Published online Feb 15, 2024. doi: 10.4239/wjd.v15.i2.232
Peer-review started: November 6, 2023
First decision: November 16, 2023
Revised: November 17, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: February 15, 2024
Glucose and lipid metabolic disorder in patients with type 2 diabetes mellitus (T2DM) is associated with the levels of serum tumor markers of the digestive tract, such as cancer antigen (CA)199. Therefore, tumor markers in T2DM are important.
To evaluate the expression of serum tumor markers [CA199, CA242, and car-cinoembryonic antigen (CEA)] and the clinical implications of the expression in T2DM.
For this observational study conducted at Hefei BOE Hospital, China, we enrolled 82 patients with first-onset T2DM and 51 controls between April 2019 and December 2020. Levels of fasting blood glucose (FBG), tumor markers (CA199, CEA, and CA242), glycosylated hemoglobin (HbA1c), etc. were measured and group index levels were compared. Moreover, FBG and HbA1c levels were correlated with tumor marker levels. Tumor markers were tested for diagnostic accuracy in patients with > 9% HbA1c using the receiver operating curve (ROC) curve.
The T2DM group had high serum FBG, HbA1c, CA199, and CEA levels (P < 0.05). A comparative analysis of the two groups based on HbA1c levels (Group A: HbA1c ≤ 9%; Group B: HbA1c > 9%) revealed significant differences in CEA and CA199 levels (P < 0.05). The areas under the ROC curve for CEA and CA199 were 0.853 and 0.809, respectively. CA199, CEA, and CA242 levels positively correlated with HbA1c (r = 0.308, 0.426, and 0.551, respectively) and FBG levels (r = 0.236, 0.231, and 0.298, respectively).
As compared to controls, serum CEA and CA199 levels were higher in patients with T2DM. HbA1c and FBG levels correlated with CA199, CEA, and CA242 levels. Patients with poorly controlled blood sugar must be screened for tumor markers.
Core Tip: Levels of serum cancer antigen (CA)199, carcinoembryonic antigen (CEA), and CA242 demonstrated close association with glycosylated hemoglobin (HbA1c) and fasting blood glucose levels in patients with type 2 diabetes mellitus. Furthermore, CA199 and CEA levels had good predictive power for HbA1c levels. These findings suggest the need for monitoring tumor marker changes in those with poorly controlled blood sugar levels.
- Citation: Meng M, Shi LL. Serum tumor markers expression (CA199, CA242, and CEA) and its clinical implications in type 2 diabetes mellitus. World J Diabetes 2024; 15(2): 232-239
- URL: https://www.wjgnet.com/1948-9358/full/v15/i2/232.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i2.232
In China, an aging population and lifestyle changes have transformed diabetes from a rare disease to an epidemic over the past four decades. The global number of individuals aged ≥ 18 years affected by diabetes is projected to increase from 425 million in 2017 to 629 million in 2045, with type 2 diabetes mellitus (T2DM) accounting for > 90% of the diabetic population[1]. Long-term hyperglycemia in patients with T2DM can cause oxidative stress-, inflammation-, and vascular endothelial function-related damage. Recent studies have highlighted the association between diabetes and cancer, demonstrating that patients with T2DM are significantly more likely to develop malignant tumors than the general population[2]. Patients with tumors may experience significant changes in the blood sugar levels during therapy. Moreover, diabetes can cause levels of specific serum tumor markers to spike. Although carcinoembryonic antigen (CEA), cancer antigen (CA)199, and CA242 are used to diagnose tumors[3], the correlation between their expression levels and blood glucose levels in patients with T2DM remains unknown.
Considering these findings, the precise relationship between the levels of tumor markers (CEA, CA199, and CA242) and T2DM needs a thorough investigation. This study aimed to address the overarching question: “What is the relationship between the expression levels and clinical significance of serum tumor markers (CEA, CA199, and CA242) in patients with T2DM?” Addressing this question is crucial for enhancing early tumor screening and improving prognostic evaluation, potentially contributing to improved clinical outcomes and management strategies for patients with T2DM and comorbid cancer conditions.
The sample size calculation for this study was based on the anticipated difference in tumor marker levels (CA199, CA242, and CEA) between patients with T2DM and the control group. Assuming a medium effect size (d = 0.5), a significance level (α) of 0.05, and a desired power of 80%, the sample size was estimated using the G*Power software. Based on these parameters, ≥ 46 participants were needed in each group. Assuming a 10% loss of data or exclusion, a minimum of 51 participants in each group was deemed necessary. Finally, 82 patients with T2DM and 51 controls were enrolled in this study.
The inclusion criteria set for this study were as follows: (1) Age ≥ 18 years; (2) patients who met the T2DM diagnostic criteria established by the guidelines for the prevention and treatment of type 2 diabetes in China (2020 Edition) formulated by the diabetes branch of the Chinese Medical Association; these included newly diagnosed patients and previously diagnosed patients with poor blood glucose control; and (3) those who or whose families provided informed consent. The exclusion criteria were as follows: (1) Patients with heart, liver, kidney and lung dysfunction, acute diabetic complications, infectious diseases, autoimmune diseases, acute and chronic inflammatory reactions, and malignant tumors; (2) patients on long-term glucocorticoid therapy, given the effect of these medications on blood sugar and lipid levels; (3) pregnant or lactating women; (4) patients with acute and chronic pancreatitis, liver cirrhosis, hepatitis, colitis, gallstones, and obstructive jaundice, given that these conditions can cause benign elevation of serum CA199 or CEA levels; and (5) patients with incomplete clinical information or inaccurate data.
We recruited 82 patients (47 men) with T2DM from BOE Technology Hospital in Hefei between April 2019 and December 2020. All patients were diagnosed with diabetes according to the 1999 World Health Organization diagnostic criteria. During the same period, 51 individuals (27 men) who underwent health examinations at our hospital's health examination center were selected as the control group. The median age was 59.5 (26–81) years in the T2DM group and 46 (27–68) years in the control group. Table 1, summarizing the general characteristics of the two groups indicates no significant inter-group differences. The exclusion criteria for the control group were as follows: (1) Individuals with type 1 diabetes, acute metabolic disorders associated with diabetes (such as ketoacidosis and hyperosmolar state), acute stroke, acute and chronic infections, thyroid disease, and cardiac insufficiency; (2) those with severe liver and kidney dysfunction; (3) those with acute and chronic hepatitis, alcoholic liver disease, cirrhosis, gallstone, pancreatitis, chole-cystitis, and other digestive system diseases; (4) those with tumors; and (5) pregnant women. The study protocol was approved by the Medical Ethics Committee and participants provided written informed consent.
| Variables | Control group (n = 51) | T2DM group (n = 82) | χ2/Z/t value | P value |
| Gender (male/female) | 27/24 | 47/35 | 0.244 | 0.621 |
| Age (yr) | 57.98 ± 11.72 | 59.02 ± 11.58 | 0.503 | 0.616 |
| ALT (U/L) | 18.40 (13.20, 28.30) | 18.75 (13.48, 30.93) | 0.558 | 0.577 |
| AST (U/L) | 18.80 (11.80, 30.10) | 16.25 (12.48, 24.80) | 0.694 | 0.488 |
| SUA (mmol/L) | 312.51 ± 119.36 | 306.20 ± 102.97 | 0.323 | 0.747 |
| Cre (mmol/L) | 68.36 ± 27.54 | 70.16 ± 28.67 | 0.357 | 0.721 |
| HbA1c (%) | 5.30 (4.30, 6.60) | 9.30 (8.18, 11.13) | 9.013 | 0 |
| FBG (mmol/L) | 4.46 ± 0.89 | 10.08 ± 4.30 | 9.199 | 0 |
| BMI (kg/m2) | 25.15 ± 4.28BMI | 25.55 ± 3.40 | 0.602 | 0.548 |
| LDL-C (mmol/L) | 2.30 (1.90, 3.30) | 2.34 (1.94, 3.23) | 0.201 | 0.84 |
| TG (mmol/L) | 1.40 (1.10, 2.30) | 1.81 (1.09, 2.68) | 1.581 | 0.144 |
| TC (mmol/L) | 4.45 ± 1.39 | 4.46 ± 1.29 | 0.049 | 0.961 |
Upon admission, the body mass index (BMI) was calculated by measuring the patient height and weight and collecting venous blood after an overnight fast. The levels of alanine transaminase (ALT), aspartate transaminase (AST), creatinine, serum uric acid, fasting blood glucose (FBG), triglycerides, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and serum tumor markers CEA, CA199, and CA242 were determined using the Roche Cobas8000 biochemical immune assembly line and the corresponding test kits. The methods for measuring the parameters were as follows: FBG, hexokinase; ALT, IFCC; AST, colorimetric; serum uric acid, colorimetric; creatinine-enzyme; triglycerides, colorimetric; TC, enzyme colorimetric; LDL-C, selective clearance; CEA (normal value < 6.5 ng/mL) and CA199 (normal value < 35 U/mL), electrochemical luminescence; and CA242 (normal value < 20 U/mL), chemiluminescence immunoassay. Glycosylated hemoglobin (HbA1c) levels were measured using a Dongcao G8 glycated hemoglobin instrument and the corresponding detection kit. All patients underwent routine abdominal ultrasonography and chest imaging [radiograph/computed tomography (CT)]. Further examinations were performed for patients with suspected tumors including CT, magnetic resonance imaging, and gastroscopy.
Statistical analyses were performed using SPSS 19.0 software. For normally distributed quantitative data, t-tests were used for comparisons, and the data are presented as mean ± SD. The Mann–Whitney rank-sum test and Spearman correlation analysis were used for skewed distribution data. A binary logistic regression analysis was conducted using FBG, HbA1c, CEA, CA199, and CA242 as independent variables to assess their predictive value for the occurrence of T2DM. The receiver operating curve (ROC) analysis was performed for variables with significant differences. Statistical significance was set at P < 0.05.
The results revealed no significant differences in the levels of liver and kidney function indicators, lipid metabolism-related indicators (AST, ALT, uric acid, creatinine, BMI, LDL-C, and TC), age, and sex distribution (P > 0.05). However, HbA1c and FBG levels were higher in the T2DM group compared to the control group (P < 0.05; Table 1).
CEA and CA199 levels were significantly higher in the T2DM group than in the control group (P < 0.001). Although CA242 levels were also elevated in the T2DM group, the difference was statistically insignificant (P = 0.068; Table 2).
| Variables | Control group (n = 51) | T2DM group (n = 82) | Z value | P value |
| CEA | 2.10 (1.40, 2.70) | 2.70 (1.90, 3.65) | 3.279 | 0.000 |
| CA199 | 7.60 (4.40, 10.10) | 11.30 (5.57, 22.13) | 3.976 | 0.000 |
| CA242 | 6.10 (3.10, 6.90) | 6.25 (4.13, 9.20) | 0.891 | 0.373 |
We investigated the association between T2DM incidence as the dependent variable and the following independent variables: FBG, HbA1C, CEA, CA199, and CA242 using a binary logistic regression analysis. T2DM occurrence was categorized as 0 (did not occur) and 1 (occurred). The results were optimized using a stepwise backward elimination method. Our findings indicated FBG [odds ratio (OR) = 43.173, 95% confidence interval (95%CI): 1.513–6.658], HbA1C (OR = 4.560, 95%CI: 1.914–10.863), CEA (OR = 1.366, 95%CI: 1.024–1.822), and CA199 (OR = 1.035, 95%CI: 1.013–1.057) as independent risk factors for the onset of T2DM, all with P values < 0.05 (Table 3).
| Factors | β value | SE | Wald | P value | OR | 95%CI |
| FBG | 1.155 | 0.378 | 9.330 | 0.002 | 3.173 | 1.513-6.658 |
| HBA1C | 1.517 | 0.443 | 11.739 | 0.001 | 4.560 | 1.914-10.863 |
| CEA | 0.312 | 0.147 | 4.505 | 0.034 | 1.366 | 1.024-1.822 |
| CA199 | 0.034 | 0.011 | 9.554 | 0.002 | 1.035 | 1.013-1.057 |
| CA242 | 0.145 | 0.115 | 1.585 | 0.208 | 1.156 | 0.923-1.448 |
Based on an HbA1c threshold value of 9%, patients with diabetes were divided into two groups: Groups A (HbA1c ≤ 9%) and B (HbA1c > 9%). Age, liver and kidney function, and lipid metabolism were compared between the two groups. The results indicated no statistical differences in age, sex, disease course, liver and kidney function, or lipid metabolism-related indicators between the two groups (P > 0.05). However, group B had higher serum uric acid, FBG, CEA, and CA199 levels than group A (P < 0.05; Table 4).
| Variables | Group A (HbA1c ≤ 9%) (n = 37) | Group B (HbA1c > 9%) (n = 45) | t/Z value | P value |
| Age (yr) | 60.08 ± 9.58 | 58.16 ± 13.05 | 0.747 | 0.457 |
| Gender (male/female) | 21/16 | 26/19 | 0.009 | 0.926 |
| Course of disease (yr) | 6 (3.00, 10.50) | 9 (4, 15.00) | 1.627 | 0.104 |
| BMI (kg/m2) | 26.09 ± 2.66 | 25.11 ± 3.88 | 1.349 | 0.181 |
| FBG (mmol/L) | 8.41 ± 2.79 | 11.46 ± 4.84 | 3.386 | 0.001 |
| LDL-C (mmol/L) | 2.33 (1.93, 3.02) | 2.38 (1.94, 3.32) | 0.680 | 0.496 |
| TG (mmol/L) | 2.00 (1.23, 3.01) | 1.57 (0.96, 2.61) | 1.142 | 0.254 |
| TC (mmol/L) | 4.54 ± 1.02 | 4.40 ± 1.48 | 0.494 | 0.623 |
| Cre (umol/L) | 71.07 ± 19.33 | 69.40 ± 34.72 | 0.261 | 0.795 |
| SUA (umol/L) | 333.64 ± 99.56 | 283.64 ± 101.27 | 2.242 | 0.028 |
| ALT (U/L) | 17.30 (12.65, 28.75) | 19.90 (14.05, 31.45) | 0.778 | 0.437 |
| CEA (ng/mL) | 1.90 (1.20, 2.60) | 3.40 (2.60, 5.25) | 5.488 | 0.000 |
| CA199 (U/mL) | 7.60 (4.15, 10.60) | 21.00 (11.85, 26.85) | 4.795 | 0.000 |
| CA242 (U/mL) | 5.90 (3.85, 7.15) | 6.50 (4.80, 9.30) | 1.622 | 0.105 |
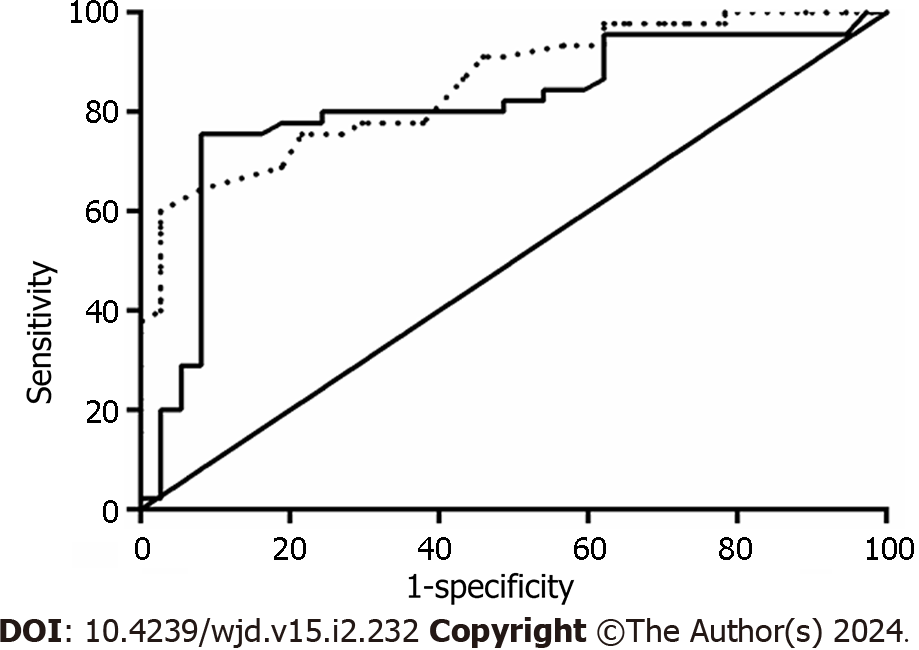
The area under the ROC curve (AUC) was calculated for both CEA and CA199 markers. For CEA, the AUC (95%CI) was identified to be 0.853 (0.774–0.933, P < 0.001; Figure 1). For CA199, the AUC (95%CI) was identified to be 0.809 (0.709–0.909, P < 0.001; Figure 1).
The results demonstrated a positive correlation between serum CA199, CEA, and CA242 levels and HbA1c levels with correlation coefficients of 0.308, 0.426, and 0.551, respectively (P < 0.001; Table 5) and FBG with correlation coefficients of 0.236, 0.231, and 0.298, respectively (P < 0.05; Table 5).
| Variables | CEA | CA199 | CA242 | |||
| r value | P value | r value | P value | r value | P value | |
| HbA1c | 0.308 | 0.000 | 0.426 | 0.000 | 0.551 | 0.000 |
| FBG | 0.236 | 0.033 | 0.231 | 0.037 | 0.298 | 0.006 |
| LDL-C | 0.138 | 0.216 | 0.238 | 0.032 | 0.240 | 0.030 |
| TG | 0.136 | 0. 222 | 0.105 | 0.346 | 0.051 | 0.649 |
| TC | 0.077 | 0.494 | 0.171 | 0.125 | 0.149 | 0.183 |
Epidemiological studies have demonstrated that the risk of certain malignancies, including hepatoma, hepatocellular carcinoma, colorectal cancer, and bladder cancer, is high in patients with T2DM[4,5]. This relationship may be attributed to long-term elevated blood glucose levels, insulin resistance, or changes in insulin-like growth factors, although the specific mechanisms remain unclear. Tumor markers, including CEA, CA199, and CA242, are mostly used for laboratory diagnosis of tumors. In patients with T2DM, chronic inflammatory lesions of beta cells in the pancreatic islets and long-term glucotoxicity and lipotoxicity can exacerbate chronic inflammation or hyperplasia of the pancreas. This process destroys normal pancreatic tissue, with subsequent replacement by adipocytes and fibrous connective tissue. Additionally the aforementioned process results in a significant release of CA199 into the bloodstream[6]. Furthermore, high blood sugar levels can affect free radical generation, increasing oxidative stress. Severe oxidative stress and high blood sugar levels may contribute to increased CEA expression[7]. Additionally, the replacement of normal pancreatic tissue by fat cells or fibrous connective tissue leads to the deposition of amyloid substances in pancreatic islet cells, followed by tissue destruction, cell degeneration, and necrosis. Hyperglycemia further exacerbates these pathological changes, releasing glycoprotein components, including CA242, into the bloodstream[8].
This study comprehensively examined tumor markers in patients with T2DM and healthy control groups. The levels of CEA and CA199 were higher in patients with T2DM than in healthy controls, indicating that blood glucose levels may be involved in the increase of serum CEA and CA199 levels, which is consistent with the findings of Lipinski et al[9]. Pancreatic tissue is affected by diabetes, which is considered an important factor that leads to a false increase in serum CA199 levels. Although the CA242 levels did not significantly differ between the two groups in this study, caution is advised when drawing conclusions owing to the limited sample size. In patients with T2DM and poorly controlled blood glucose levels, a benign increase in the concentration of CA199 and CEA can occur, which does not necessarily indicate the presence of malignant tumors. The benign increase in tumor marker CA199 and CEA levels in patients with poor blood glucose control can be attributed to “glucotoxicity” damage. However, whether this increase leads to malignant tumor development cannot be determined. Therefore, patients must actively control their blood glucose levels to avoid further increases in CA199 and CEA levels[10], thereby reducing the risk of developing malignant tumors. Similarly, the slight increase in serum CA199 and CEA levels may be due to glucose metabolism disorders in patients with diabetes. Hence, increasing the cutoff value for the “normal” levels of CA199 and CEA may be necessary, for distinguishing benign digestive tract diseases from malignant digestive tract tumors in patients with diabetes.
HbA1c has a marked effect in promoting CA199 and CEA elevation, providing insight into blood sugar control during the previous 3 months in patients[11,12]. In this study, patients with diabetes were divided into two subgroups based on their HbA1c levels. CEA and CA199 levels in Group B (HbA1c > 9%) patients were significantly different from those in Group A (HbA1c ≤ 9%) patients, with positive correlations observed between serum CA199, CEA, CA242, and HbA1c levels. Notably, the positive correlation between serum CA199 and HbA1c levels in T2DM has been demonstrated previously[13]. Furthermore, we observed that LDL-C levels positively correlated with CA199 levels. Increased HbA1c levels can lead to tissue hypoxia, elevated plasma low-density lipoprotein levels, tissue collagen glycosylation, increased blood viscosity, blood stasis, abnormal anticoagulation mechanisms, and enhanced production of free radicals. Moreover, these factors can collectively cause pancreatic tissue damage, leading to elevated CA199 levels[14]. The significant relationship between increased serum CA199 and CEA and HbA1c levels in patients with T2DM underscores the diagnostic value of CA199 and CEA levels for HbA1c percentage. Hence, when clinically using CA199 and CEA to identify malignant tumors in patients with T2DM, hypoglycemic treatment should be prioritized to stabilize blood sugar levels before tumor marker detection and observation[15].
This study also observed an outstanding dependence between CEA and CA199 levels and hyperglycemia, indicating that CEA and CA199 may be related to poor blood sugar and lipid control. Previous studies have displayed that elevated CEA levels are associated with oxidative stress, which can be induced by high blood sugar levels[16]. However, increased FBG levels in patients with T2DM may contribute to upregulated CEA and CA199 expression, which could be significantly associated with a high incidence of pancreatic cancer in these patients[17]. Repetitive injury to pancreatic tissue caused by chronic glucose toxicity may be a major factor contributing to the occurrence and progression of pancreatic cancer. Active blood sugar control and early screening for pancreatic cancer could potentially reduce the risk of malignant tumors in such patients[18]. Additionally, CA199 and CEA have high diagnostic values for digestive system tumors and also demonstrate certain diagnostic values for T2DM.
Although our study highlights the association between elevated CEA, CA199, and CA242 levels and T2DM, the broad clinical implications are paramount. In a real-world setting, these tumor markers could be early indicators for potential complications in patients with T2DM. Regular monitoring of these markers could provide clinicians with actionable insights, aiding in therapeutic decisions and possibly leading to timely interventions. The correlation of these markers with metabolic indicators, such as HbA1c and FBG, further positions them as potential prognostic tools in T2DM management. As our understanding of T2DM deepens, these markers may emerge as vital tools in refining clinical strategies and bridging the gap between epidemiological data and hands-on patient care.
Some limitations of this study should be considered. A limited extrapolation of results could occur owing to all the study samples being from the same center. Furthermore, considering the relatively small sample size, a cautious interpretation of results is warranted.
Our study detected elevated serum CEA and CA199 levels in patients with T2DM. Additionally, CA199, CEA, and CA242 levels showcased significant correlations with HbA1c and FBG levels. These findings transcend mere epidemiological associations. In the clinical context, the elevated levels of the aforementioned tumor markers in patients with T2DM could indicate potential underlying pathologies or complications. Incorporating routine CA199, CEA, and CA242 assessments in patients with T2DM care might provide clinicians with valuable insights, aiding in therapeutic decisions, especially for those struggling with blood sugar management. Such proactive monitoring could lead to timely interventions, potentially mitigating complications and improving patient outcomes. As our understanding of these markers in the T2DM landscape improves, they might emerge as pivotal tools in refining patient management strategies and improving overall care.
Glucose and lipid metabolic disorder in patients with type 2 diabetes mellitus (T2DM) is closely related to the level of serum tumor markers [such as cancer antigen (CA)199] in the digestive tract. Therefore, tumor markers of T2DM are important.
To assess the expression and clinical significance of serum tumor markers [CA199, CA242, and carcinoembryonic antigen (CEA)] in T2DM.
To study the expression of serum tumor markers (CA199, CA242, and CEA) and its clinical implications in T2DM.
We conducted an observational study at Hefei BOE Hospital, Anhui, China, between April 2019 and December 2020 and enrolled 82 patients with first-onset T2DM and 51 controls. Levels of fasting blood glucose (FBG), tumor markers (CA199, CEA, and CA242), glycosylated hemoglobin (HbA1c), and other metabolic indicators were measured and group index levels were compared. FBG and HbA1c levels were correlated with tumor marker levels. Tumor markers were tested for diagnostic accuracy in patients with high HbA1c (> 9%) using the receiver operating curve (ROC) curve.
Compared to the control group, the T2DM group had higher serum FBG, HbA1c, CA199, and CEA levels (P < 0.05). A comparative analysis of the two groups based on HbA1c levels (Group A: HbA1c ≤ 9%; Group B: HbA1c > 9%) revealed significant differences in CEA and CA199 levels (P < 0.05). The areas under the ROC curve for CEA and CA199 were 0.853 and 0.809, respectively. Moreover, CA199, CEA, and CA242 levels were positively correlated with HbA1c (r = 0.308, 0.426, and 0.551, respectively) and FBG (r = 0.236, 0.231, and 0.298, respectively) levels.
Serum CEA and CA199 levels were high in patients with T2DM. HbA1c and FBG levels correlated with CA199, CEA, and CA242 levels. Patients with poorly controlled blood sugar levels require tumor marker screening.
Serum CEA and CA199 levels were higher in patients with T2DM than in controls. HbA1c and FBG levels correlated with CA199, CEA, and CA242 levels.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rajeswari VD, India; Strain WD, United Kingdom S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27S:S139-S146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Ling S, Zaccardi F, Issa E, Davies MJ, Khunti K, Brown K. Inequalities in cancer mortality trends in people with type 2 diabetes: 20 year population-based study in England. Diabetologia. 2023;66:657-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Chen K, Jiao DA, Zheng S, Zhou L, Yu H, Yuan YC, Yao KY, Ma XY, Zhang Y. Diagnostic value of occult fecal blood testing for colorectal cancer screening. World J Gastroenterol. 1997;3:166-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, Rizos EC, Monori G, Ward HA, Kyrgiou M, Gunter MJ, Tsilidis KK. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol Biomarkers Prev. 2021;30:1218-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 5. | Scherübl H. [Type-2-diabetes and cancer risk]. Dtsch Med Wochenschr. 2021;146:1218-1225. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 6. | Zelenko Z, Gallagher EJ, Tobin-Hess A, Belardi V, Rostoker R, Blank J, Dina Y, LeRoith D. Silencing vimentin expression decreases pulmonary metastases in a pre-diabetic mouse model of mammary tumor progression. Oncogene. 2017;36:1394-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Zayed AA, Beano AM, Amer FN, Maslamani JM, Zmaili MA, Al-Khudary TH, Momani MS, Yousef AF. Serum levels of carcinoembryonic antigen in patients with type 2 diabetes. Endocr Pract. 2016;22:1310-1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Dou H, Sun G, Zhang L. CA242 as a biomarker for pancreatic cancer and other diseases. Prog Mol Biol Transl Sci. 2019;162:229-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J. 2016;37:536-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Shang X, Song C, Du X, Shao H, Xu D, Wang X. The serum levels of tumor marker CA19-9, CEA, CA72-4, and NSE in type 2 diabetes without malignancy and the relations to the metabolic control. Saudi Med J. 2017;38:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Linkeviciute-Ulinskiene D, Patasius A, Zabuliene L, Stukas R, Smailyte G. Increased Risk of Site-Specific Cancer in People with Type 2 Diabetes: A National Cohort Study. Int J Environ Res Public Health. 2019;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Rong F, Dai H, Wu Y, Li J, Liu G, Chen H, Zhang X. Association between thyroid dysfunction and type 2 diabetes: a meta-analysis of prospective observational studies. BMC Med. 2021;19:257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Tong W, Gao H, Wei X, Mao D, Zhang L, Chen Q, Zhang Z, Li Y. Correlation of serum CA199 levels with glycemic control and microvascular complications in patients with type 2 diabetes mellitus. Am J Transl Res. 2021;13:3302-3308. [PubMed] [Cited in This Article: ] |
| 14. | Chen PC, Lin HD. Reversible high blood CEA and CA19-9 concentrations in a diabetic patient. Libyan J Med. 2012;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Ata N, Dal K, Kucukazman M, Yeniova AÖ, Karakaya S, Unsal O, Dagdeviren M, Akın KO, Baser S, Beyan E, Ertugrul DT. The effect of glycemic control on CEA, CA 19-9, amylase and lipase levels. Open Med (Wars). 2015;10:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hasan M, Mohieldein A. Association between serum carcinoembryonic antigen level and oxidative stress parameters among diabetic females. Int J Clin Exp Med. 2015;8:6489-6494. [PubMed] [Cited in This Article: ] |
| 17. | Qin S, Lu Y, Chen S, Hu Z, Chen H, Zhong J, Li S, Chen Z. The Relationship of Neutrophil-to-Lymphocyte Ratio or Platelet-to-Lymphocyte Ratio and Pancreatic Cancer in Patients with Type 2 Diabetes. Clin Lab. 2019;65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31 Suppl 2:S161-S164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |