Published online Mar 15, 2024. doi: 10.4239/wjd.v15.i3.318
Peer-review started: December 15, 2023
First decision: January 15, 2024
Revised: January 15, 2024
Accepted: February 7, 2024
Article in press: February 7, 2024
Published online: March 15, 2024
The bidirectional association between type 2 diabetes mellitus (T2DM) and periodontitis is now well established, resulting in periodontal disease being considered as the 6th major complication of diabetes mellitus (DM) after car-diovascular disease, eye disease, neuropathy, nephropathy, and peripheral vascular disease. DM can worsen the virulence and invasiveness of pathogenic oral microbial flora aggravating the local inflammation and infection in those with periodontal disease. On the other hand, the chemical and immunological mediators released into the circulation as part of periodontal inflammation worsen the systemic insulin resistance with worsening of T2DM. Periodontitis if undiagnosed or left untreated can also result in eventual tooth loss. A study by Xu et al in the World Journal of Diabetes examined the predictive factors associated with periodontitis in Chinese patients with T2DM. The prevalence of periodontitis was found to be 75.7% in this study. Based on logistic regression analysis, the predictive factors for higher risk were low tooth brushing frequency [odds ratio (OR) = 4.3], high triglycerides (TG; OR = 3.31), high total cholesterol (TC; OR = 2.87), higher glycated hemoglobin (HbA1c; OR = 2.55), and higher age (OR = 1.05) while higher education level was protective (OR = 0.53). However, the most influential variables were HbA1c followed by age, TC, TG, low education level, brushing frequency, and sex on the random forest model (this model showed higher sensitivity for predicting the risk). A good understanding of the predictors for periodontitis in T2DM patients is important in prevention, early detection of susceptible patients, and intervention to improve periodontal health and enable long-term glycaemic control as observed by Xu et al.
Core Tip: The bidirectional association between type 2 diabetes mellitus (T2DM) and periodontitis is now well established, resulting in periodontal disease being considered as the 6th major complication of diabetes mellitus (DM) after cardiovascular disease, eye disease, neuropathy, nephropathy, and peripheral vascular disease. Periodontal inflammation worsens systemic insulin resistance with worsening of DM. A higher prevalence of periodontitis is seen in patients with poor glycemic control presumably from increased level of inflammation and risk of tissue destruction in these patients. Periodontitis if undiagnosed or left untreated can result in eventual tooth loss. A study by Xu et al in the recent issue of the World Journal of Diabetes highlights the predictive factors associated with periodontitis in patients with T2DM to enable readers to have a better understanding of both diseases.
- Citation: Kudiyirickal MG, Pappachan JM. Periodontitis: An often-neglected complication of diabetes. World J Diabetes 2024; 15(3): 318-325
- URL: https://www.wjgnet.com/1948-9358/full/v15/i3/318.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i3.318
Gingival and dental health are very sensitive markers of systemic health and well-being, though healthcare providers often underrecognize their importance largely because of inadequate awareness about this interlink. The dental and gingival disease can result in the worsening of several systemic illnesses including diabetes mellitus (DM), and DM can aggravate oral ill-health in a vicious circle[1]. The oral cavity is the habitat for billions of microorganisms, and changes in this biological ecosystem may be associated with several local and systemic disorders that can cause serious human health issues.
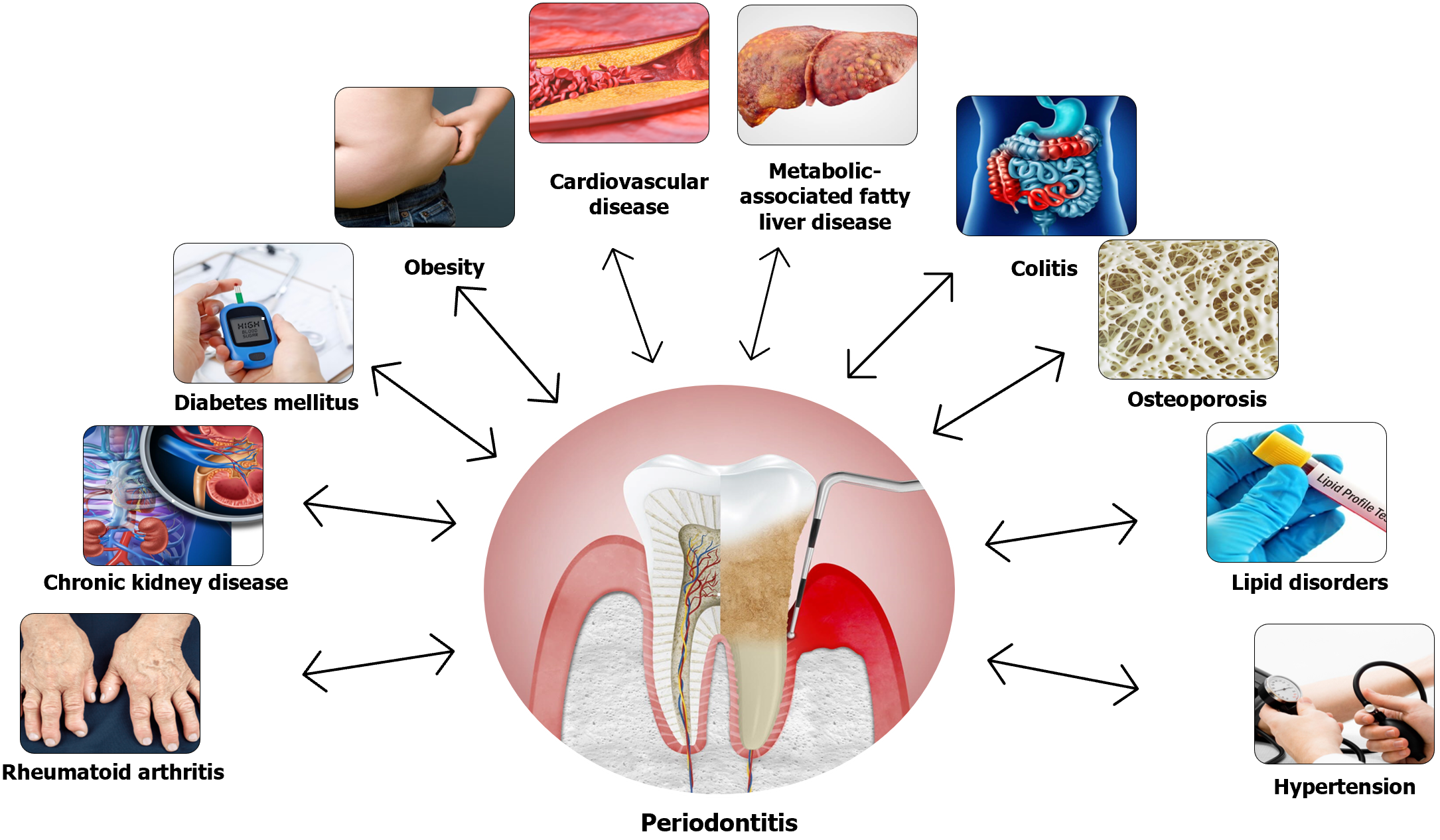
Periodontitis is a chronic inflammatory disorder affecting supportive tissues of teeth which results in tooth loss unless appropriate management is instituted on time[2]. The disease severity may range from mild illness without serious consequences to extreme cases with tooth loss as an unavoidable complication. Periodontitis affects more than 50% of the adult global population with an estimated 1.1 billion severe cases in the year 2019, and a 68% increase in the prevalence of severe disease from 1990 to 2019[3]. Apart from local complications, periodontitis can also be associated with several systemic diseases such as cardiovascular disease (CVD), lung disorders, chronic kidney disease (CKD), cirrhosis, obesity, colitis, rheumatoid arthritis, osteoporosis, malignancies, type 2 DM (T2DM), and even Alzheimer’s disease[4,5]. Although the exact mechanisms interlinking these systemic diseases and periodontitis are not fully elucidated, population-based studies reveal clear associations. Figure 1 shows the association between periodontitis and various systemic diseases[5].
Periodontitis-related inflammation of gum tissues and the host response to microbial dysbiosis results in the gradual destruction of the dental supporting structures with loosening of the tooth and expulsion over time. This inflammation also evokes the release of several immune and inflammatory cytokines and other chemical factors into the circulation which might in turn initiate or perpetuate the worsening of several systemic diseases.
A recent systematic review suggested a 7.2% prevalence of CVD and its significant association in men (OR = 1.22) and women (OR = 1.11) with periodontal disease[6]. The highly vascular nature of gums and periodontal issues result in the dissemination of bacteria and their toxins into circulation regularly. These initiate and potentiate systemic inflammatory responses and acceleration of atherosclerotic process as a direct consequence of endotoxemia and indirectly through various cytokines like C-reactive protein (CRP), interleukin-6, tumor necrosis factor-alpha and interferon-gamma[7,8]. Atherosclerosis is the main cause of most of the CVD subtypes such as hypertension, coronary heart disease, heart failure, and stroke.
The strong interlink between lung disease and periodontitis was reported by a recent systematic review which found statistically significant positive associations with chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea, while few studies revealed positive benefits of treatment of periodontitis in those with COPD, asthma, and bacterial pneumonia[9]. Micro-aspiration of oral microbes and systemic immune mechanisms related to oral inflammation impacting a harmful immune response in the respiratory system might explain these interlinks[5]. Moreover, oral infection with systemic bacteremia can sometimes result in pulmonary infections and even a lung abscess.
A recent meta-analysis showed a significant association (OR: 2.36) between CKD and periodontitis[10]. Another meta-analysis revealed improvement in glomerular filtration rate, markers of inflammation, and possibly even mortality rate in those patients receiving periodontal treatment[11]. These study results emphasize the importance of appropriate dental care for patients with CKD from the therapeutic and prevention perspectives.
Similar humoral and immune mechanisms are involved in the aetio-pathogenesis of other diseases such as cirrhosis, obesity, colitis, rheumatoid arthritis, osteoporosis, malignancies, T2DM, and even Alzheimer’s disease[5,12]. Inflammation of periodontal tissues from the local oral microbiome activates lymphocytes with the release of several immune factors into the circulation which results in initiation or aggravation of the systemic comorbidities. On the other hand, these systemic disorders such as DM aggravate the periodontal disease in a vicious circle potentiating both the local and systemic disease[12]. Therefore, clinicians should be vigilant to address both issues in patients they care for. Table 1 summarises the pathobiological interlink between periodontitis and various systemic disorders[5-12].
| Systemic disease | Interlink between periodontitis and the disease | Potential mechanisms |
| CVD | Accelerated atherosclerosis resulting in ischemic heart disease, stroke, peripheral vascular disease, and heart failure | Aggravation of dyslipidemia, insulin resistance, and endothelial dysfunction from release of various toxic cytokines and microbial products |
| Pulmonary disorders | Pneumonia caused by systemic spread of pathogens from periodontal tissues; Exacerbation of asthma and COPD from spread of pathogens from mouth; Sleep apnoea with high cytokine load worsens periodontitis | Direct and indirect results of oral microbes on pulmonary diseases and systemic cytokines worsening periodontal inflammation |
| CKD | CKD can worsen periodontitis and vice versa | Immunological and inflammatory responses from either disease |
| MAFLD & cirrhosis | Periodontitis can increase insulin resistance and advanced liver disease can worsen periodontal inflammation | Inflammatory mediators such as cytokines and chemokines aggravate either disorder |
| Obesity | Unhealthy lifestyles lead to obesity and periodontitis | Both diseases have inflammatory pathogenesis and can perpetuate each other |
| Colitis | Oral and gut microbiota are closely interlinked and can perpetuate disease processes in either region | Inflammatory and immune alterations in gut and periodontal tissues can aggravate both diseases |
| Rheumatoid arthritis | Inflammation of joints and oral tissues can modulate both diseases | Various humoral and immunological factors and cytokines worsen diseases |
| Osteoporosis | Pathogenesis of systemic and alveolar bone loss are closely linked | Inflammatory processes in periodontal tissues can potentially impact bone loss |
| Malignancies | Inflammation in periodontal areas can increase risk of malignancy | Various immunological and inflammatory factors from periodontitis can worsen the risk of cancer |
| Alzheimer’s disease | Periodontal disease can worsen Alzheimer’s disease and dementia can worsen mouth care and worsen periodontal disease | Increased deposition of β-amyloid and hyperphosphorylation of Tau protein is seen in patients with periodontal disease |
| Diabetes | Both T1DM and T2DM can worsen periodontitis and periodontitis can worsen glycaemic control | Aggravation of insulin resistance in periodontal disease and poor glycaemic control can worsen oral infections |
Since 1993, severe periodontal disease has been considered the 6th major complication of DM after CVD, eye disease, neuropathy, nephropathy, and peripheral vascular disease[13]. Being a state of immunocompromise, DM can worsen the virulence and invasiveness of pathogenic oral microbial flora aggravating the local inflammation and infection in those with periodontal disease[1,14]. Moreover, hyperglycemia-induced oxidative stress and the generation of reactive oxygen species in the periodontal tissues can aggravate local tissue damage and worsen periodontitis[14]. On the other hand, the chemical and immunological mediators released into the circulation as part of periodontal inflammation worsen systemic insulin resistance (IR) with worsening of DM.
Metabolic syndrome (MetS) and IR are the pathophysiological hallmarks of the disease in most patients with T2DM. The inflammatory process in periodontal tissues may release several cytokines into the systemic circulation along with bacterial toxins potentially aggravating the IR. There is also clear evidence for a significant association between MetS and periodontitis as evidenced by a very recent systematic review examining the interlink[15]. This meta-analysis, including 24567 participants from 14 studies, showed that the adjusted odds ratio (OR) for MetS among patients with moderate periodontitis was 1.26 and those with severe periodontitis was 1.50.
The prevalence of periodontitis among type 1 DM (T1DM) patients was 18.5% higher with an OR of 2.51 compared to the general population[16]. It was found that the markers of severity of periodontitis such as plaque index, bleeding on probing, pocket depth, gingival index, and clinical attachment loss were significantly higher among children and adolescents with T1DM[17]. There is also evidence for a higher prevalence of periodontitis in patients with poor glycemic control presumably from increased level of inflammation and risk of tissue destruction in these patients[18]. Local mechanisms of tissue defense against inflammation may be poor with higher microbial activity among patients with poor control of T1DM making them more vulnerable to severe periodontal disease.
It is crucial to understand the bidirectional relationship between periodontitis and DM for optimal management of patients in day-to-day clinical practice. A prompt collaboration between the dentist and the diabetologist should enhance patient outcomes when both diseases co-exist. Regular annual screening for micro- and macro-vascular complications of diabetes is meticulously performed by most clinicians. However, a thorough dental examination is often ignored even among patients with poor glycemic control. Although the annual dental review is recommended in adults with DM, a recent large population-based United States study showed that only 60% of patients performed annual dental check-ups[19].
This underscores the importance of increasing the awareness of oral health among diabetics. Primary care physicians, internists, and diabetologists should encourage DM patients to have annual dental evaluations and utilize the opportunity to refer patients for urgent dental reviews when they notice moderate to severe dental disease during their clinic visits for diabetes care.
Poor oral hygiene results in plaque biofilm accumulation on the tooth and surrounding gingival tissues resulting in inflammation and progressive damage of supporting periodontal tissues and bone in susceptible patients[20]. The surface area of the palm is the approximate size of the surface area of periodontal tissue inflammation and ulceration in a periodontitis patient. Periodontitis if undiagnosed or left untreated can result in bleeding gums, periodontal pocket, clinical attachment loss resulting in recession, furcation involvement, suppuration, tooth mobility, and eventual loss of the affected tooth[21].
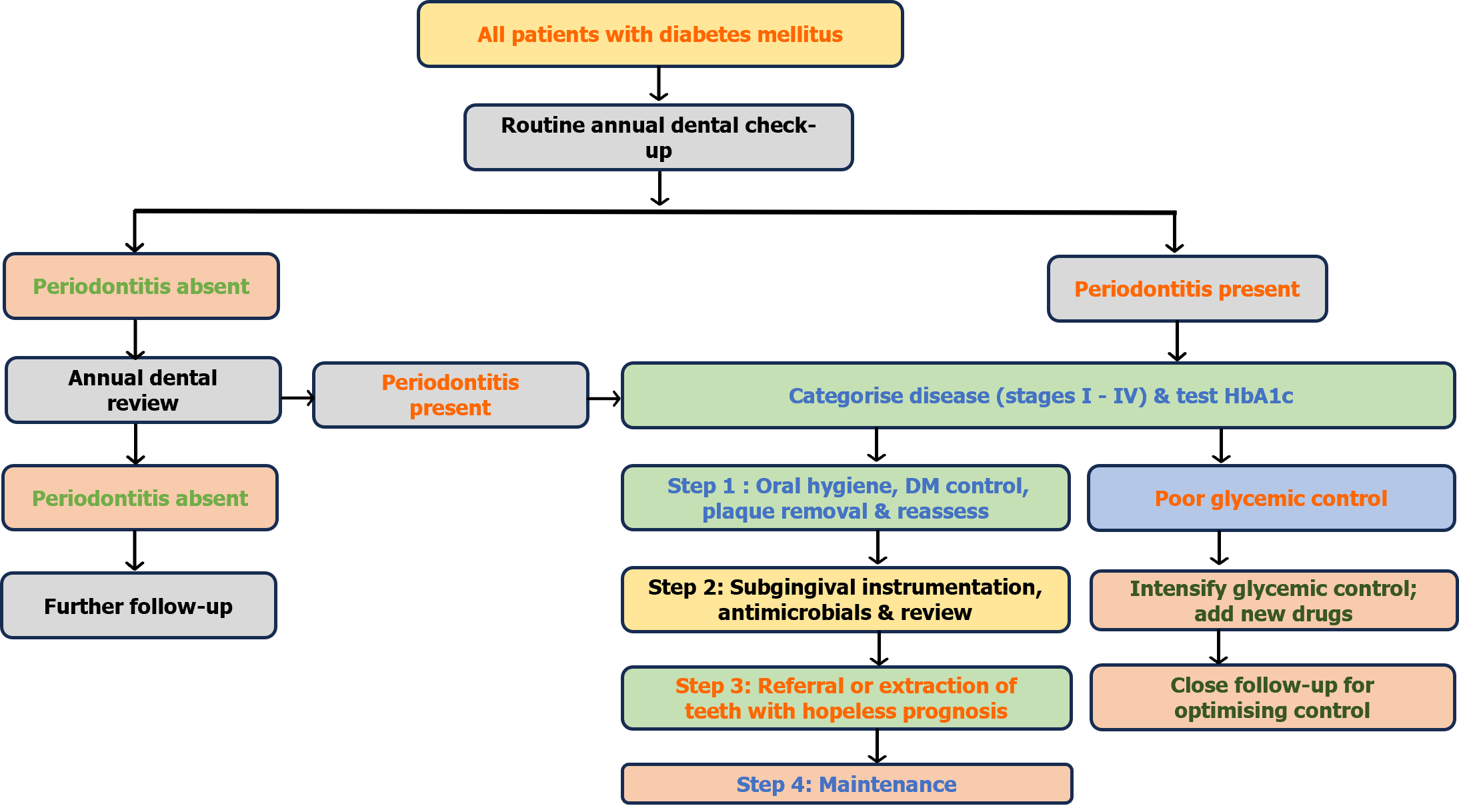
The revised classification of periodontitis is based on the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions[22]. This resulted in defining periodontal health and gingivitis based on whether there was bleeding on probing. Gingival health was considered if there were < 10% bleeding sites and less than or equal to 3 mm of probing depth. Staging of periodontitis into stages I, II, III, or IV was based on clinical attachment loss, depth of periodontal pocket, or both[22]. The classification included systemic diseases like DM as modifying periodontal disease and is now stated in the diagnosis along with the staging and grading of periodontitis[23].
A meticulous clinical examination during the annual dentist review for patients with DM involves a basic periodontal examination (BPE) to initially screen for periodontal disease[24]. A BPE score of 3 or 4 would need further investigation which involves a six-point pocket depth charting, bleeding and plaque scores, mobility, recession, furcation involvement, and appropriate radiographs. It is now well established that if an individual has periodontitis, then it is a life-long diagnosis of the disease despite treatment since regular supportive care is essential to maintain healthy periodontal tissues[24].
The pretreatment and post-treatment charting of periodontal indices helps to assess the periodontal disease progression. These scores are repeated annually for patients who are stable following periodontal treatment.
A Cochrane review update published in 2022 on periodontal management for diabetic patients has reported a moderate level of evidence for improvement in glycaemic control until one year following non-surgical periodontal treatment which involves plaque and calculus removal in subgingival areas by ultrasonic or manual method[25]. There was a significant change in glycaemic control in individuals with diabetes and periodontitis in comparison to untreated individuals or those who received normal treatment. The results from thirty studies included in this review revealed that in patients with DM, there was a 0.43% reduction in the level of glycated hemoglobin (HbA1c; from 7.43% to 7%) after a period of 3 to 4 months following periodontal treatment when compared to patients with routine care or no intervention. This difference was 0.30% after 6 months in 12 trials, whereas one study revealed a 0.5% reduction in HbA1c after 12 months[25].
A well-integrated pathway for medical and dental care of diabetic patients for evaluation of periodontal health and provision of appropriate periodontal treatment is widely recommended[26]. Preliminary sequential management of periodontitis involves patient education involving home care instructions on oral hygiene to enable long-term control of plaque, microbial biofilm, and factors causing periodontal disease, followed by supragingival professional mechanical plaque removal.
In the next stage, subgingival mechanical debridement is done to remove biofilm and calculus deposits using ultrasonic or manual instruments or in combination. The previous terminology ‘scaling and root planning’ is now replaced by ‘subgingival instrumentation’ based on the European Federation for Periodontology S3 treatment guidelines[27].
In advanced cases of periodontitis where the above non-surgical management has failed to show signs of improvement, the next stage of treatment involves surgical management by a periodontist. This involves direct access to subgingival sites of residual disease in patients who have good oral hygiene. Multiple visits may be needed in some cases. However, teeth that do not respond to treatment and have poor prognoses will need extraction to minimize the systemic effects of disease progression and to restore periodontal health[27].
Adjuncts like antiseptic mouthwash like chlorhexidine, and local and systemic antibiotics have been used to treat periodontitis. Currently, there is no clear evidence for the routine use of these antimicrobials along with periodontal therapy[27].
A systematic review and meta-analysis by Cao et al[28] looked at no treatment and different modalities of periodontal treatment which included antimicrobial photodynamic therapy, sub-antimicrobial dose of doxycycline, antibiotics (metronidazole, amoxicillin doxycycline), local drug delivery (simvastatin gel, chlorhexidine gel, atorvastatin gel), diode laser and subgingival instrumentation. It was found that a combination of antimicrobial photodynamic therapy, and doxycycline along with subgingival instrumentation was the most effective treatment modality for decreasing HbA1c% in non-smokers with chronic periodontitis who did not have severe complications of T2DM, though the authors of the review acknowledge that the certainty of evidence of this observation was low, or very low.
Thus, patient motivation, home care instructions on oral hygiene, professional mechanical plaque removal combined with surgical or non-surgical management, and use of local or systemic antimicrobial drug delivery, and supportive therapy are important for active intervention and long-term maintenance of periodontal health. Figure 2 summarises the management algorithm for patients with diabetes and periodontal disease.
The bidirectional association between T2DM and periodontitis is well established as pointed out by a systematic review by Wu et al[29] examining 53 observational studies. The adjusted OR for the prevalence of T2DM in patients with periodontitis was 4.04, while the adjusted OR for the prevalence of periodontitis in patients with T2DM was 1.56 in this study. However, several confounding factors such as the triglyceride level, white blood cell count, CRP, hypertension, waist circumference, body mass index, sex, education, age, income, and dental check-ups might have weakened the evidence level.
As mentioned in the previous section, several factors predispose to the development of periodontitis in patients with T2DM. A study by Xu et al[30] in the recent issue of World Journal of Diabetes, examined the predictive factors associated with periodontitis in patients with T2DM. The study reported a very high prevalence of periodontitis (75.7%) among Chinese patients. The predictive factors for higher risk were low tooth brushing frequency (OR = 4.3), high triglycerides (OR = 3.31), high cholesterol (OR = 2.87), higher HbA1c (OR = 2.55), and higher age (OR = 1.05) while higher education level was protective (OR = 0.53) on logistic regression analysis, while the most influential variables were HbA1c followed by age, total cholesterol, triglycerides, low education level, brushing frequency, and sex on the random forest model. In the study, the random forest statistical model showed a higher sensitivity for predicting the risk compared to the logistic regression model [area under the curve (AUC) 1.000 vs 0.851; P < 0.05] in their training dataset, while the validation dataset did not show such a difference (AUC = 0.946 vs 0.915; P > 0.05). Although other studies looked at the factors associated with periodontitis and T2DM, the study by Xu et al[30] provides us reasonably comprehensive evidence for the predictive factors for the disease in the diabetic population. However, we need larger multi-centre prospective cohort studies and randomized controlled trials to generate more robust evidence for clearly proving the definite association between these predictive factors and periodontitis.
There is no doubt among scientific professionals regarding the bidirectional association between periodontitis and DM. Periodontal disease is now considered as the 6th major complication of DM after CVD, eye disease, neuropathy, nephropathy, and peripheral vascular disease. Both DM and periodontitis can perpetuate the disease process of each other, and without the appropriate and adequate care, each condition can worsen the other disease to cause significant health issues among sufferers. Periodontitis is also associated with several systemic diseases which can directly or indirectly worsen the periodontal disease process. Thorough understanding about the pathobiology, clinical presentation and diagnostic work-up of patients with periodontitis and DM is important among healthcare professionals managing these diseases to improve clinical outcomes. The study by Xu et al[30] in the Journal examining the predictive factors associated with periodontitis and DM is such an attempt to empower clinicians caring for such patients across the globe.
We thank Dr. Cornelius J Fernandez for helping to construct the pictures of this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Horowitz M, Australia S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Kudiyirickal MG, Pappachan JM. Diabetes mellitus and oral health. Endocrine. 2015;49:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 891] [Cited by in F6Publishing: 901] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 3. | Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990-2019: An analysis of the Global Burden of Disease Study 2019. J Clin Periodontol. 2021;48:1165-1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 4. | Pai SI, Matheus HR, Guastaldi FPS. Effects of periodontitis on cancer outcomes in the era of immunotherapy. Lancet Healthy Longev. 2023;4:e166-e175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Hajishengallis G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol 2000. 2022;89:9-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 6. | Leng Y, Hu Q, Ling Q, Yao X, Liu M, Chen J, Yan Z, Dai Q. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front Cardiovasc Med. 2023;10:1114927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 7. | Priyamvara A, Dey AK, Bandyopadhyay D, Katikineni V, Zaghlol R, Basyal B, Barssoum K, Amarin R, Bhatt DL, Lavie CJ. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr Atheroscler Rep. 2020;22:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Yang S, Zhao LS, Cai C, Shi Q, Wen N, Xu J. Association between periodontitis and peripheral artery disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2018;18:141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Molina A, Huck O, Herrera D, Montero E. The association between respiratory diseases and periodontitis: A systematic review and meta-analysis. J Clin Periodontol. 2023;50:842-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Serni L, Caroti L, Barbato L, Nieri M, Serni S, Cirami CL, Cairo F. Association between chronic kidney disease and periodontitis. A systematic review and metanalysis. Oral Dis. 2023;29:40-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Delbove T, Gueyffier F, Juillard L, Kalbacher E, Maucort-Boulch D, Nony P, Grosgogeat B, Gritsch K. Effect of periodontal treatment on the glomerular filtration rate, reduction of inflammatory markers and mortality in patients with chronic kidney disease: A systematic review. PLoS One. 2021;16:e0245619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21:426-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 467] [Article Influence: 155.7] [Reference Citation Analysis (0)] |
| 13. | Kidambi S, Patel SB. Diabetes mellitus: considerations for dentistry. J Am Dent Assoc. 2008;139 Suppl:8S-18S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Zhao M, Xie Y, Gao W, Li C, Ye Q, Li Y. Diabetes mellitus promotes susceptibility to periodontitis-novel insight into the molecular mechanisms. Front Endocrinol (Lausanne). 2023;14:1192625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 15. | Rosário-Dos-Santos HL, Miranda SS, Gomes-Filho IS, Cruz SSD, Figueiredo ACMG, Souza ES, Hintz AM, Loomer PM, Passos-Soares JS. Periodontitis severity relationship with metabolic syndrome: A systematic review with meta-analysis. Oral Dis. 2023;29:2512-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Dicembrini I, Serni L, Monami M, Caliri M, Barbato L, Cairo F, Mannucci E. Type 1 diabetes and periodontitis: prevalence and periodontal destruction-a systematic review. Acta Diabetol. 2020;57:1405-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Jensen E, Allen G, Bednarz J, Couper J, Peña A. Periodontal risk markers in children and adolescents with type 1 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2021;37:e3368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Costa R, Ríos-Carrasco B, Monteiro L, López-Jarana P, Carneiro F, Relvas M. Association between Type 1 Diabetes Mellitus and Periodontal Diseases. J Clin Med. 2023;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 19. | Baccaglini L, Kusi Appiah A, Ray M, Yu F. US adults with diabetes mellitus: Variability in oral healthcare utilization. PLoS One. 2021;16:e0251120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Abusleme L, Hoare A, Hong BY, Diaz PI. Microbial signatures of health, gingivitis, and periodontitis. Periodontol 2000. 2021;86:57-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 21. | Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontol. 1998;3:108-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 206] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, Geisinger ML, Genco RJ, Glogauer M, Goldstein M, Griffin TJ, Holmstrup P, Johnson GK, Kapila Y, Lang NP, Meyle J, Murakami S, Plemons J, Romito GA, Shapira L, Tatakis DN, Teughels W, Trombelli L, Walter C, Wimmer G, Xenoudi P, Yoshie H. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45 Suppl 20:S68-S77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 23. | Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018;89 Suppl 1:S159-S172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 700] [Cited by in F6Publishing: 1018] [Article Influence: 203.6] [Reference Citation Analysis (0)] |
| 24. | Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, Geisinger ML, Genco RJ, Glogauer M, Goldstein M, Griffin TJ, Holmstrup P, Johnson GK, Kapila Y, Lang NP, Meyle J, Murakami S, Plemons J, Romito GA, Shapira L, Tatakis DN, Teughels W, Trombelli L, Walter C, Wimmer G, Xenoudi P, Yoshie H. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89 Suppl 1:S74-S84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 25. | Simpson TC, Clarkson JE, Worthington HV, MacDonald L, Weldon JC, Needleman I, Iheozor-Ejiofor Z, Wild SH, Qureshi A, Walker A, Patel VA, Boyers D, Twigg J. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2022;4:CD004714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45:138-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 27. | Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47 Suppl 22:4-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 538] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 28. | Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health. 2019;19:176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, An ZJ, Chen SY, Wu YZ, Han B, Li CJ, Li LJ. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20:204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 30. | Xu HM, Shen XJ, Liu J. Establishment of models to predict factors influencing periodontitis in patients with type 2 diabetes mellitus. World J Diabetes. 2023;14:1793-1802. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |