diabetic nephropathy and modulates high glucose-induced glomerular endothelial cell dysfunction via targeting fibroblast growth factor-16. Arch Med Sci. 2023;19:703-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 64. | Chen C, Zhao C, Gu C, Cui X, Wu J. MiRNA-144-3p inhibits high glucose induced cell proliferation through suppressing FGF16. Biosci Rep. 2019;39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | He W, Liu X, Luo Z, Li L, Fang X. FGF16 regulated by miR-520b enhances the cell proliferation of lung cancer. Open Med (Wars). 2021;16:419-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Ling Y, Du Q. FGF10/FGF17 as prognostic and drug response markers in acute myeloid leukemia. Curr Res Transl Med. 2022;70:103316. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 67. | Gauglhofer C, Sagmeister S, Schrottmaier W, Fischer C, Rodgarkia-Dara C, Mohr T, Stättner S, Bichler C, Kandioler D, Wrba F, Schulte-Hermann R, Holzmann K, Grusch M, Marian B, Berger W, Grasl-Kraupp B. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53:854-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Behr B, Sorkin M, Manu A, Lehnhardt M, Longaker MT, Quarto N. Fgf-18 is required for osteogenesis but not angiogenesis during long bone repair. Tissue Eng Part A. 2011;17:2061-2069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Ishihara J, Ishihara A, Fukunaga K, Sasaki K, White MJV, Briquez PS, Hubbell JA. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat Commun. 2018;9:2163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 70. | Song N, Zhong J, Hu Q, Gu T, Yang B, Zhang J, Yu J, Ma X, Chen Q, Qi J, Liu Y, Su W, Feng Z, Wang X, Wang H. FGF18 Enhances Migration and the Epithelial-Mesenchymal Transition in Breast Cancer by Regulating Akt/GSK3β/Β-Catenin Signaling. Cell Physiol Biochem. 2018;49:1019-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Sonvilla G, Allerstorfer S, Stättner S, Karner J, Klimpfinger M, Fischer H, Grasl-Kraupp B, Holzmann K, Berger W, Wrba F, Marian B, Grusch M. FGF18 in colorectal tumour cells: autocrine and paracrine effects. Carcinogenesis. 2008;29:15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Zhang J, Zhou Y, Huang T, Wu F, Pan Y, Dong Y, Wang Y, Chan AKY, Liu L, Kwan JSH, Cheung AHK, Wong CC, Lo AKF, Cheng ASL, Yu J, Lo KW, Kang W, To KF. FGF18, a prominent player in FGF signaling, promotes gastric tumorigenesis through autocrine manner and is negatively regulated by miR-590-5p. Oncogene. 2019;38:33-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Tsuchiya Y, Seki T, Kobayashi K, Komazawa-Sakon S, Shichino S, Nishina T, Fukuhara K, Ikejima K, Nagai H, Igarashi Y, Ueha S, Oikawa A, Tsurusaki S, Yamazaki S, Nishiyama C, Mikami T, Yagita H, Okumura K, Kido T, Miyajima A, Matsushima K, Imasaka M, Araki K, Imamura T, Ohmuraya M, Tanaka M, Nakano H. Fibroblast growth factor 18 stimulates the proliferation of hepatic stellate cells, thereby inducing liver fibrosis. Nat Commun. 2023;14:6304. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 74. | Perry RJ, Lee S, Ma L, Zhang D, Schlessinger J, Shulman GI. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Commun. 2015;6:6980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Gómez-Ambrosi J, Gallego-Escuredo JM, Catalán V, Rodríguez A, Domingo P, Moncada R, Valentí V, Salvador J, Giralt M, Villarroya F, Frühbeck G. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr. 2017;36:861-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Barutcuoglu B, Basol G, Cakir Y, Cetinkalp S, Parildar Z, Kabaroglu C, Ozmen D, Mutaf I, Bayindir O. Fibroblast growth factor-19 levels in type 2 diabetic patients with metabolic syndrome. Ann Clin Lab Sci. 2011;41:390-396. [PubMed] [Cited in This Article: ] |
| 77. | Hu J, Tang Y, Liu H, Li Y, Li X, Huang G, Xiao Y, Zhou Z. Decreased serum fibroblast growth factor 19 level is a risk factor for type 1 diabetes. Ann Transl Med. 2021;9:376. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 78. | Chen Y, An N, Zhou X, Mei L, Sui Y, Chen G, Chen H, He S, Jin C, Hu Z, Li W, Wang Y, Lin Z, Chen P, Jin L, Guan X, Wang X. Fibroblast growth factor 20 attenuates pathological cardiac hypertrophy by activating the SIRT1 signaling pathway. Cell Death Dis. 2022;13:276. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 79. | Md Dom ZI, Satake E, Skupien J, Krolewski B, O'Neil K, Willency JA, Dillon ST, Wilson JM, Kobayashi H, Ihara K, Libermann TA, Pragnell M, Duffin KL, Krolewski AS. Circulating proteins protect against renal decline and progression to end-stage renal disease in patients with diabetes. Sci Transl Med. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Wang X, Sun X, Zhang X, Li H, Xie A. Quantitative assessment of the effect of FGF20 rs12720208 variant on the risk of Parkinson's disease: a meta-analysis. Neurol Res. 2017;39:374-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | You L, Hong X, Wu H, Liang D, Li F, Zheng D, Zhang X, Liu D, Chen Q, Yan L, Ren M, Wang W. The association of FGF-21 with the risk of newly diagnosed type-2 diabetes mellitus: a cross-sectional study in Southern China. BMC Endocr Disord. 2023;23:188. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 82. | Ying L, Li N, He Z, Zeng X, Nan Y, Chen J, Miao P, Ying Y, Lin W, Zhao X, Lu L, Chen M, Cen W, Guo T, Li X, Huang Z, Wang Y. Fibroblast growth factor 21 Ameliorates diabetes-induced endothelial dysfunction in mouse aorta via activation of the CaMKK2/AMPKα signaling pathway. Cell Death Dis. 2019;10:665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 83. | Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78:223-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 540] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 84. | Aljović A, Jacobi A, Marcantoni M, Kagerer F, Loy K, Kendirli A, Bräutigam J, Fabbio L, Van Steenbergen V, Pleśniar K, Kerschensteiner M, Bareyre FM. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol Med. 2023;15:e16111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 85. | Zhu S, Chen M, Ye J, Ying Y, Wu Q, Dou H, Bai L, Mao F, Ni W, Yu K. Fibroblast Growth Factor 22 Inhibits ER Stress-Induced Apoptosis and Improves Recovery of Spinal Cord Injury. Front Pharmacol. 2020;11:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Cho HW, Jin HS, Eom YB. FGFRL1 and FGF genes are associated with height, hypertension, and osteoporosis. PLoS One. 2022;17:e0273237. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 87. | Muras K, Masajtis-Zagajewska A, Nowicki M. Diabetes modifies effect of high-phosphate diet on fibroblast growth factor-23 in chronic kidney disease. J Clin Endocrinol Metab. 2013;98:E1901-E1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Yilmaz MI, Sonmez A, Saglam M, Kurt YG, Unal HU, Karaman M, Gok M, Cetinkaya H, Eyileten T, Oguz Y, Vural A, Mallamaci F, Zoccali C. Ramipril lowers plasma FGF-23 in patients with diabetic nephropathy. Am J Nephrol. 2014;40:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |