Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.348
Peer-review started: October 25, 2023
First decision: December 8, 2023
Revised: December 14, 2023
Accepted: January 18, 2024
Article in press: January 18, 2024
Published online: March 15, 2024
Processing time: 142 Days and 8.4 Hours
As a common hyperglycemic disease, type 1 diabetes mellitus (T1DM) is a complicated disorder that requires a lifelong insulin supply due to the immune-mediated destruction of pancreatic β cells. Although it is an organ-specific autoimmune disorder, T1DM is often associated with multiple other autoimmune disorders. The most prevalent concomitant autoimmune disorder occurring in T1DM is autoimmune thyroid disease (AITD), which mainly exhibits two extremes of phenotypes: hyperthyroidism [Graves' disease (GD)] and hypo-thyroidism [Hashimoto's thyroiditis, (HT)]. However, the presence of comorbid AITD may negatively affect metabolic management in T1DM patients and thereby may increase the risk for potential diabetes-related complications. Thus, routine screening of thyroid function has been recommended when T1DM is diagnosed. Here, first, we summarize current knowledge regarding the etiology and pathogenesis mechanisms of both diseases. Subsequently, an updated review of the association between T1DM and AITD is offered. Finally, we provide a relatively detailed review focusing on the application of thyroid ultrasonography in diagnosing and managing HT and GD, suggesting its critical role in the timely and accurate diagnosis of AITD in T1DM.
Core Tip: Although type 1 diabetes mellitus (T1DM) is an organ-specific autoimmune disease, patients with this disease are more prone to develop other autoimmune disorder, and the most prevalent autoimmune disorder in T1DM patients is autoimmune thyroid disease (AITD). Undiagnosed and untreated AITD may lead to metabolic disturbances and impair diabetes care in T1DM patients, warranting regular and long-term observation. We herein offer an updated review of the basic characteristics of both diseases and factors contribute to their concomitant presence. Additionally, we focus on the role of thyroid ultrasonography in the diagnosis and management of AITD.
- Citation: Wang J, Wan K, Chang X, Mao RF. Association of autoimmune thyroid disease with type 1 diabetes mellitus and its ultrasonic diagnosis and management. World J Diabetes 2024; 15(3): 348-360
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/348.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.348
As a common childhood-onset chronic disorder, type 1 diabetes mellitus (T1DM) affects 1:300 children, and the disease incidence has continued to increase in recent decades[1,2]. The incidence of T1DM is not uniform across the world, and it tends to be higher in higher-income countries than in lower-income countries[3]. As a result of the autoimmune attack predominantly driven by T cells, T1DM occurs in genetically predisposed individuals exposed to environmental and stochastic factors, leading to the dysfunction and death of pancreatic β-cells, with subsequent hyperglycemia[4]. However, although T1DM is an organ-specific autoimmune disorder, individuals with T1DM often exhibit a higher risk of additional autoimmune disorders[5]. The concomitant presentation of T1DM and another autoimmune disorder may complicate diabetes management and result in varying clinical symptoms, thus seriously influencing patient quality of life[6]. Among these additional autoimmune disorders co-occurring among children and adolescents with T1DM, autoimmune thyroid disease (AITD) accounts for the highest proportion[7]. As the most prevalent organ-specific immune-mediated disorder in the world, AITD is characterized by autoreactive lymphocyte infiltration in the thyroid and the presence of autoantibodies targeting thyroid antigens[8]. Clinically, thyroid dysfunctions, which include hyperthyroidism and hypothyroidism[9], lead to metabolic disturbances and may impair diabetes management in T1DM. Therefore, it is important for individuals with T1DM to regularly screen for thyroid disorders, allowing for the early detection, early diagnosis and intervention of thyroid dysfunction. Benefiting from advances in ultrasound technology, ultrasonography has been widely used to evaluate and treat thyroid diseases[10]. Here, we aim to provide an updated review about the relationship between T1DM and AITD as well as the current status of ultrasonography application in AITD.
As described above, the estimated incidence of T1DM is increasing in many areas around the world. However, these incidences of T1DM may be underestimated. These numbers do not include many adults with T1DM, as almost all incidence data are derived from registered individuals under 20 years of age[11]. Additionally, there is a clear male predominance in T1DM individuals, and this may be associated with the protective role of estrogen[12].
Although the etiology and pathogenesis mechanisms of T1DM have many unknow and large knowledge gaps, our understanding of its pathological process has greatly improved during the last two decades. It has been suggested that a complicated interaction among genetic, environmental, and immunologic factors induces a T-cell-regulated immune attack directed against pancreatic β cells[4,13]. Genetic studies have revealed that T1DM genetic susceptibility exhibits a polygenic nature. Currently, there are more than 60 gene loci linked to T1DM[4]. Among them, the human leukocyte antigen (HLA) class II genes involved in antigen presentation exhibit a major risk factor for T1DM, and HLA-DR and HLA-DQ show the strongest relationship with this disease[14]. In addition, various other immune-related loci (non-HLA) connected to T1DM are recognized, such as CTLA4 (cytotoxic T-lymphocyte antigen 4) and PTPN22 (protein tyrosine phosphatase non-receptor type 22). Furthermore, various candidate genes as well as noncoding RNAs have been identified based on genome-wide association studies (GWAS)[15]. This strong genetic component of T1DM has stimulated efforts to develop a T1DM genetic risk score based on single-nucleotide polymorphism genotyping, as it would be useful for evaluating and predicting islet autoimmunity progression as well as T1DM development in high-risk individuals[16].
However, compared to genetic factors, environmental influences remain poorly understood despite intensive research. The increasing incidence, twin studies, and immigrant studies indicate that environmental factors also exhibit a major role in contributing to T1DM development[17]. Numerous research findings have indicated that the environmental triggers connected to T1DM mainly include climatic conditions, diet, lifestyle, obesity, toxins, vitamin D sufficiency, and infections[17,18]. All these factors may lead to gut microbiota dysbiosis and influence the interrelationship between the intestinal microbiota and host immune system, potentially contributing to T1DM[19,20]. However, some research results related to the role of various environmental factors are largely controversial[21,22], and this may reflect the heterogeneity of T1DM. Therefore, further work concerning the role of gene-environment interactions in contributing to T1DM development is needed.
Influenced by potential genetic and environmental factors, β cell-directed autoimmunity, which includes humoral and cell-mediated autoimmunity, is triggered during the initiation of the development of T1DM. Before clinical symptoms present, there is a long preclinical stage, characterized by the production of disease-specific autoantibodies and reduced insulin and C-peptide production and secretion. The best-characterized autoantibodies connected to T1DM are those that recognize islet cells, insulin, glutamic acid decarboxylase 65, islet tyrosine phosphatase 2, and zinc transporter 8[23]. These nonpathogenic autoantibodies can be viewed as biomarkers of the autoimmune process. Therefore, according to the appearance of autoantibody(ies) and clinical manifestations, a disease staging classification system has been introduced to evaluate and predict T1DM progression in genetically at-risk individuals[24]. Three stages have been defined, starting from serological autoimmunity (≥ 2 disease-related autoantibodies with normoglycemia, stage 1) to a second stage of dysglycemia (stage 2), and to definitive diagnosis of T1DM (stage 3). As it is important to guide the predication and prevention of T1DM, this classification scheme should be further revised by identifying novel stage-specific biomarkers[25,26]. In nonobese diabetic mice, both CD4+ and CD8+ T cells contribute to T1DM development, and in individuals with T1DM, T cells targeting T1DM-related autoantigens can be observed in the pancreatic lymph nodes and islets[27,28]. The participation of these potentially pathogenic T cells in the immune attack toward β-cells suggests the failure of immune system regulation. Of note, the gatekeeper role of regulatory T cells (Tregs) is important to maintain immunological tolerance and prevent autoimmune disease[29]. Thus, a reduction in different Treg populations, especially CD4+CD25+Foxp3+ Tregs, contributes to the development of T1DM[30]. As a result, various immune cells infiltrate the islets, resulting in insulitis. Insulitis is an early pathologic hallmark of this autoimmune disorder and eventually causes the death of β-cells and a reduction in insulin[31]. In addition, it has been proposed that β-cells are not merely passive targets of autoimmune reactions but also contribute to the initiation of this complex autoimmune process[32,33].
At present, there are no widely accepted and validated diagnostic criteria for T1DM. Instead, its clinical diagnosis still mainly depends on two main features, including insulin deficiency as well as the presence of the corresponding autoantibodies. However, additional criteria are needed as the diagnostic accuracy of the above criteria in individuals who develop diabetes over the age of 20 years is less informative[34]. Once diagnosed, individuals with T1DM must rely on exogenous insulin for glycemic control to avoid ketoacidosis and hyperglycemia-related complications[35]. However, insulin therapy does not represent a cure and often fails to achieve optimal blood sugar management in many patients. Based on the understanding of its heterogeneity and early-stage development as described above, more personalized medicine approaches should be designed to diagnose, prevent, and hopefully treat T1DM[36,37]. However, as an autoimmune disease, the ultimate optimal goal of T1DM treatment is to restore immune tolerance toward disease-specific autoantigens to avoid autoimmune attack against β-cells. For this purpose, combination therapy based on antigen-specific immunotherapy exhibits promising prospects[38,39].
As the most prevalent organ-specific autoimmune disorder all over the world and the most prevalent pathological condition associated with the thyroid gland, AITD affects approximately 5% of the total world population[40]. Graves' disease (GD) and Hashimoto's thyroiditis (HT) represent its two main clinical manifestations. The incidence of HT in females and males is approximately 3.5/1000 and 0.6/1000, respectively, with a global prevalence of 2% to 3%. GD influences 1% to 2% of females and 0.1% to 0.2% of males[40]. In contrast to the male predominance in T1DM, AITD shows a strong female preponderance, which may result from the immune-enhancing activity provided by estrogenic sex steroids[41]. Thus, the reasons behind these sex differences in these autoimmune diseases deserve more attention and research in the future.
As a result of immune imbalance, tolerance toward thyroid-specific autoantigens, such as thyroglobulin (Tg), thyroperoxidase (TPO) as well as thyroid-stimulating hormone receptor (TSHR), lost, leading to an immune destruction of thyroid tissue, yielding AITD[40]. Autoreactive T and B lymphocyte infiltrates within the thyroid and the presence of antibodies targeting the above thyroid self-antigens (anti-Tg, anti-TPO, and anti-TSHR antibodies) can directly confirm that autoimmune reactions occurr in both GD and HT. Compared to those in GD, lymphocyte infiltrates in HT are more severe, and therefore, HT patients exhibit the destruction of thyroid follicles, leading to low thyroid function (hypothyroidism)[42]. However, as the production of TSHR-specific stimulating antibodies (TSAbs) is redundant in GD, thyrocyte proliferation, thyroid growth, and the production of thyroid hormones are induced, finally inducing hyperthyroidism[43]. Both diseases exhibit different clinical manifestations. However, HT and GD share similar immunogenetic mechanisms, and conversion between conditions can occur[44,45].
During the last two decades, major progress on the mechanisms underlying the development of AITD has been made based on extensive research. Generally, it is believed that a complicated interaction between genetic susceptibility and environmental risk factors, together with various epigenetic factors, contributes to the pathogenesis of AITD[40,42,43]. Among these factors, genetic factors predominate, as they account for 70% to 80% of the risk of developing thyroid autoimmunity based on twin/family studies. Environmental factors account for the remaining 20% to 30%[46,47]. The identification of genes associated with AITD susceptibility has contributed to a better understanding of disease-causing mechanisms and has indicated that the presence of the related genes exacerbates AITD risk[48]. The main known AITD susceptibility genes can be mechanistically divided into general immune-regulatory genes (such as HLA-DR3, CTLA-4, and PTPN22) as well as thyroid-specific genes, such as the genes encoding the corresponding autoantigens (Tg, TPO, and TSHR). In addition, various novel candidate risk genes for AITD, such as FCRL3 (FCReceptor-Like-3), SCGB3A2 (secretoglobin 3A2), and TNFR 2 (tumor necrosis factor receptor 2), have been described by GWAS and immunochip analysis[40,49]. As genetic factors play a major role in triggering AITD, individuals with family members who develop this disease exhibit a high risk of AITD. Therefore, to get a precise answer to the question asked by individuals with AITD “Will my daughter or my sister also get this disorder?”, the Thyroid Hormones Event Amsterdam (THEA) score was designed and applied for predicting AITD risk in healthy female subjects who had at least one relative with AITD based on the various baseline characteristics. This THEA score performs accurately and seems to be useful for young women of AITD families[50]. However, this THEA score still needs to be further validated externally.
In addition, for a given genetic risk factor in AITD, epigenetic modifications mediated by DNA methylation[51], histone modifications[52], and noncoding RNAs[53] may be necessary to trigger AITD. However, the promoting mechanism of such epigenetic modifications in AITD have not been fully elucidated, and therefore, more research should be done to further investigate their roles in AITD pathogenesis and to develop better diagnostic, prognostic, and therapeutic tools. Some environmental factors may induce corresponding epigenetic modifications, and subsequently trigger AITD in genetically susceptible individuals, indicating that epigenetic modifications seem to narrow this gap between genetic and environmental factors[54,55]. Several AITD-related environmental factors have been confirmed, such as iodine status, smoking, alcohol intake, selenium supplementation, vitamin D deficiency, infections, stress, and drugs[47]. Thus, preventive interventions, namely, the modulation of exposure to particular environmental risk factors, may diminish the corresponding risk in individuals at risk for developing AITD. However, there are few effective preventive interventions to diminish this risk, and these few options are not always feasible[47].
As a result of the interaction between the above various factors, the balance of immune homeostasis is disrupted, inducing a loss of tolerance toward thyroid-specific autoantigens and finally the onset of AITD[56]. Effector T cells and their secreted cytokines contribute greatly to the pathogenic development of HT and GD[57,58]. Traditionally, Th1/Th2 cell imbalance is viewed as the main driver of autoimmunity in AITD. Th1 cells may induce apoptotic pathways in thyroid follicular cells by secreting IFN-γ and IL-2, resulting in the destruction of thyroid cells. Th2 cells, which mainly produce IL-4, IL-5, and IL-13, may induce thyroid growth and overactivity by enhancing TSAbs release[59,60]. In addition, numerous recent studies have demonstrated the pathogenic functions of IL-17 and Th17 cells and Th17/Treg imbalance in both HT and GD[61]. This is important for future research to discover Th17-related therapeutic targets.
Accurately diagnosing GD or HT is important, and this mainly relies on the measurement of serum levels of thyroid stimulating hormone, free thyroid hormones (FT3, FT4) as well as the corresponding autoantibody levels. In addition, cytological examination, thyroid ultrasonography, and radiological evaluation may be needed in some cases[62,63]. If a definitive diagnosis was established, the most appropriate patient management decision could be made. For GD treatment, mainly including thyroidectomy, radioiodine therapy, antithyroid drugs, and β-blockers, there have been no major changes in recent years[62]. For HT treatment, oral administration of a synthetic hormone is used to control hypothyroidism. In addition, diet management is advised[63]. Although these available treatments are effective for HT and GD, there are still some limitations. Thyroid hormone substitution therapy in HT does not target the disease process[64]. Available treatments performed in GD may have the potential to cause some side effects[62,65]. Therefore, the clinical management of AITD remains an active area that requires further investigation, especially by improving understanding of its pathophysiology to discover therapeutic approaches targeting the underlying autoimmune process.
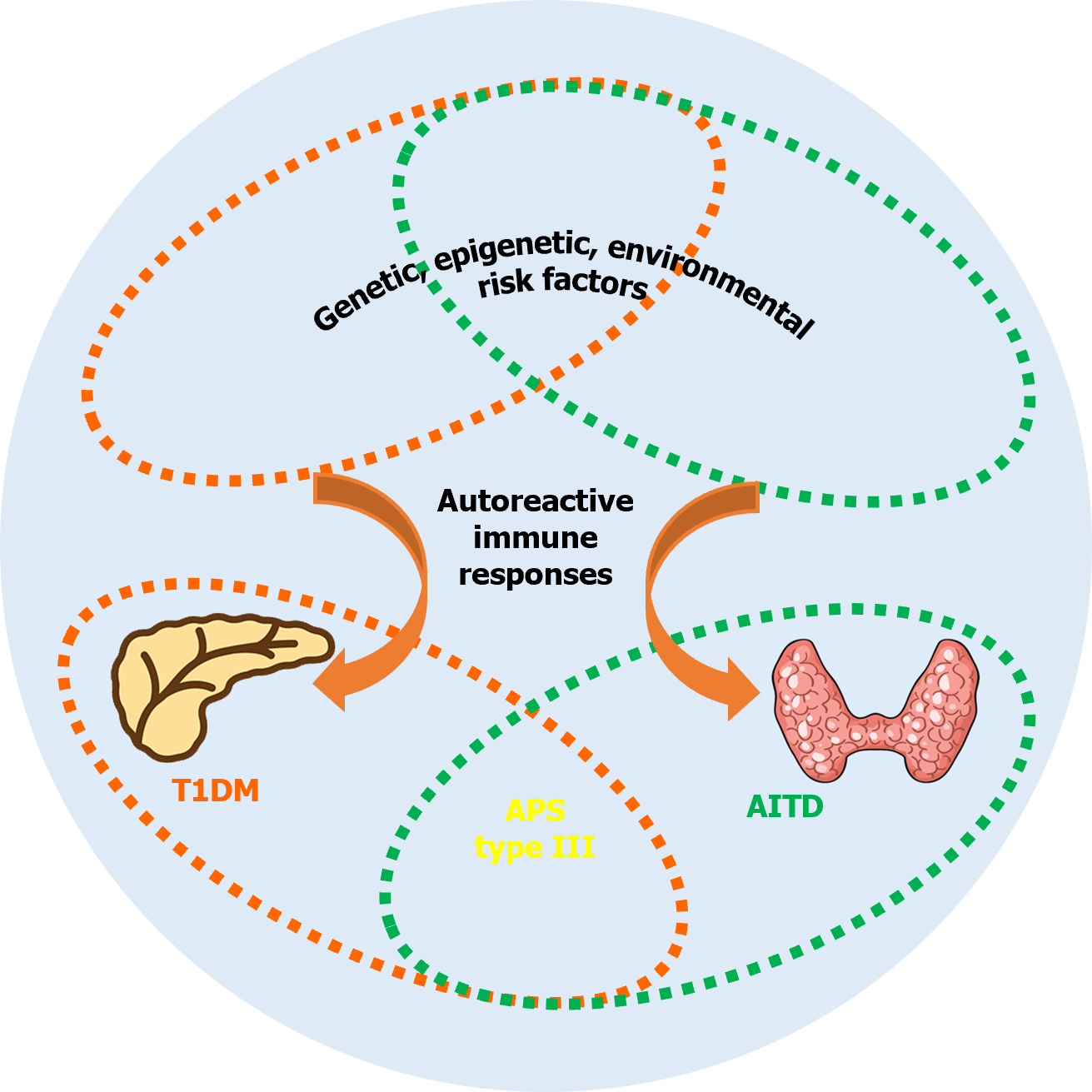
The occurrence of one autoimmune disorder enhances the risk for the development of others. Therefore, the coexistence of two or more autoimmune endocrinopathies is termed autoimmune polyendocrine syndrome (APS). However, sometimes there may be additional (non)glandular autoimmune disease(s) present[66]. There are two major types of APS, including juvenile type I and adult APS with three variants or subtypes (type II to IV)[66,67]. An economic evaluation of the costs for patients with APS in Germany has shown that T1DM is the main cost driver in APS[68]. APS type III, encompassing T1DM and AITD (HT or GD), is the most prevalent APS type, and it can often be associated with other (non)glandular autoimmune disorders, excluding Addison’s disease[69,70]. Various studies have observed a higher rate of thyroid disorder among T1DM patients compared with the general population, suggesting that AITD represents the most prevalent autoimmune disorder concomitant with T1DM[5,71,72]. Existing data show that approximately one-third of T1DM individuals develop AITD within a few years, and this proportion increases up to 50% in anti-TPO autoantibody-positive T1DM individuals. Additionally, the incidence of HT among T1DM individuals is relatively higher than that of GD[73,74]. Conversely, the prevalence of T1DM is also enhanced in patients with HT or GD, and the incidence of T1DM in HT individuals is relatively higher than that in GD individuals[75,76].
As described above, both T1DM and AITD are common organ-specific autoimmune disorders, and a complicated interaction between genetic factors and environmental stimuli, together with various immune events or epigenetic factors, induces the autoimmune process to destroy the target tissue (the β-cells in T1DM and the thyroid in AITD; Figure 1). While differences in the pathogenesis responsible for both disorders persist, the relatively high concomitant presence rate of T1DM and AITD in the same individual or family indicates that these two diseases may share pathogenic factors within the induction of the corresponding autoimmune process[77]. Various genes have been confirmed to contribute to the risk of both T1DM and AITD; these are referred to as joint susceptibility genes for APS type III (Figure 1)[73,77-79]. Among these susceptibility genes, HLA genes remain the most important contributor[73,77]. Based on the interaction with susceptibility genes, environmental factors are necessary to trigger autoimmune responses in both T1DM and AITD. It has been shown that infection (such as Helicobacter pylori infection), vitamin D deficiency, as well as multiple chemokine (C-X-C motif) ligands could confer susceptibility to both diseases[77]. Therefore, the combined influence of these susceptibility risk factors may stimulate the corresponding autoimmune processes in various organs of the same individual or in families (Figure 1). As there may be a rather long time interval between the first occurrence of one autoimmune endocrinopathy and the other, long-term monitoring and regular evaluation of patients and their relatives is warranted, such as the detection of associated autoantibodies[80] and thyroid ultrasound[81].
As it is noninvasive without known detrimental bioeffects and affordable, ultrasound has been widely applied in the clinic for decades. Low-resolution B-mode ultrasound was first introduced for thyroid imaging in 1967[82], and ultrasonography is currently considered crucial in the diagnosis and management of thyroid disorders, including AITD[81,83].
As mentioned above, the cellular and humoral immunity involved in the development of HT results in morphologic and microscopic changes in thyroid tissue, such as thyroid enlargement, lymphoplasmacytic infiltration, fibroplastic proliferation, lymphatic follicular formation, calcification, vascular proliferation, and parenchymal atrophy[63]. These changes influence the ultrasonographic characteristics of HT. Generally, a moderate grayscale uniform echo image, with a higher signal compared to the surrounding muscles, can be observed in the structurally normal thyroid. As a result of thyroid infiltration in HT, a heterogeneously hypoechoic thyroid can be observed, and thus, this hypoechogenicity can be used for clarifying diagnosis[84,85]. In addition, pseudonodules and inhomogeneous parenchyma can also be observed, which could be due to fibroplastic proliferation[86].
However, the sonographic appearances detected in HT vary greatly and may be indistinguishable from other thyroid disorders[87,88]. Therefore, in some atypical cases, multiple sonographic characteristics obtained from various ultrasound imaging technologies should be considered. The vascularity type of “focal inferno” observed by color Doppler ultrasound is a characteristic of focal HT, which is a special form of HT, and this is crucial to determine the corresponding treatment strategy[89]. In anti-TPO autoantibody-positive euthyroid subjects, comprehensive parameters obtained by ultrasound and power Doppler ultrasound exhibited a diagnostic accuracy of 87.2%, sensitivity of 90%, specificity of 84.8%, negative predictive value (NPV) of 90.7%, and positive predictive value (PPV) of 83.7% for the diagnosis of HT[90]. The cutoff value for thyroid tissue elasticity obtained from real-time ultrasound elastography for diagnosing HT showed 96% sensitivity and 67% specificity in adults[91], as well as 97.4% sensitivity and 100% specificity in children[92]. Based on ultrasound 2D shear-wave elastography, thyroid stiffness measured by shear-wave dispersion performed somewhat better in diagnosing HT than thyroid viscosity measured by shear-wave dispersion[93]. Compared with conventional ultrasound examination, high-frequency ultrasonic elastography exhibited a significantly higher diagnostic accuracy of HT (sensitivity, 92.16%; specificity, 92.86%; NPV, 86.67%; PPV, 95.92%)[94]. A recent meta-analysis indicated that ultrasound-based shear wave elastography plays an important role and should be encouraged for use in diagnosing pediatric HT[95].
In addition, ultrasound acquisition and interpretation are highly subjective and somewhat operator dependent, even irreproducible in some cases[96]. To avoid subjective differences, a computer-assisted diagnostic system based on feature extraction and classification as well as a machine learning algorithm was proposed to provide objective and reproducible interpretation results in the diagnosis of HT, yielding a diagnostic accuracy of 80%[97], 85%[98], and 79%[99]. Recently, artificial intelligence (AI)-aided diagnosis of thyroid disorders has attracted growing interest[100,101]. A convolutional neural network-based computer-aided HT diagnostic system was evaluated and validated in a large number of samples, including 39280 ultrasonic images from 21110 individuals. The results show that this strategy significantly improved the radiologists’ diagnostic efficiency of HT, as it exhibited high performance (89.2% accuracy, 89% sensitivity, and 89.5% specificity)[102]. A later report in 2022 developed a deep learning-based diagnostic system for HT (HTNet) through training and testing in a larger number of samples, and HTNet significantly exceeded the performance of radiologists in terms of accuracy and sensitivity. The corresponding diagnostic performance of HTNet can be further improved by integrating serologic markers[103]. Therefore, these computer-assisted ultrasound diagnostic systems based on novel AI show promising prospects in HT management and thus could be tested in prospective clinical trials.
Cervical lymph nodes (CLNs) are often observed in HT patients[104]. Fine needle aspiration biopsy (FNAB), an invasive intervention, has been regarded as the gold standard to diagnose, differentiate, and recognize CLNs as true nodules or pseudonodules[105]. To avoid the use of unnecessary invasive biopsies, sonoelastography should be applied, as it can detect true thyroid nodules (TNs) with a similar accuracy and sensitivity to FNAB[106]. An enhanced number of enlarged CLNs without a significant increase in lymph node size was observed on the sonographic images of HT patients[107], and an enhanced frequency of CLNs with abnormal ultrasonographic characteristics has been observed in HT patients[108]. Therefore, further understanding of the sonographic characteristics of CLNs in HT patients may be useful to improve the diagnosis of HT and avoid unnecessary invasive tests.
In addition, TNs can be frequently detected among HT patients, and these nodules often exhibit poor uptake of radioisotopes, indicating the possibility of malignancy and suggesting a possible association between HT and thyroid cancer[109,110]. However, whether HT increases thyroid cancer risk in individuals with TNs is controversial and remains to be defined[111,112]. Therefore, to avoid overtreatment with surgery in HT patients with TNs without any other evidence of malignancy as well as to predict the malignancy risk of these TNs accurately, various ultrasound-based diagnostic classification systems, which have been developed for differentiating benign and malignant TNs, may represent a critical role in detecting malignant TNs in HT individuals[113-116]. Moreover, in some cases with difficult diagnoses, ultrasound-guided FNAB can be used as an effective, less-invasive approach to confirm the nature of the lesion and propose the most beneficial/optimal treatment[117,118]. For the treatment of benign TNs in HT patients, ultrasound-guided microwave ablation shows a promising trend[119].
As described above, autoantibodies against TSHR (TSAbs) drive GD pathogenesis. However, the role of TSAbs in GD is different from that of autoantibodies causing tissue damage in many other autoimmune disorders. TSAbs stimulate the thyroid and increase the production and secretion of thyroid hormones, therefore causing goiter and hyperthyroidism[62]. Apart from clinical presentations and laboratory findings, Doppler ultrasound measuring thyroid blood flow is widely applied in diagnosing GD[120]. However, it should be noted that the application of ultrasound in GD management, which mainly focuses on academic interest, has not gained much clinical importance thus far compared with that of some other thyroid disorders, such as thyroid cancer[81,121].
Features of an increased thyroid gland volume, diffusely low thyroid echogenicity as well as hypervascularity have been shown in GD[121,122]. At variance with the hypoechogenicity resulting from diffuse lymphocytic infiltration in HT as described above, the hypoechogenic pattern observed in GD may result from decreased colloid content with enhanced cellularity and a decrease in the cell-colloid interface and/or from enhanced blood flow[122,123]. Alternatively, it can be said that hypoechogenicity is not specific for HT, as it can also be observed in GD. Therefore, it has been shown that conventional grayscale ultrasound exhibits a high specificity with low sensitivity in diagnosing and differentiating GD and HT, and it is difficult to differentiate between both disorders using conventional grayscale ultrasound alone as a result of those significant overlaps in ultrasonographic images[124].
During the late 20th/early 21st centuries, Doppler ultrasonography, including color Doppler and power Doppler, has been widely studied to diagnose, evaluate, and manage GD, and the characteristic intense Doppler flow referred to as the “thyroid inferno” pattern has been well defined in this disease, yielding a high specificity in differentiating GD from other triggers of hyperthyroidism[125-130]. However, at that time, little effort was made to emphasize the role of Doppler ultrasonography in GD, leading to its underutilization in diagnosing this thyroid disorder. Therefore, a call to include an ultrasound protocol with Doppler patterns in the clinical diagnosis of GD was raised in 2009[131]. Since then, various methods based on Doppler ultrasonography have been widely and further investigated for their roles in GD management. The diagnostic utility of the peak systolic and/or end-diastolic velocities (PSV and EDV) in the superior and/or inferior thyroid artery measured by color Doppler ultrasonography is comparable to the performance of TSAb and Tc-99m pertechnetate uptake to differentiate GD from painless (or silent) thyroiditis[132,133]. Compared to EDV, PSV is a more useful parameter in differentiating GD from HT[88]. Although thyroid ultrasound is less accurate than both autoantibody immunoassays and thyroid scintigraphy in diagnostic testing for Graves’ or non-Graves’ hyper-thyroidism, the “thyroid inferno” pattern shows a high PPV toward GD[134]. However, as these methods are highly operator-dependent and subjective, the interobserver variability as well as the difficulty in quantifying the corresponding results objectively remain their major limitations. Therefore, a newly developed analysis software that can quantify color Doppler signals, entitled “Color Quantification” (CQ), has been introduced. The results show that the increased CQ values help diagnose GD, and therefore, the CQ technique exhibits promise in diagnosing GD[135]. In addition, a new-generation Doppler designed for improving diagnostic sensitivity, microvascular ultrasonography, has also been tested regarding its ability in the differential diagnosis of GD and HT[136] or destructive thyroiditis[137] in a quantitative and real-time manner with low intra- or inter-observer variability. In addition, some tests analyzing the ability of shear-wave elastography in diagnosing GD show that it can be applied as a complementary technique to facilitate the diagnosis of GD[138] or the differential diagnosis of GD and HT[139].
Apart from the diagnosis or differential diagnosis of GD, ultrasound contributes a lot to treat and manage this thyroid disorder. The sonographic appearance of the thyroid gland can be used to classify GD into different clinical courses and autoimmune activities[140-142]. Therefore, color pixel density calculated based on the color-flow maps obtained with color duplex ultrasonography can be used to evaluate the optimal dose of antithyroid drugs to maintain euthyroid status in GD[143]. In addition, it is important to predict outcome in GD patients after drug withdrawal. Thus, color Doppler ultrasonography may be a useful tool to detect a relapsing course of hyperthyroidism and, therefore, facilitate the offering of an adequate therapeutic approach[126,128]. As described above, thyroidectomy may need to be performed in some GD patients, and preoperative color Doppler sonography evaluating the superior thyroid artery may be useful to identify those individuals who are more prone to bleeding intraoperatively[144]. Concurrent differentiated thyroid cancer occurs in pediatric GD patients, and it has been suggested that ultrasound examination should be included for those with an abnormal thyroid at palpation to select patients for appropriate definitive therapy, such as thyroidectomy[145,146]. In addition, surgery and radioactive iodine (RAI) therapy are recommended for individuals with persistent/relapsed GD[147]. However, many patients may not want to accept surgery or RAI therapy as a result of the possible risks from surgery and radiation[148,149]. Therefore, one preliminary study applied and evaluated ultrasound-guided high-intensity focused ultrasound ablation as a novel manner to treat medically refractory GD, and the results show that this strategy may be a safe and efficacious method for treating persistent/relapsed GD[150]. This usefulness was confirmed based on the outcomes (specifically, disease relapse and safety) over the two years of follow-up[151].
T1DM and AITD (HT and GD) represent the two most frequent autoimmune endocrine disorders. Accumulating evidence indicates that T1DM and AITD share similar immunogenetic susceptibilities; therefore, both diseases often cluster in individuals as well as families. AITD has been the most prevalent comorbid autoimmune disease of T1DM. Thus, a timely and accurate diagnosis of AITD in T1DM patients is particularly crucial for diabetes management. For this purpose, thyroid ultrasonography exhibits a critical role in the diagnosis and management of AITD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aslam M, India; Saha S, India; Shao JQ, China S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, Castell C, Schoenle EJ, Bingley PJ, Dahlquist G, Jarosz-Chobot PK, Marčiulionytė D, Roche EF, Rothe U, Bratina N, Ionescu-Tirgoviste C, Weets I, Kocova M, Cherubini V, Rojnic Putarek N, deBeaufort CE, Samardzic M, Green A. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 2. | Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, Ogle GD. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 330] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 3. | Green A, Hede SM, Patterson CC, Wild SH, Imperatore G, Roglic G, Beran D. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. 2021;64:2741-2750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Quattrin T, Mastrandrea LD, Walker LSK. Type 1 diabetes. Lancet. 2023;401:2149-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 5. | Nederstigt C, Uitbeijerse BS, Janssen LGM, Corssmit EPM, de Koning EJP, Dekkers OM. Associated auto-immune disease in type 1 diabetes patients: a systematic review and meta-analysis. Eur J Endocrinol. 2019;180:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 6. | Gimenez-Perez G, Vlacho B, Navas E, Mata-Cases M, Real J, Cos X, Franch-Nadal J, Mauricio D. Comorbid autoimmune diseases and burden of diabetes-related complications in patients with type 1 diabetes from a Mediterranean area. Diabetes Res Clin Pract. 2022;191:110031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Araujo DB, Barone B, Melleti NF, Dantas JR, Oliveira MM, Zajdenverg L, Tortora RP, Vaisman M, Milech A, Oliveira JE, Rodacki M. Thyroid disorders are common in first-degree relatives of individuals with type 1 diabetes mellitus. Arch Endocrinol Metab. 2015;59:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 751] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 10. | Lantz M, Almquist M, Koutouridou E, Pellby D, Planck T, Tsoumani K, Mijovic Z. [Thyroid ultrasound and its role in the investigation of thyroid disease]. Lakartidningen. 2022;119. [PubMed] |
| 11. | Vanderniet JA, Jenkins AJ, Donaghue KC. Epidemiology of Type 1 Diabetes. Curr Cardiol Rep. 2022;24:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 12. | Ostman J, Lönnberg G, Arnqvist HJ, Blohmé G, Bolinder J, Ekbom Schnell A, Eriksson JW, Gudbjörnsdottir S, Sundkvist G, Nyström L. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med. 2008;263:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 942] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 14. | Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 15. | Klak M, Gomółka M, Kowalska P, Cichoń J, Ambrożkiewicz F, Serwańska-Świętek M, Berman A, Wszoła M. Type 1 diabetes: genes associated with disease development. Cent Eur J Immunol. 2020;45:439-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Redondo MJ, Geyer S, Steck AK, Sharp S, Wentworth JM, Weedon MN, Antinozzi P, Sosenko J, Atkinson M, Pugliese A, Oram RA; Type 1 Diabetes TrialNet Study Group. A Type 1 Diabetes Genetic Risk Score Predicts Progression of Islet Autoimmunity and Development of Type 1 Diabetes in Individuals at Risk. Diabetes Care. 2018;41:1887-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15:635-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 18. | Zajec A, Trebušak Podkrajšek K, Tesovnik T, Šket R, Čugalj Kern B, Jenko Bizjan B, Šmigoc Schweiger D, Battelino T, Kovač J. Pathogenesis of Type 1 Diabetes: Established Facts and New Insights. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 19. | Del Chierico F, Rapini N, Deodati A, Matteoli MC, Cianfarani S, Putignani L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 20. | Dedrick S, Sundaresh B, Huang Q, Brady C, Yoo T, Cronin C, Rudnicki C, Flood M, Momeni B, Ludvigsson J, Altindis E. The Role of Gut Microbiota and Environmental Factors in Type 1 Diabetes Pathogenesis. Front Endocrinol (Lausanne). 2020;11:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 447] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 22. | Zorena K, Michalska M, Kurpas M, Jaskulak M, Murawska A, Rostami S. Environmental Factors and the Risk of Developing Type 1 Diabetes-Old Disease and New Data. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Lampasona V, Liberati D. Islet Autoantibodies. Curr Diab Rep. 2016;16:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 695] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 25. | Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Primavera M, Giannini C, Chiarelli F. Prediction and Prevention of Type 1 Diabetes. Front Endocrinol (Lausanne). 2020;11:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Anderson AM, Landry LG, Alkanani AA, Pyle L, Powers AC, Atkinson MA, Mathews CE, Roep BO, Michels AW, Nakayama M. Human islet T cells are highly reactive to preproinsulin in type 1 diabetes. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, Hering BJ, Hafler DA. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 29. | Rajendeeran A, Tenbrock K. Regulatory T cell function in autoimmune disease. J Transl Autoimmun. 2021;4:100130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 30. | Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes: what's broken and how can we fix it? Diabetologia. 2017;60:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Pugliese A. Insulitis in the pathogenesis of type 1 diabetes. Pediatr Diabetes. 2016;17 Suppl 22:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Erdem N, Montero E, Roep BO. Breaking and restoring immune tolerance to pancreatic beta-cells in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2021;28:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 499] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 34. | Powers AC. Type 1 diabetes mellitus: much progress, many opportunities. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 35. | Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 36. | Akil AA, Yassin E, Al-Maraghi A, Aliyev E, Al-Malki K, Fakhro KA. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med. 2021;19:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 37. | Mameli C, Triolo TM, Chiarelli F, Rewers M, Zuccotti G, Simmons KM. Lessons and gaps in the prediction and prevention of type 1 diabetes. Pharmacol Res. 2023;193:106792. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Wesley JD, Pagni PP, Bergholdt R, Kreiner FF, von Herrath M. Induction of antigenic immune tolerance to delay type 1 diabetes - challenges for clinical translation. Curr Opin Endocrinol Diabetes Obes. 2022;29:379-385. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Chuzho N, Mishra N, Tandon N, Kumar N. Therapies for Type 1 Diabetes: Is a Cure Possible? Curr Diabetes Rev. 2023;19:e021222211565. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 580] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 41. | Grossman CJ, Roselle GA, Mendenhall CL. Sex steroid regulation of autoimmunity. J Steroid Biochem Mol Biol. 1991;40:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, Churilov LP, Ferrari SM, Antonelli A. Hashimotos' thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019;33:101367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 322] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 43. | Antonelli A, Ferrari SM, Ragusa F, Elia G, Paparo SR, Ruffilli I, Patrizio A, Giusti C, Gonnella D, Cristaudo A, Foddis R, Shoenfeld Y, Fallahi P. Graves' disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract Res Clin Endocrinol Metab. 2020;34:101387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 44. | Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of autoimmune thyroid diseases: A comprehensive review. J Autoimmun. 2015;64:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 45. | Daramjav N, Takagi J, Iwayama H, Uchino K, Inukai D, Otake K, Ogawa T, Takami A. Autoimmune Thyroiditis Shifting from Hashimoto's Thyroiditis to Graves' Disease. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 46. | Saranac L, Zivanovic S, Bjelakovic B, Stamenkovic H, Novak M, Kamenov B. Why is the thyroid so prone to autoimmune disease? Horm Res Paediatr. 2011;75:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Wiersinga WM. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinol Metab (Seoul). 2016;31:213-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 48. | Hwangbo Y, Park YJ. Genome-Wide Association Studies of Autoimmune Thyroid Diseases, Thyroid Function, and Thyroid Cancer. Endocrinol Metab (Seoul). 2018;33:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Vargas-Uricoechea H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 50. | Strieder TG, Tijssen JG, Wenzel BE, Endert E, Wiersinga WM. Prediction of progression to overt hypothyroidism or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Intern Med. 2008;168:1657-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Lafontaine N, Wilson SG, Walsh JP. DNA Methylation in Autoimmune Thyroid Disease. J Clin Endocrinol Metab. 2023;108:604-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 52. | Yan N, Mu K, An XF, Li L, Qin Q, Song RH, Yao QM, Shao XQ, Zhang JA. Aberrant Histone Methylation in Patients with Graves' Disease. Int J Endocrinol. 2019;2019:1454617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Martínez-Hernández R, Marazuela M. MicroRNAs in autoimmune thyroid diseases and their role as biomarkers. Best Pract Res Clin Endocrinol Metab. 2023;37:101741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Wang B, Shao X, Song R, Xu D, Zhang JA. The Emerging Role of Epigenetics in Autoimmune Thyroid Diseases. Front Immunol. 2017;8:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Coppedè F. Epigenetics and Autoimmune Thyroid Diseases. Front Endocrinol (Lausanne). 2017;8:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | McLachlan SM, Rapoport B. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev. 2014;35:59-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 57. | Janyga S, Marek B, Kajdaniuk D, Ogrodowczyk-Bobik M, Urbanek A, Bułdak Ł. CD4+ cells in autoimmune thyroid disease. Endokrynol Pol. 2021;72:572-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Li Q, Wang B, Mu K, Zhang JA. The pathogenesis of thyroid autoimmune diseases: New T lymphocytes - Cytokines circuits beyond the Th1-Th2 paradigm. J Cell Physiol. 2019;234:2204-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 59. | McIver B, Morris JC. The pathogenesis of Graves' disease. Endocrinol Metab Clin North Am. 1998;27:73-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Ramos-Leví AM, Marazuela M. Pathogenesis of thyroid autoimmune disease: the role of cellular mechanisms. Endocrinol Nutr. 2016;63:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Wang Y, Fang S, Zhou H. Pathogenic role of Th17 cells in autoimmune thyroid disease and their underlying mechanisms. Best Pract Res Clin Endocrinol Metab. 2023;37:101743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 62. | Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, Eckstein AK, Stagnaro-Green A, Kahaly GJ. Graves' disease. Nat Rev Dis Primers. 2020;6:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 63. | Ralli M, Angeletti D, Fiore M, D'Aguanno V, Lambiase A, Artico M, de Vincentiis M, Greco A. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19:102649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 64. | Topliss DJ. Clinical Update in Aspects of the Management of Autoimmune Thyroid Diseases. Endocrinol Metab (Seoul). 2016;31:493-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Azizi F, Abdi H, Amouzegar A, Habibi Moeini AS. Long-term thionamide antithyroid treatment of Graves' disease. Best Pract Res Clin Endocrinol Metab. 2023;37:101631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Frommer L, Kahaly GJ. Autoimmune Polyendocrinopathy. J Clin Endocrinol Metab. 2019;104:4769-4782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Kahaly GJ, Frommer L. Polyglandular autoimmune syndromes. J Endocrinol Invest. 2018;41:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 68. | Radermacher LK, Ponto K, Merkesdal S, Pomart V, Frommer L, Pfeiffer N, König J, Kahaly GJ. Type I Diabetes is the Main Cost Driver in Autoimmune Polyendocrinopathy. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Horie I, Kawasaki E, Ando T, Kuwahara H, Abiru N, Usa T, Yamasaki H, Ejima E, Kawakami A. Clinical and genetic characteristics of autoimmune polyglandular syndrome type 3 variant in the Japanese population. J Clin Endocrinol Metab. 2012;97:E1043-E1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Hansen MP, Matheis N, Kahaly GJ. Type 1 diabetes and polyglandular autoimmune syndrome: A review. World J Diabetes. 2015;6:67-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Schloot NC, Pham MN, Hawa MI, Pozzilli P, Scherbaum WA, Schott M, Kolb H, Hunter S, Schernthaner G, Thivolet C, Seissler J, Leslie RD; Action LADA Group. Inverse Relationship Between Organ-Specific Autoantibodies and Systemic Immune Mediators in Type 1 Diabetes and Type 2 Diabetes: Action LADA 11. Diabetes Care. 2016;39:1932-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Biondi B, Kahaly GJ, Robertson RP. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr Rev. 2019;40:789-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 287] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 73. | Frommer L, Kahaly GJ. Type 1 Diabetes and Autoimmune Thyroid Disease-The Genetic Link. Front Endocrinol (Lausanne). 2021;12:618213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 74. | Riquetto ADC, de Noronha RM, Matsuo EM, Ishida EJ, Vaidergorn RE, Soares Filho MD, Calliari LEP. Thyroid function and autoimmunity in children and adolescents with Type 1 Diabetes Mellitus. Diabetes Res Clin Pract. 2015;110:e9-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Sharma H, Sahlot R, Purwar N, Garg U, Saran S, Sharma B, Mathur SK. Co-existence of type 1 diabetes and other autoimmune ailments in subjects with autoimmune thyroid disorders. Diabetes Metab Syndr. 2022;16:102405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Ruggeri RM, Trimarchi F, Giuffrida G, Certo R, Cama E, Campennì A, Alibrandi A, De Luca F, Wasniewska M. Autoimmune comorbidities in Hashimoto's thyroiditis: different patterns of association in adulthood and childhood/adolescence. Eur J Endocrinol. 2017;176:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 77. | Li L, Liu S, Yu J. Autoimmune thyroid disease and type 1 diabetes mellitus: same pathogenesis; new perspective? Ther Adv Endocrinol Metab. 2020;11:2042018820958329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Dittmar M, Kahaly GJ. Immunoregulatory and susceptibility genes in thyroid and polyglandular autoimmunity. Thyroid. 2005;15:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Levin L, Tomer Y. The etiology of autoimmune diabetes and thyroiditis: evidence for common genetic susceptibility. Autoimmun Rev. 2003;2:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Nelson HA, Joshi HR, Straseski JA. Mistaken Identity: The Role of Autoantibodies in Endocrine Disease. J Appl Lab Med. 2022;7:206-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Levine RA. History of Thyroid Ultrasound. Thyroid. 2023;33:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 82. | Fujimoto Y, Oka A, Omoto R, Hirose M. Ultrasound scanning of the thyroid gland as a new diagnostic approach. Ultrasonics. 1967;5:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 40] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Ruchała M, Szmyt K, Sławek S, Zybek A, Szczepanek-Parulska E. Ultrasound sonoelastography in the evaluation of thyroiditis and autoimmune thyroid disease. Endokrynol Pol. 2014;65:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Zantour B, Sfar MH, Alaya W, Chebbi W, Chatti K, Jerbi S. Hashimoto's thyroiditis and severe hypothyroidism, associated with a single hot nodule. Rev Esp Med Nucl. 2011;30:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Mazziotti G, Sorvillo F, Iorio S, Carbone A, Romeo A, Piscopo M, Capuano S, Capuano E, Amato G, Carella C. Grey-scale analysis allows a quantitative evaluation of thyroid echogenicity in the patients with Hashimoto's thyroiditis. Clin Endocrinol (Oxf). 2003;59:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Wu G, Zou D, Cai H, Liu Y. Ultrasonography in the diagnosis of Hashimoto's thyroiditis. Front Biosci (Landmark Ed). 2016;21:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Anderson L, Middleton WD, Teefey SA, Reading CC, Langer JE, Desser T, Szabunio MM, Hildebolt CF, Mandel SJ, Cronan JJ. Hashimoto thyroiditis: Part 1, sonographic analysis of the nodular form of Hashimoto thyroiditis. AJR Am J Roentgenol. 2010;195:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 88. | Liu Y, Liu X, Wu N. A Review of Testing for Distinguishing Hashimoto's Thyroiditis in the Hyperthyroid Stage and Grave's Disease. Int J Gen Med. 2023;16:2355-2363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Fu X, Guo L, Zhang H, Ran W, Fu P, Li Z, Chen W, Jiang L, Wang J, Jia J. "Focal thyroid inferno" on color Doppler ultrasonography: a specific feature of focal Hashimoto's thyroiditis. Eur J Radiol. 2012;81:3319-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Ceylan I, Yener S, Bayraktar F, Secil M. Roles of ultrasound and power Doppler ultrasound for diagnosis of Hashimoto thyroiditis in anti-thyroid marker-positive euthyroid subjects. Quant Imaging Med Surg. 2014;4:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 91. | Menzilcioglu MS, Duymus M, Gungor G, Citil S, Sahin T, Boysan SN, Sarica A. The value of real-time ultrasound elastography in chronic autoimmune thyroiditis. Br J Radiol. 2014;87:20140604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Bakırtaş Palabıyık F, İnci E, Papatya Çakır ED, Hocaoğlu E. Evaluation of Normal Thyroid Tissue and Autoimmune Thyroiditis in Children Using Shear Wave Elastography. J Clin Res Pediatr Endocrinol. 2019;11:132-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Stoian D, Borlea A, Sporea I, Popa A, Moisa-Luca L, Popescu A. Assessment of Thyroid Stiffness and Viscosity in Autoimmune Thyroiditis Using Novel Ultrasound-Based Techniques. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 94. | Wang R, Yu Z, Li J, Gao Z, Xu Z, Liu Z. High-frequency ultrasound elastography improves the effect of determining the nature of lesions during the diagnosis of Hashimoto's thyroiditis and thyroid cancer. Minerva Med. 2023;114:267-269. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 95. | Decker T, Schnittka E, Stolzenberg L, Yalowitz J. Shear-Wave Elastography for the Diagnosis of Pediatric Hashimoto's Thyroiditis: A Systematic Review and Meta-Analysis. Cureus. 2023;15:e35490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 96. | Kim I, Kim EK, Yoon JH, Han KH, Son EJ, Moon HJ, Kwak JY. Diagnostic role of conventional ultrasonography and shearwave elastography in asymptomatic patients with diffuse thyroid disease: initial experience with 57 patients. Yonsei Med J. 2014;55:247-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Acharya UR, Vinitha Sree S, Mookiah MR, Yantri R, Molinari F, Zieleźnik W, Małyszek-Tumidajewicz J, Stępień B, Bardales RH, Witkowska A, Suri JS. Diagnosis of Hashimoto's thyroiditis in ultrasound using tissue characterization and pixel classification. Proc Inst Mech Eng H. 2013;227:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 98. | Acharya UR, Sree SV, Krishnan MM, Molinari F, Zieleźnik W, Bardales RH, Witkowska A, Suri JS. Computer-aided diagnostic system for detection of Hashimoto thyroiditis on ultrasound images from a Polish population. J Ultrasound Med. 2014;33:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Kim GR, Kim EK, Kim SJ, Ha EJ, Yoo J, Lee HS, Hong JH, Yoon JH, Moon HJ, Kwak JY. Evaluation of Underlying Lymphocytic Thyroiditis With Histogram Analysis Using Grayscale Ultrasound Images. J Ultrasound Med. 2016;35:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Aversano L, Bernardi ML, Cimitile M, Maiellaro A, Pecori R. A systematic review on artificial intelligence techniques for detecting thyroid diseases. PeerJ Comput Sci. 2023;9:e1394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 101. | Cao CL, Li QL, Tong J, Shi LN, Li WX, Xu Y, Cheng J, Du TT, Li J, Cui XW. Artificial intelligence in thyroid ultrasound. Front Oncol. 2023;13:1060702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 102. | Zhao W, Kang Q, Qian F, Li K, Zhu J, Ma B. Convolutional Neural Network-Based Computer-Assisted Diagnosis of Hashimoto's Thyroiditis on Ultrasound. J Clin Endocrinol Metab. 2022;107:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 103. | Zhang Q, Zhang S, Pan Y, Sun L, Li J, Qiao Y, Zhao J, Wang X, Feng Y, Zhao Y, Zheng Z, Yang X, Liu L, Qin C, Zhao K, Liu X, Li C, Zhang L, Yang C, Zhuo N, Zhang H, Liu J, Gao J, Di X, Meng F, Wang Y, Duan Y, Shen H, Li Y, Yang M, Yang Y, Xin X, Wei X, Zhou X, Jin R, Song F, Zheng X, Gao M, Chen K, Li X. Deep learning to diagnose Hashimoto's thyroiditis from sonographic images. Nat Commun. 2022;13:3759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 104. | Sahlmann CO, Meller J, Siggelkow H, Homayounfar K, Ozerden M, Braune I, Kluge G, Meller B. Patients with autoimmune thyroiditis. Prevalence of benign lymphadenopathy. Nuklearmedizin. 2012;51:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Mittendorf EA, Tamarkin SW, McHenry CR. The results of ultrasound-guided fine-needle aspiration biopsy for evaluation of nodular thyroid disease. Surgery. 2002;132:648-53; discussion 653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 106. | Yildirim D, Gurses B, Gurpinar B, Ekci B, Colakoglu B, Kaur A. Nodule or pseudonodule? Differentiation in Hashimoto's thyroiditis with sonoelastography. J Int Med Res. 2011;39:2360-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 107. | Jones MR, Mohamed H, Catlin J, April D, Al-Qurayshi Z, Kandil E. The presentation of lymph nodes in Hashimoto's thyroiditis on ultrasound. Gland Surg. 2015;4:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 108. | Lyu GR, Zheng WK, Lin WL, Zheng LP, Guo HX, Li LY. Sonographic Features of Cervical Lymph Nodes in Patients With Hashimoto Thyroiditis and the Impacts From the Levothyroxine With Prednisone Therapy. Ultrasound Q. 2018;34:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Del Rio P, Montana Montana C, Cozzani F, Rossini M, Loderer T, Dall'Aglio E, Cataldo S, Marina M, Graziano C. Is there a correlation between thyroiditis and thyroid cancer? Endocrine. 2019;66:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Noureldine SI, Tufano RP. Association of Hashimoto's thyroiditis and thyroid cancer. Curr Opin Oncol. 2015;27:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 111. | Keefe G, Culbreath K, Cherella CE, Smith JR, Zendejas B, Shamberger RC, Richman DM, Hollowell ML, Modi BP, Wassner AJ. Autoimmune Thyroiditis and Risk of Malignancy in Children with Thyroid Nodules. Thyroid. 2022;32:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Kassi GN, Evangelopoulou CC, Papapostolou KD, Karga HJ. Benign and malignant thyroid nodules with autoimmune thyroiditis. Arch Endocrinol Metab. 2022;66:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 113. | Wang D, Du LY, Sun JW, Hou XJ, Wang H, Wu JQ, Zhou XL. Evaluation of thyroid nodules with coexistent Hashimoto's thyroiditis according to various ultrasound-based risk stratification systems: A retrospective research. Eur J Radiol. 2020;131:109059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 114. | Peng Q, Niu C, Zhang M, Peng Q, Chen S. Sonographic Characteristics of Papillary Thyroid Carcinoma with Coexistent Hashimoto's Thyroiditis: Conventional Ultrasound, Acoustic Radiation Force Impulse Imaging and Contrast-Enhanced Ultrasound. Ultrasound Med Biol. 2019;45:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Zhao T, Xu S, Zhang X, Xu C. Comparison of Various Ultrasound-Based Malignant Risk Stratification Systems on an Occasion for Assessing Thyroid Nodules in Hashimoto's Thyroiditis. Int J Gen Med. 2023;16:599-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 116. | Wang B, Ou X, Yang J, Zhang H, Cui XW, Dietrich CF, Yi AJ. Contrast-enhanced ultrasound and shear wave elastography in the diagnosis of ACR TI-RADS 4 and 5 category thyroid nodules coexisting with Hashimoto's thyroiditis. Front Oncol. 2022;12:1022305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | Todsen T, Bennedbaek FN, Kiss K, Hegedüs L. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Head Neck. 2021;43:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Tarigan TJE, Anwar BS, Sinto R, Wisnu W. Diagnostic accuracy of palpation versus ultrasound-guided fine needle aspiration biopsy for diagnosis of malignancy in thyroid nodules: a systematic review and meta-analysis. BMC Endocr Disord. 2022;22:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 119. | Chen Y, Liu W, Jin C, Xu X, Xu L, Lu J, Zheng J, Sun X, Feng J, Chen S, Li Z, Gong X. Ultrasound-guided microwave ablation for benign thyroid nodules results in earlier and faster nodule shrinkage in patients with Hashimoto's thyroiditis than in those with normal thyroid function. Front Surg. 2023;10:1077077. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 120. | Committee on Pharmaceutical Affairs; Japanese Society for Pediatric Endocrinology; and the Pediatric Thyroid Disease Committee; Japan Thyroid Association (Taskforce for the Revision of the Guidelines for the Treatment of Childhood-Onset Graves’ Disease); Minamitani K, Sato H, Ohye H, Harada S, Arisaka O. Guidelines for the treatment of childhood-onset Graves' disease in Japan, 2016. Clin Pediatr Endocrinol. 2017;26:29-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | English C, Casey R, Bell M, Bergin D, Murphy J. The Sonographic Features of the Thyroid Gland After Treatment with Radioiodine Therapy in Patients with Graves' Disease. Ultrasound Med Biol. 2016;42:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 122. | Vitti P. Grey scale thyroid ultrasonography in the evaluation of patients with Graves' disease. Eur J Endocrinol. 2000;142:22-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Müller HW, Schröder S, Schneider C, Seifert G. Sonographic tissue characterisation in thyroid gland diagnosis. A correlation between sonography and histology. Klin Wochenschr. 1985;63:706-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Pishdad P, Pishdad GR, Tavanaa S, Pishdad R, Jalli R. Thyroid Ultrasonography in Differentiation between Graves' Disease and Hashimoto's Thyroiditis. J Biomed Phys Eng. 2017;7:21-26. [PubMed] |
| 125. | Cappelli C, Pirola I, De Martino E, Agosti B, Delbarba A, Castellano M, Rosei EA. The role of imaging in Graves' disease: a cost-effectiveness analysis. Eur J Radiol. 2008;65:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 126. | Saleh A, Cohnen M, Fürst G, Mödder U, Feldkamp J. Prediction of relapse after antithyroid drug therapy of Graves' disease: value of color Doppler sonography. Exp Clin Endocrinol Diabetes. 2004;112:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Arslan H, Unal O, Algün E, Harman M, Sakarya ME. Power Doppler sonography in the diagnosis of Graves' disease. Eur J Ultrasound. 2000;11:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Varsamidis K, Varsamidou E, Mavropoulos G. Doppler ultrasonography in predicting relapse of hyperthyroidism in Graves' disease. Acta Radiol. 2000;41:45-48. [PubMed] |
| 129. | Morosini PP, Simonella G, Mancini V, Argalia G, Lucarelli F, Montironi R, Diamanti L, Suraci V. Color Doppler sonography patterns related to histological findings in Graves' disease. Thyroid. 1998;8:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Ralls PW, Mayekawa DS, Lee KP, Colletti PM, Radin DR, Boswell WD, Halls JM. Color-flow Doppler sonography in Graves disease: "thyroid inferno". AJR Am J Roentgenol. 1988;150:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 110] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Aldasouqi S, Sheikh A, Klosterman P. Doppler ultrasonography in the diagnosis of Graves disease: a non-invasive, widely under-utilized diagnostic tool. Ann Saudi Med. 2009;29:323-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 132. | Zuhur SS, Ozel A, Kuzu I, Erol RS, Ozcan ND, Basat O, Yenici FU, Altuntas Y. The Diagnostic Utility of Color Doppler Ultrasonography, Tc-99m Pertechnetate Uptake, and TSH-Receptor Antibody for Differential Diagnosis of Graves' Disease and Silent Thyroiditis: A Comparative Study. Endocr Pract. 2014;20:310-319. [PubMed] [DOI] [Full Text] |
| 133. | Hiraiwa T, Tsujimoto N, Tanimoto K, Terasaki J, Amino N, Hanafusa T. Use of color Doppler ultrasonography to measure thyroid blood flow and differentiate graves' disease from painless thyroiditis. Eur Thyroid J. 2013;2:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 134. | Scappaticcio L, Trimboli P, Keller F, Imperiali M, Piccardo A, Giovanella L. Diagnostic testing for Graves' or non-Graves' hyperthyroidism: A comparison of two thyrotropin receptor antibody immunoassays with thyroid scintigraphy and ultrasonography. Clin Endocrinol (Oxf). 2020;92:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 135. | Yuksekkaya R, Celikyay F, Gul SS, Yuksekkaya M, Kutluturk F, Ozmen C. Quantitative Color Doppler Ultrasonography Measurement of Thyroid Blood Flow in Patients with Graves' Disease. Curr Med Imaging. 2020;16:1111-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 136. | Bayramoglu Z, Kandemirli SG, Akyol Sarı ZN, Kardelen AD, Poyrazoglu S, Bas F, Darendeliler F, Adaletli I. Superb Microvascular Imaging in the Evaluation of Pediatric Graves Disease and Hashimoto Thyroiditis. J Ultrasound Med. 2020;39:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | Baek HS, Park JY, Jeong CH, Ha J, Kang MI, Lim DJ. Usefulness of Real-Time Quantitative Microvascular Ultrasonography for Differentiation of Graves' Disease from Destructive Thyroiditis in Thyrotoxic Patients. Endocrinol Metab (Seoul). 2022;37:323-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 138. | Kılınçer A, Durmaz MS, Baldane S, Kıraç CO, Cebeci H, Koplay M. Evaluation of the Stiffness of Thyroid Parenchyma With Shear Wave Elastography Using a Free-Region of Interest Technique in Graves Disease. J Ultrasound Med. 2021;40:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 139. | Hefeda MM. Value of the New Elastography Technique using Acoustic Radiation Force Impulse in Differentiation between Hashimoto's Thyroiditis and Graves' Disease. J Clin Imaging Sci. 2019;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 140. | Shih SR, Chang JS, Lin LC, Chang YC, Li HY, Lee CY, Chen CM, Chang TC. The relationship between thyrotropin receptor antibody levels and intrathyroid vascularity in patients with Graves' disease. Exp Clin Endocrinol Diabetes. 2013;121:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 141. | Baldini M, Orsatti A, Bonfanti MT, Castagnone D, Cantalamessa L. Relationship between the sonographic appearance of the thyroid and the clinical course and autoimmune activity of Graves' disease. J Clin Ultrasound. 2005;33:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 142. | Brancatella A, Torregrossa L, Viola N, Sgrò D, Casula M, Basolo F, Materazzi G, Marinò M, Marcocci C, Santini F, Latrofa F. In Graves' disease, thyroid autoantibodies and ultrasound features correlate with distinctive histological features. J Endocrinol Invest. 2023;46:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 143. | Saleh A, Fürst G, Feldkamp J, Godehardt E, Grust A, Mödder U. Estimation of antithyroid drug dose in Graves' disease: value of quantification of thyroid blood flow with color duplex sonography. Ultrasound Med Biol. 2001;27:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 144. | Huang SM, Chow NH, Lee HL, Wu TJ. The value of color flow Doppler ultrasonography of the superior thyroid artery in the surgical management of Graves disease. Arch Surg. 2003;138:146-51; discussion 151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 145. | Nys P, Cordray JP, Sarafian V, Lefort-Mossé È, Merceron RÉ. Screening for thyroid cancer according to French recommendations with thyroid ultrasound in newly diagnosed Graves' disease without palpable nodule is not useful. Ann Endocrinol (Paris). 2015;76:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 146. | Kovatch KJ, Bauer AJ, Isaacoff EJ, Prickett KK, Adzick NS, Kazahaya K, Sullivan LM, Mostoufi-Moab S. Pediatric Thyroid Carcinoma in Patients with Graves' Disease: The Role of Ultrasound in Selecting Patients for Definitive Therapy. Horm Res Paediatr. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 147. | Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26:1343-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 1475] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 148. | Bartalena L, Burch HB, Burman KD, Kahaly GJ. A 2013 European survey of clinical practice patterns in the management of Graves' disease. Clin Endocrinol (Oxf). 2016;84:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 149. | Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves' disease. J Clin Endocrinol Metab. 2012;97:4549-4558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 150. | Lang BH, Woo YC, Wong IY, Chiu KW. Single-Session High-Intensity Focused Ultrasound Treatment for Persistent or Relapsed Graves Disease: Preliminary Experience in a Prospective Study. Radiology. 2017;285:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 151. | Lang BH, Woo YC, Chiu KW. Two-year outcomes of single-session high-intensity focused ultrasound (HIFU) treatment in persistent or relapsed Graves' disease. Eur Radiol. 2019;29:6690-6698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |