Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.179
Peer-review started: December 25, 2022
First decision: January 9, 2023
Revised: January 17, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 15, 2023
Gestational diabetes mellitus (GDM) is a common pregnancy complication strongly associated with poor maternal-fetal outcomes. Its incidence and prevalence have been increasing in recent years. Women with GDM typically give birth through either vaginal delivery or cesarean section, and the maternal-fetal outcomes are related to several factors such as cervical level, fetal lung maturity, the level of glycemic control still present, and the mode of treatment for the condition. We categorized women with GDM based on the latter two factors. GDM that is managed without medication when it is responsive to nutrition- and exercise-based therapy is considered diet- and exercise-controlled GDM, or class A1 GDM, and GDM managed with medication to achieve adequate glycemic control is considered class A2 GDM. The remaining cases in which neither medical nor nutritional treatment can control glucose levels or patients who do not control their blood sugar are categorized as class A3 GDM. We investigated the optimal time of delivery for women with GDM according to the classification of the condition. This review aimed to address the benefits and harms of giving birth at different weeks of gestation for women with different classes of GDM and attempted to provide an analytical framework and clearer advice on the optimal time for labor.
Core Tip: The global incidence of gestational diabetes mellitus (GDM) is increasing, and GDM is closely related to adverse maternal-fetal outcomes. Therefore, the time of delivery for women with GDM has gained increasing attention in recent years. The maternal and fetal outcomes of pregnancy in women with GDM are closely related to the level of glycemic control and modality of treatment. This review aims to summarize current research on the classification of GDM, discuss the benefits and harms of delivery at different gestational weeks in women with GDM, and determine the optimal time of delivery for women with different classes of GDM.
- Citation: Li X, Li TT, Tian RX, Fei JJ, Wang XX, Yu HH, Yin ZZ. Gestational diabetes mellitus: The optimal time of delivery. World J Diabetes 2023; 14(3): 179-187
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/179.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.179
Gestational diabetes mellitus (GDM) is a glucose intolerance disorder first diagnosed during pregnancy[1]. Recently, the global incidence rate of GDM has been increasing, and the incidence rate in some regions is as high as 14%. The occurrence of GDM is related to obesity, changes in diet structure, reduced exercise, and other factors[2-4]. Moreover, history of GDM and family history of diabetes can also increase the probability of GDM occurrence[5].
GDM is one of the most common metabolic disorder syndromes during pregnancy and is related to adverse maternal and fetal outcomes[6]. Studies have shown that GDM can lead to an increased risk of embryonic disease in early pregnancy. A retrospective study by Zawiejska et al[7] showed that early occurrence of GDM can lead to a significantly increased risk of congenital malformations, especially in the heart. Data have also shown that patients with GDM have a significantly increased risk of preeclampsia, premature rupture of membranes, amniotic fluid contamination, stillbirth, macrosomia, and fetal growth restriction in the third trimester[8,9]. The risk of stillbirth in women with GDM increases with gestational weeks, and prenatal fetal death among patients with diabetes occurs mainly in the third trimester or after 40 wk[10]. GDM also triggers a delay in fetal lung maturation, making the process take up to 38.5 wk[11].
Based on a study of hyperglycemia and adverse pregnancy outcome in 2008, the higher the blood glucose value obtained using the 75-g oral glucose tolerance test (OGTT) at 24-32 wk of gestation, the higher the risk of adverse maternal-fetal outcomes[12]. Therefore, it is recommended that all pregnant women should be screened for GDM. Currently, for GDM screening and diagnosis, most guidelines recommend the “one-step method” or the “two-step method”[4,13-16].
In 1973, O'Sullivan et al[17] first proposed the “two-step method” to diagnose GDM. The 50-g OGTT was first performed, followed by a 100-g OGTT if the one-hour plasma glucose result was abnormal. The 2018 guidelines of the American College of Obstetricians and Gynecologists (ACOG) and the 2019 guidelines of the Society of Obstetricians and Gynecologists of Canada (SOGC) currently recommend the “two-step method” for the diagnosis of GDM[4,16].
Subsequently, the International Association of Diabetes and Pregnancy Study Groups (IADPSG)[13] proposed the “one-step method” in 2010, which comprises a 75-g OGTT under a fasting state. This screening method is recommended by most guidelines, including those of the World Health Organization (2013), Australasian Diabetes in Pregnancy Society (2014), and International Federation of Gynecology and Obstetrics (2015)[14,15]. Nevertheless, the two diagnostic criteria have some diff-erences. Mirghani Dirar and Doupis[18] made a simple comparison of the two competing diagnostic criteria for GDM and supported using the IADPSG criteria as an international standard approach.
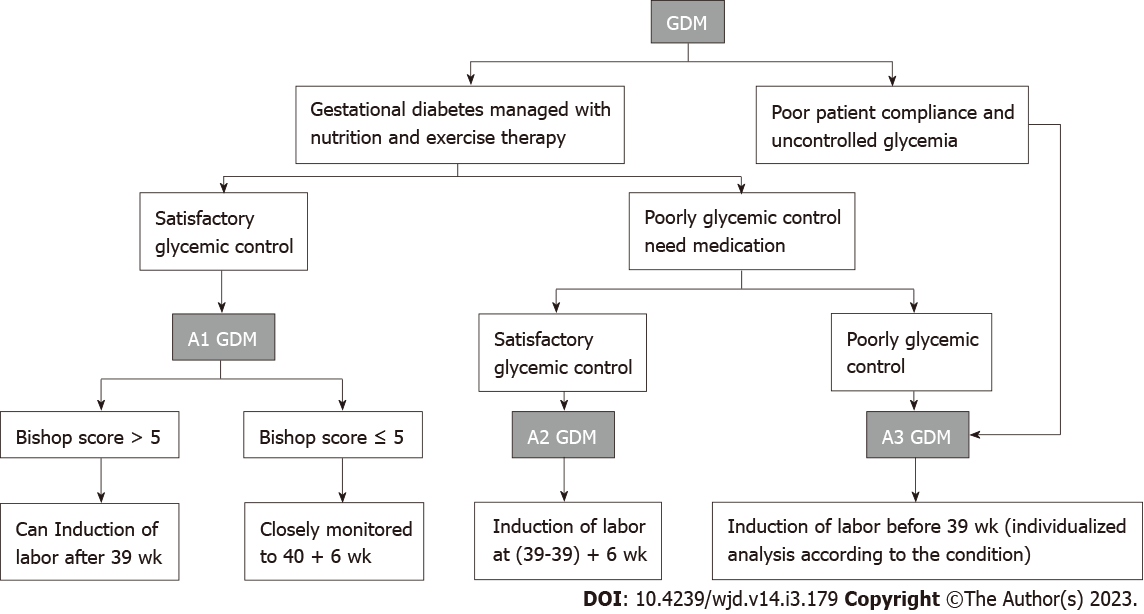
GDM that is responsive to nutrition and exercise therapy and managed without medication is considered diet- and exercise-controlled GDM or class A1 GDM. GDM managed by medication to achieve adequate glycemic control is considered class A2 GDM[19]. The remaining cases in which neither medical nor nutritional treatment can control glucose levels or patients who do not control their blood sugar are categorized as class A3 GDM.
Compared to that during normal pregnancies, the incidence of complications in the third trimester among patients with GDM is significantly higher, resulting in more adverse effects with regard to maternal and infant health. Data in the literature have shown that the risk of adverse pregnancy outcomes among patients with GDM is related to maternal glycemic level[20]. The three GDM subtypes all exhibit different characteristics in terms of maternal glycemic control, and thus their adverse pregnancy risks are also different. The current guidelines and related studies on choice of delivery time for different classes of GDM, however, are still inconsistent. Therefore, we sought in this review to discuss the optimal time of delivery to reduce the risk of adverse events for the three different types of GDM.
Pregnancy conclusion includes either vaginal delivery or cesarean section. Vaginal delivery can either be spontaneous or medically induced[16]. It has been suggested that the goal of GDM management should be to achieve optimal maternal and fetal outcomes with minimal interventions, under strict glycemic control[21]. Patients with GDM who do not spontaneously undergo vaginal delivery can end their pregnancies by either induction of labor or cesarean section, which can minimize maternal-fetal risks.
A1 GDM is the most common type of GDM (> 70%), for which the optimal time of delivery is of great concern[22]. Several national clinical practice guidelines make recommendations regarding the time of delivery for patients with A1 GDM. ACOG, for example, does not recommend delivery before 39 wk and instead, to wait up to 40 + 6 wk for spontaneous delivery[16]. SOGC, in contrast, believes that induction of labor at 40 wk may be beneficial[4]. The Chinese Society of Perinatal Medicine recommends that pregnancies in patients with A1 GDM should be completed at 40-41 wk[23]. In Sweden, patients with A1 GDM who have normal blood glucose and no other indications are expected to be managed before 42 wk of gestation, and labor is induced if spontaneous delivery does not occur[24]. Overall, then, there is no uniformity in the clinical guidelines for the optimal delivery time in patients with A1 GDM[4,16,24].
Whether patients with A1 GDM are at additional risk of maternal-fetal complications that require earlier completion of pregnancy than normal patients remains controversial based on published reports of studies. Several studies have compared the differences in the maternal and fetal outcomes of patients with A1 GDM or GDM with good glycemic control compared to normal pregnancies[25-27]. Han et al[25] compared the differences in maternal and fetal outcomes between 120 patients with A1 GDM and 200 normal patients who underwent delivery at (31-38) + 6 wk of gestation and found that acceleration time (AT), injection time (ET), and AT/ET, which reflect fetal lung maturity, were not significantly different between the two groups and positively correlated with gestational week. The incidence of neonatal respiratory distress syndrome, neonatal pneumonia, neonatal hypoglycemia, and neonatal asphyxia is not significantly different between A1 GDM and normal pregnancies. Tartaglione et al[26] continuously monitored and adjusted blood glucose levels in 46 patients with GDM and 53 normal controls and found no differences in maternal and fetal outcomes between them when blood glucose levels were consistently controlled. Hochberg et al[27] compared the differences in maternal and fetal outcomes after induction of labor from 37-38 wk in 193 non-GDM patients and 39-40 wk in 237 singleton pregnant women with well-controlled GDM and found no differences between gestational week intervals. However, these prospective studies generally suffered from inadequate sample sizes.
Valgeirsdóttir et al[24] reviewed the occurrence of adverse maternal and fetal outcomes in patients with GDM compared to that of normal subjects in Sweden over 14 years and found that patients with A1 GDM had significantly higher rates of cesarean section and obstructed shoulder births and significantly lesser gestational duration before birth than normal subjects. Familiari et al[28] investigated the predictive role of fetal Doppler parameters on maternal and fetal outcomes in patients with GDM and found that the middle cerebral artery pulsatility index (MCA PI) was significantly associated with adverse perinatal outcomes such as the need for cesarean section birth. They concluded that, instead of solely focusing on the glycemic aspect of the disease, the assessment of fetal Doppler, especially MCA PI, is necessary for patients with GDM. Simeonova-Krstevska et al[29] compared maternal and fetal outcomes among patients with A1 and A2 GDM and found that patients with A1 GDM who gained more weight during pregnancy were at a higher risk of large-for-gestational-age (LGA) fetuses than patients with A2 GDM. These findings demonstrate that the risk of adverse maternal-fetal outcomes remains higher than normal in patients with A1 GDM and that more indicators need to be combined to determine pregnancy status in these patients.
Several studies have shown that the rate of cesarean section, fetal death, and stillbirth and the risk of neonatal admission to the neonatal intensive care unit in patients with A1 GDM are all associated with the time of pregnancy[21,30,31]. Šimják et al[21] found that the highest rate of neonatal complications occurred upon induction of labor at (37-37) + 6 wk of gestation in patients with A1 GDM. Melamed et al[30] found a significantly increased risk of neonatal hypoglycemia, jaundice requiring phototherapy, and admission to the neonatal intensive care unit with induction of labor at ≤ (38 + 6) wk of gestation among patients with low-risk GDM. Niu et al[32] used a theoretical cohort model to study the optimal time of labor induction in patients with A1 GDM and concluded that fetal death could be minimized when labor was induced at (38-38) + 6 wk of gestation. However, the study focused on fetal mortality as the main factor for analysis and did not consider cesarean section rates or other maternal and fetal outcomes, and the authors agreed that the study was a simulation and not representative of real-world conditions. Our previous study also concluded that deliveries at ≤ (38 + 6) wk of gestation did not show a benefit compared to those at (39-40) + 6 wk of gestation[33]. We also found a significant increase in the rate of LGA and caesarean section (CS) at ≥ 41 wk of gestation, which was similar to the findings of Šimják et al[21] and Sutton et al[31] found that the rate of CS was three times higher when labor was induced at ≥ 41 wk of gestation than at (39-39) + 6 wk of gestation in patients with mild GDM. A survey of obstetricians[34] found that only 10% of patients with A1 GDM actually delivered at (38-38) + 6 wk. Therefore, we believe that the time of labor induction in patients with A1 GDM should be (39-40) + 6 wk of gestation.
A1 GDM is determined only based on the mode and level of glycemic control[16], but some studies have found that maternal and fetal outcomes in patients with A1 GDM are not only related to the level of glycemia but also to the cervical development status, number of births, and other factors[30,33,35].
Our study found that the induction of labor at (39-39) + 6 wk of gestation in patients with A1 GDM with Bishop scores of ≥ 5 was associated with a significantly higher rate of vaginal delivery compared to that upon induction of labor at (38-38) + 6, (40-40) + 6, and (41-41) + 6 wk of gestation[33]. Melamed et al[30] also found that the induction of labor at (39-39) + 6 wk of gestation significantly reduced the rate of cesarean section in patients with GDM; however, this study included pregnant women with better glycemic control and did not classify patients into A1/A2 GDM subgroups. Feghali et al[35] found a significantly higher Cesarean delivery rate among patients subjected to labor inductions at (39-39) + 6 wk of gestation than among those who underwent expectant management in transitional patients with GDM and a Bishop score < 5. Our previous study also found a significantly higher Cesarean delivery rate upon labor induction at (39-39) + 6 wk in patients with a Bishop score < 4[33]. For this population, those with poor cervical statuses are not suitable for induction of labor and should wait for spontaneous delivery. Overall, we believe that patients undergoing induction of labor need to be carefully assessed for Bishop score, with indicators such as cervical maturity kept in mind. Induction of labor at (39-39) + 6 wk of gestation is a recommended option for patients with A1 GDM who exhibit good cervical statuses.
Patients with A1 GDM who have poor cervical scores or are unwilling to induce labor at (39-39) + 6 wk of gestation should be closely monitored and allowed to continue their pregnancies until 40 + 6 wk. We previously found that the Cesarean delivery rate was significantly lower in A1 GDM patients with spontaneous delivery at (40-40) + 6 wk of gestation than in patients with induced labor[33]. Šimják et al[21] found a significant reduction in the incidence of neonatal hypoglycemia and macrosomia in patients with A1 GDM subjected to labor induction at (40-40) + 6 wk of gestation and concluded that induction of labor at (40-40) + 6 wk resulted in the best neonatal outcomes. Karmon et al[36] found that the stillbirth rate was even lower in patients with A1 GDM at (40-40) + 6 wk than in their normal counterparts, showing the benefits of delivering at (40-40) + 6 wk among patients with A1 GDM. However, several studies have shown that patients with A1 GDM who are still not experiencing spontaneous contractions at (40-40) + 6 wk of gestation have a significantly higher rate of needing cesarean sections after induction of labor[31,33]. A survey of clinicians found that approximately 57% of patients, in fact, delivered at (40-40) + 6 wk of gestation[34]. Therefore, we believe that it is beneficial to wait until (40-40) + 6 wk for spontaneous delivery and that even if patients at (40-40) + 6 wk of gestation require induction of labor for spontaneous delivery, the stillbirth rate at this time is lower than that of normal pregnancies[36].
We believe that the best time to induce labor in patients with A1 GDM is between (39-39) + 6 wk and (40-40) + 6 wk of gestation; the time should be determined individually based on the patient’s cervical Bishop score, as well as other indicators. There are two main options for this decision: Induction of labor at (39-39) + 6 wk or waiting until spontaneous delivery before 40 + 6 wk of gestation and inducing labor if spontaneous delivery does not occur. The data from existing studies do not control well for confounding factors, resulting in shortcomings in the reliabilities of the findings. Moreover, the numbers and sample sizes of studies on A1 GDM stratified by the week of gestation are currently small, and higher quality randomized controlled trial (RCT) studies with larger sample sizes are warranted to further confirm and refine existing findings.
For women with A2 GDM, current guidelines from the American College of Obstetricians and Gynecologists recommend insulin as the standard therapy for treating pregestational diabetes and GDM[37]. However, insulin requires to be injected and is associated with hypoglycemia, excessive gestational weight gain, and increased CS rates[38]. Insulin therapy also increases the chances of LGA[39]. An alternative approach is the use of oral medication, which includes glibenclamide and metformin[40]. One meta-analysis demonstrated that metformin was superior to insulin in terms of perinatal outcomes and that glibenclamide was inferior to insulin and metformin due to an increased risk of adverse perinatal outcomes. Therefore, if insulin or metformin is available, glibenclamide should not be used in treating women with GDM[41].
As with A1 GDM, delivery time considerations in women with A2 GDM should focus on balancing the increased risk of neonatal morbidity or mortality associated with early delivery and the increased risk of stillbirth associated with expected management. The National Institute of Child Health and Human Development (NICHD) recommends delivery at 39 wk of gestation[42]. The ACOG recommends a time of delivery of (39-39) + 6 wk for patients with A2 GDM[43]. A retrospective cohort study showed a significantly increased risk of perinatal death among women with GDM expecting to be managed compared to those who delivered at 39 wk of gestation. Neonatal morbidity did not appear to be higher for deliveries induced at 39 wk than at 40 wk in that study, supporting the preference for delivery at 39 wk of gestation in women with GDM, although the study did not strictly differentiate between A1 and A2 GDM[9]. The main clinical study investigating the optimal time of delivery for patients with A2 GDM is a prospective RCT by Kjos et al[44] in 1993, which compared labor induction to expectant treatment at 38 wk. The expectant treatment group was found to have a significantly higher incidence of LGA fetuses and shoulder dystocia. This study supported the induction of labor at 38 wk in patients with insulin-dependent GDM[44]. Similarly, a prospective study in 1996 showed that the incidence of shoulder dystocia could be reduced by selective labor induction at 38-39 wk of gestation in women with diabetes requiring insulin[45]. From these studies, we can conclude that the most widely recommended time of delivery for patients with A2 GDM is currently (39-39) + 6 wk of gestation.
Although we currently believe that the optimal delivery time for patients with A2 GDM is (39-39) + 6 wk of gestation, due to the inadequacy of the current literature on the subject, newer and more robust clinical evidence to support this view is warranted. Moreover, the cervical status and fetal lung maturity should not be ignored when considering the time of delivery for patients with A2 GDM. The cervical status is an important factor for the success of labor induction[46], and fetal lung maturity is also closely related to the incidence of complications in newborns. Patients with GDM are more likely to give birth to babies with neonatal respiratory distress syndrome[47]. In the above-mentioned clinical studies regarding the delivery time of patients with type A2 GDM, amniocentesis was performed to check fetal lung maturity, Bishop scores were calculated to evaluate the cervical statuses, and labor induction time and method were adjusted according to these parameters[44,45]. Therefore, the induction time for women with A2-type GDM also needs to be adjusted according to the cervical condition and fetal lung maturity. Moreover, in the current studies on optimal delivery time for patients with A2 GDM, most of the drugs that pregnant women receive to control blood glucose levels are insulin injections. In clinical practice, whether the delivery time of patients with GDM should be changed according to the actual situation in combination with oral hypoglycemic drug therapy and insulin therapy to manage blood glucose remains to be studied.
For pregnant women with poor glycemic control, NICHD recommends delivery between 34 and 39 + 6 wk of gestation[48], and ACOG states that delivery should be delayed for women with poor glycemic control or diabetic complications, even in the hospital. The recommended time for delivery is between 37 wk and 38 + 6 wk[16].
Women with poor glycemic control are at an increased risk of maternal and neonatal-perinatal complications compared to that of women with good glycemic control, with poor glycemic control being strongly associated with poor maternal-fetal outcomes[49]. Infants from mothers with GDM often develop complications related to maternal hyperglycemia, including neonatal hypoglycemia, respiratory disturbances, hypocalcemia, polycythemia, hyperbilirubinemia, cardiac hypertrophy, and LGA[50].
When considering the optimal time for labor in pregnant women with GDM who have poor glycemic control or complications, it is important to weigh the advantages and disadvantages of waiting for labor vs induction of labor. A RCT has shown that delivery at 38 wk reduced macrosomia and obstructed shoulder labor[51]. Metcalfe et al[52] concluded that, among women with GDM, iatrogenic delivery was associated with an increased risk of neonatal morbidity/mortality compared to expectant management at 36 and 37 wk of gestation and a lower risk of neonatal morbidity/mortality at 38, 39, and 40 wk of gestation. However, perinatal mortality decreases with improved glycemic control[53,54]. Experts believe that the optimal time of delivery for women with poor glycemic control should actually be earlier[43,55], but there is currently a lack of specific guidance on the degree of glycemic control in women with A3 GDM, and more reliable prospective randomized studies are needed to determine the optimal time of delivery[42].
Before delivery, attention should also be paid to assessing the maternal cervical status and determining fetal lung maturity. The key to successful labor induction is the readiness of the cervix for labor, and a higher Bishop score is strongly associated with a successful procedure. The MohawksJakub study found that women with a Bishop score < 6 had fourfold higher risks of CS during labor[56]. A higher Bishop score and a shorter cervix based on vaginal ultrasound reduce the probability of a cesarean section being necessary for delivery and are associated with lower maternal and neonatal morbidity as well as shorter hospitalization time[46,57]. The risk of neonatal respiratory distress syndrome in pregnant women with GDM is six times higher than that in normal pregnant women[47], and several studies have demonstrated that poor glycemic control in pregnant women with GDM is associated with delayed fetal lung maturation, meaning that it is particularly important to measure fetal lung maturation before delivery in patients with A3 GDM[25,58].
Therefore, for women with poor glycemic control, we recommend delivery before 39 wk, considering the cervical status and fetal lung maturity. Since the risk of perinatal complications and fetal death is increasing for this population, the optimal time of delivery should be “individualized” by clinicians for this group.
The optimal time of labor for patients with different classes of GDM is determined by a combination of maternal and fetal factors, and the choice should be made considering the advantages and disadvantages of inducing labor compared to waiting for a spontaneous contraction to optimize both maternal and fetal outcomes (check recommendation in Figure 1). In the future, more prospective randomized studies should be conducted on the time of labor in patients with different classes of GDM, incorporating factors such as type of diabetes, level of glycemic control, Bishop score, fetal lung maturity, and presence of complications in order to provide better quality data for decision making.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adela R, India; Thandassery RB, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol Metab. 2018;29:743-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 386] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 2. | Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 224] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 3. | Yuen L, Wong VW. Gestational diabetes mellitus: Challenges for different ethnic groups. World J Diabetes. 2015;6:1024-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 97] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 4. | Berger H, Gagnon R, Sermer M. Guideline No. 393-Diabetes in Pregnancy. J Obstet Gynaecol Can. 2019;41:1814-1825.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Diabetes Canada Clinical Practice Guidelines Expert Committee; Feig DS, Berger H, Donovan L, Godbout A, Kader T, Keely E, Sanghera R. Diabetes and Pregnancy. Can J Diabetes. 2018;42 Suppl 1:S255-S282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Vince K, Perković P, Matijević R. What is known and what remains unresolved regarding gestational diabetes mellitus (GDM). J Perinat Med. 2020;48:757-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Zawiejska A, Wróblewska-Seniuk K, Gutaj P, Mantaj U, Gomulska A, Kippen J, Wender-Ozegowska E. Early Screening for Gestational Diabetes Using IADPSG Criteria May Be a Useful Predictor for Congenital Anomalies: Preliminary Data from a High-Risk Population. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Mistry SK, Das Gupta R, Alam S, Kaur K, Shamim AA, Puthussery S. Gestational diabetes mellitus (GDM) and adverse pregnancy outcome in South Asia: A systematic review. Endocrinol Diabetes Metab. 2021;4:e00285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Rosenstein MG, Cheng YW, Snowden JM, Nicholson JM, Doss AE, Caughey AB. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol. 2012;206:309.e1-309.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Kapustin R, Arzhanova O, Alekseenkova E, Glotov A. Time and mode of delivery in diabetic pregnancy: a review. Gynecol Endocrinol. 2020;36:58-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66 Suppl 2:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 445] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 12. | HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3783] [Cited by in F6Publishing: 3405] [Article Influence: 212.8] [Reference Citation Analysis (0)] |
| 13. | International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2198] [Cited by in F6Publishing: 2769] [Article Influence: 197.8] [Reference Citation Analysis (1)] |
| 14. | Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 458] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 15. | Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, Cabero Roura L, McIntyre HD, Morris JL, Divakar H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131 Suppl 3:S173-S211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 507] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 16. | ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131:e49-e64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 891] [Article Influence: 148.5] [Reference Citation Analysis (0)] |
| 17. | O'Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116:895-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 269] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Mirghani Dirar A, Doupis J. Gestational diabetes from A to Z. World J Diabetes. 2017;8:489-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 116] [Cited by in F6Publishing: 101] [Article Influence: 14.4] [Reference Citation Analysis (6)] |
| 19. | Quintanilla Rodriguez BS, Mahdy H. Gestational Diabetes. 2022 Sep 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] [Cited in This Article: ] |
| 20. | Modzelewski R, Stefanowicz-Rutkowska MM, Matuszewski W, Bandurska-Stankiewicz EM. Gestational Diabetes Mellitus-Recent Literature Review. J Clin Med. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 21. | Šimják P, Krejčí H, Hornová M, Mráz M, Pařízek A, Kršek M, Haluzík M, Anderlová K. Establishing the Optimal Time for Induction of Labor in Women with Diet-Controlled Gestational Diabetes Mellitus: A Single-Center Observational Study. J Clin Med. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 22. | Oskovi-Kaplan ZA, Ozgu-Erdinc AS. Management of Gestational Diabetes Mellitus. Adv Exp Med Biol. 2021;1307:257-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Obstetrics Subgroup, Chinese Society of Obstetrics and Gynecology; Chinese Medical Association; Chinese Society of Perinatal Medicine, Chinese Medical Association; Committee of Pregnancy with Diabetes Mellitus, China Maternal and Child Health Association. [Guideline of diagnosis and treatment of hyperglycemia in pregnancy (2022) [Part two]]. Zhonghua Fu Chan Ke Za Zhi. 2022;57:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Valgeirsdóttir IR, Hanson U, Schwarcz E, Simmons D, Backman H. Diet-Treated Gestational Diabetes Mellitus Is an Underestimated Risk Factor for Adverse Pregnancy Outcomes: A Swedish Population-Based Cohort Study. Nutrients. 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 25. | Han T, Jin XD, Yang JF, Tang Y. Clinical Analysis of Fetal Lung Development Index and Pregnancy Outcome in Pregnant Women with Gestational Diabetes Mellitus with Satisfactory Blood Glucose Control. Contrast Media Mol Imaging. 2022;2022:5777804. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 26. | Tartaglione L, di Stasio E, Sirico A, Di Leo M, Caputo S, Rizzi A, Caneschi A, De Carolis S, Pitocco D, Lanzone A. Continuous Glucose Monitoring in Women with Normal OGTT in Pregnancy. J Diabetes Res. 2021;2021:9987646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Hochberg A, Pardo A, Oron G, Krispin E, Amikam U, Wiznitzer A, Hadar E, Salman L. Perinatal outcome following induction of labor in patients with good glycemic controlled gestational diabetes: does timing matter? Arch Gynecol Obstet. 2019;300:299-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Familiari A, Neri C, Vassallo C, Di Marco G, Garofalo S, Martino C, Degennaro V, Lanzone A. Fetal Doppler Parameters at Term in Pregnancies Affected by Gestational Diabetes: Role in the Prediction of Perinatal Outcomes. Ultraschall Med. 2020;41:675-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Simeonova-Krstevska S, Bogoev M, Bogoeva K, Zisovska E, Samardziski I, Velkoska-Nakova V, Livrinova V, Todorovska I, Sima A, Blazevska-Siljanoska V. Maternal and Neonatal Outcomes in Pregnant Women with Gestational Diabetes Mellitus Treated with Diet, Metformin or Insulin. Open Access Maced J Med Sci. 2018;6:803-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Melamed N, Ray JG, Geary M, Bedard D, Yang C, Sprague A, Murray-Davis B, Barrett J, Berger H. Induction of labor before 40 weeks is associated with lower rate of cesarean delivery in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2016;214:364.e1-364.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Sutton AL, Mele L, Landon MB, Ramin SM, Varner MW, Thorp JM Jr, Sciscione A, Catalano P, Harper M, Saade G, Caritis SN, Sorokin Y, Grobman WA; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Delivery timing and cesarean delivery risk in women with mild gestational diabetes mellitus. Am J Obstet Gynecol. 2014;211:244.e1-244.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Niu B, Lee VR, Cheng YW, Frias AE, Nicholson JM, Caughey AB. What is the optimal gestational age for women with gestational diabetes type A1 to deliver? Am J Obstet Gynecol. 2014;211:418.e1-418.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Yin Z, Li T, Zhou L, Fei J, Su J, Li D. Optimal delivery time for patients with diet-controlled gestational diabetes mellitus: a single-center real-world study. BMC Pregnancy Childbirth. 2022;22:356. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 34. | Ogunyemi DA, Fong A, Rad S, Fong S, Kjos SL. Attitudes and practices of healthcare providers regarding gestational diabetes: results of a survey conducted at the 2010 meeting of the International Association of Diabetes in Pregnancy Study Group (IADPSG). Diabet Med. 2011;28:976-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Feghali MN, Caritis SN, Catov JM, Scifres CM. Timing of delivery and pregnancy outcomes in women with gestational diabetes. Am J Obstet Gynecol. 2016;215:243.e1-243.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Karmon A, Levy A, Holcberg G, Wiznitzer A, Mazor M, Sheiner E. Decreased perinatal mortality among women with diet-controlled gestational diabetes mellitus. Int J Gynaecol Obstet. 2009;104:199-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Sleeman A, Odom J, Schellinger M. Comparison of Hypoglycemia and Safety Outcomes With Long-Acting Insulins Versus Insulin NPH in Pregestational and Gestational Diabetes. Ann Pharmacother. 2020;54:669-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;11:CD012037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Dunne F, Newman C, Devane D, Smyth A, Alvarez-Iglesias A, Gillespie P, Browne M, O'Donnell M. A randomised placebo-controlled trial of the effectiveness of early metformin in addition to usual care in the reduction of gestational diabetes mellitus effects (EMERGE): study protocol. Trials. 2022;23:795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 40. | Sweeting A, Wong J, Murphy HR, Ross GP. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43:763-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 142] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 41. | Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 42. | Caughey AB, Valent AM. When to Deliver Women with Diabetes in Pregnancy? Am J Perinatol. 2016;33:1250-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Society for Maternal-Fetal Medicine. Medically Indicated Late-Preterm and Early-Term Deliveries: ACOG Committee Opinion, Number 831. Obstet Gynecol. 2021;138:e35-e39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 44. | Kjos SL, Henry OA, Montoro M, Buchanan TA, Mestman JH. Insulin-requiring diabetes in pregnancy: a randomized trial of active induction of labor and expectant management. Am J Obstet Gynecol. 1993;169:611-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 163] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 45. | Lurie S, Insler V, Hagay ZJ. Induction of labor at 38 to 39 weeks of gestation reduces the incidence of shoulder dystocia in gestational diabetic patients class A2. Am J Perinatol. 1996;13:293-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Ezebialu IU, Eke AC, Eleje GU, Nwachukwu CE. Methods for assessing pre-induction cervical ripening. Cochrane Database Syst Rev. 2015;2015:CD010762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Robert MF, Neff RK, Hubbell JP, Taeusch HW, Avery ME. Association between maternal diabetes and the respiratory-distress syndrome in the newborn. N Engl J Med. 1976;294:357-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 203] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Diabetes in pregnancy: management from preconception to the postnatal period. London: National Institute for Health and Care Excellence (NICE); 2020-Dec-16 . [PubMed] [Cited in This Article: ] |
| 49. | Horvath K, Koch K, Jeitler K, Matyas E, Bender R, Bastian H, Lange S, Siebenhofer A. Effects of treatment in women with gestational diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;340:c1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 50. | Cheng YW, Caughey AB. Gestational diabetes: diagnosis and management. J Perinatol. 2008;28:657-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Witkop CT, Neale D, Wilson LM, Bass EB, Nicholson WK. Active compared with expectant delivery management in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113:206-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Metcalfe A, Hutcheon JA, Sabr Y, Lyons J, Burrows J, Donovan LE, Joseph KS. Timing of delivery in women with diabetes: A population-based study. Acta Obstet Gynecol Scand. 2020;99:341-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Bassaw B, Ataullah I, Roopnarinesingh S, Sirjusingh A. Diabetes in pregnancy. Int J Gynaecol Obstet. 1995;50:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477-2486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2150] [Cited by in F6Publishing: 2012] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 55. | Spong CY, Mercer BM, D'Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 56. | Jakub M, Marta M, Jagoda G, Kamila G, Stanislaw G. Is Unfavourable Cervix prior to Labor Induction Risk for Adverse Obstetrical Outcome in Time of Universal Ripening Agents Usage? J Pregnancy. 2020;2020:4985693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Vimercati A, Greco P, Lopalco P, Loizzi V, Scioscia M, Mei L, Rossi AC, Selvaggi L. The value of ultrasonographic examination of the uterine cervix in predicting post-term pregnancy. J Perinat Med. 2001;29:317-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Piper JM. Lung maturation in diabetes in pregnancy: if and when to test. Semin Perinatol. 2002;26:206-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |