Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1877
Peer-review started: August 26, 2023
First decision: October 9, 2023
Revised: October 19, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 15, 2023
Maturity-onset diabetes of the young 10 caused by the c.4G>A (p.Ala2Thr) mutation is extremely rare, with only two reported studies to date. Herein, we report another case that differs from previous cases in phenotype.
The proband developed diabetes at the age of 27 years, despite having a normal body mass index (BMI). She exhibited partial impairment of islet function, tested positive for islet antibodies, and required high doses of insulin. Her sister also carried the c.4G>A (p.Ala2Thr) mutation, and their mother was strongly suspected to carry the mutated gene. Her sister developed diabetes around 40 years of age and required high doses of insulin, while the mother was diagnosed in her 20s and was managed with oral hypoglycemic agents; neither of them were obese.
p.Ala2Thr mutation carriers often experience relatively later onset and normal BMI. Treatment regimens vary between individuals.
Core Tip: Maturity-onset diabetes of the young (MODY) 10 is uncommon, especially when caused by the c.4G>A (p.Ala2Thr) mutation, and thus, our knowledge of this disease is limited. Herein, we present an atypical MODY10 case resulting from the p.Ala2Thr mutation, which differs from previous reports and deviates from the prevalent phenotype of MODY. This patient exhibited insulin resistance and positive islet autoantibodies, as well as demonstrated significant familial inheritance and hearing impairment, which increased the potential for misdiagnosis.
- Citation: Chen H, Fei SJ, Deng MQ, Chen XD, Wang WH, Guo LX, Pan Q. Maturity-onset diabetes of the young type 10 caused by an Ala2Thr mutation of INS: A case report. World J Diabetes 2023; 14(12): 1877-1884
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1877.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1877
Maturity-onset diabetes of the young (MODY) is an autosomal dominant monogenic diabetes, characterized by islet cell dysfunction or impaired insulin synthesis and secretion[1]. Most individuals have early age onset diabetes and usually do not require insulin during the early stages of the disease. MODY accounts for approximately 1%-5% of diabetes, but is often misdiagnosed as type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus for various reasons[2].
At least 14 subtypes of MODY have been identified[3]. MODY10 is relatively rare, and is caused by a mutation of the 11p15.5 site on chromosome 11 encoding insulin[4]. Preproinsulin is synthesized by the transcription and translation of INS, and subsequently cleaved to secrete insulin[5]. Therefore, INS mutations are strongly associated with abnormal insulin generation and glucose metabolism. Two studies have reported the c.4G>A (p.Ala2Thr) mutation in MODY10 patients and confirmed that this mutation was closely related to preproinsulin cleavage and insulin synthesis[6,7]. Here, we report another clinical case of MODY10 caused by the c.4G>A (p.Ala2Thr) mutation in a Chinese pedigree, and review the literature to summarize the clinical characteristics of MODY10 resulting from INS c.4G>A (p.Ala2Thr).
This case report describes a 53-years-old woman who had suffered from polyphagia, polydipsia, polyuria, and weight loss for 26 years, as well as repeated dizziness, cold sweats, and palpitations for one week.
Symptoms including recurrent dizziness, cold sweats, and palpitations started one week before the patient presented to the hospital. Blood glucose levels were often < 3.9 mmol/L during these episodes.
The individual presented with typical hyperglycemic symptoms and was diagnosed with T1DM in 1996 when she was 27 years old. Both fasting C-peptide (FCP) and postprandial C-peptide (PCP) levels were low, although details on the specific data were unavailable. Islet-related antibodies and hemoglobin A1c (HbA1c) levels could not be recalled. Due to an early age onset, as well as being non-obese and exhibiting pancreatic insufficiency, the patient was diagnosed with T1DM. Insulin therapy was initiated (12 U, 8 U, 8 U Novolin-R before three meals, 0.56 U/kg/d). Treatment regimens were subsequently adjusted according to the patient’s blood glucose levels. After three years, the regimen was modified to Novolin-R 50/50 (18 U before breakfast and 12 U before dinner, 0.625 U/kg/d). However, since her blood glucose levels remained high, doses were gradually increased to 20 U and 18 U. Five years later, the proband’s HbA1c levels were 6.8%, fasting blood glucose (FBG) levels were 5.32 mmol/L, and FCP levels were 430 pmol/L. The proband was positive for both glutamic acid decarboxylase antibody (GADA) and islet cell antibody. Despite the absence of foamy urine, the urine albumin-creatinine ratio was 240.90 mg/g and 214.19 mg/g, and the estimated glomerular rate (eGFR) was 71.59 mL/min/(1.73 m²). She was diagnosed with T1DM with diabetic kidney disease (DKD) (G2A2 stage). The patient exhibited higher blood glucose levels (10-12 mmol/L) after lunch and dinner, but fasting glucose (around 7 mmol/L) and post-breakfast glucose (around 8 mmol/L) levels were normal. The patient’s treatment regimen was switched to Novolin 70/30, and gradually increased to 30 U before breakfast and 18 U before dinner (1 U/kg/d). Following this treatment, her FBG levels were 4.5-5 mmol/L, and 2 h postprandial blood glucose (PBG) levels were 6.7-7.8 mmol/L.
Twelve years after disease onset, the patient complained of numbness in her toes without pain and abnormal sweating. Electromyography revealed a decreased amplitude in her left superficial peroneal nerve. DKD progressed to G2A3 stage. Islet function appeared to be stable with FCP levels of 317 pmo1/L and PCP levels of 619 pmo1/L. Because of the high insulin dosage requirements and the absence of progressive pancreatic function decline, MODY was considered, and the patient began combined oral hypoglycemic therapy. Thus, the treatment regimen was switched to metformin [0.5 g ter in die (TID)], acarbose (50 mg TID) and insulin aspart 30 (20 U before breakfast and 10 U before dinner, 0.64 U/kg/d). Under this treatment regimen, the proband’s HbA1c levels fluctuated between 6.8% and 8%.
In 2020, 24 years after disease onset, ultrasound doppler showed intima-media thickening in the carotid arteries and atherosclerotic plaques in multiple arteries. The patient suffered from fluctuating blood glucose levels and was frequently hypoglycemic. At this time, the hypoglycemic regimen was changed to metformin (0.5 g bis in die) combined with four daily insulin injections (4 U, 6 U, 5 U insulin aspart before three meals and 9 U insulin degludec before bedtime, 0.5 U/kg/d). Although the patient’s HbA1c levels fluctuated between 7% and 9%, she often experienced hypoglycemia one hour after meals.
The proband had a history of hypertension, dyslipidemia, Hashimoto’s thyroiditis, bilateral sensorineural deafness (average hearing 50 dB), pre-excitation syndrome, and purpura nephritis (cured).
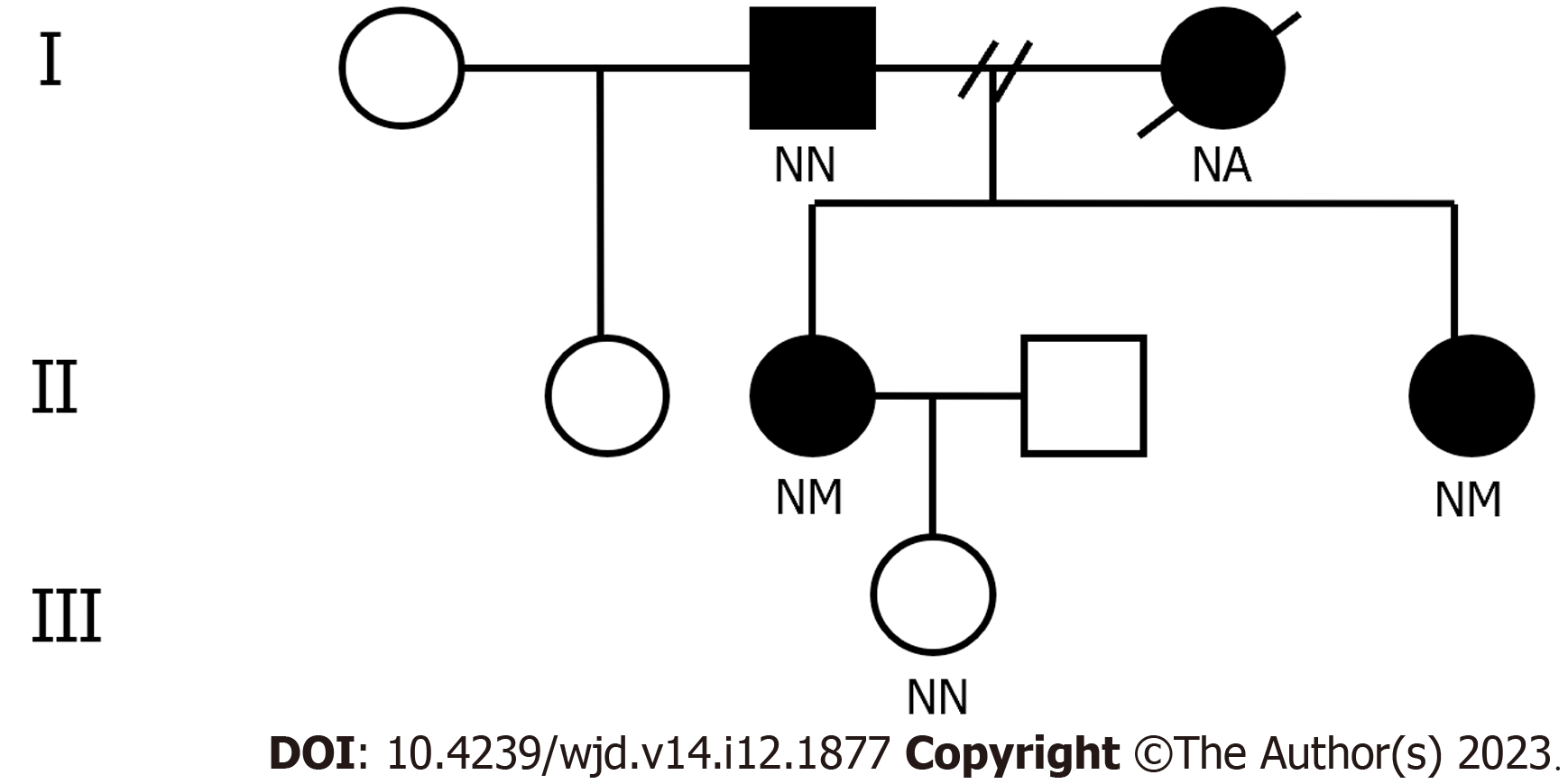
The proband’s daughter was healthy. Her father was diagnosed with diabetes mellitus at 60-years-old. Her mother was thin and suffered from chronic kidney disease (diagnosed in her 20s), diabetes (diagnosed in her 30s), hearing loss (details unknown), and died of kidney failure at the age of 42 years. Her mother was insulin-independent. Details regarding the mother’s diabetic complications are unclear, but it is known she never complained of numbness or pain, blurred vision, and abnormal sweating. The proband has two siblings: Her sister who was normal in size was diagnosed with diabetes around 40 years old, while her half-sister was healthy. The diabetic sibling suffered hearing loss and hypertension, but no diabetic complications. Her auto-antibodies and islet function were unknown and she was treated with insulin aspart 30 (a total dose of 27 U, 0.54 U/kg/d). The child of the diabetic sibling was healthy.
Physical examination revealed that her body mass index (BMI) was 20.24 kg/m2, waist circumference was 75 cm, and waist-hip ratio was 0.91. No abnormal signs were found during cardiopulmonary and abdominal examinations, except for a surgical scar on her abdomen. Diabetic peripheral neuropathy (DPN) screening and dorsalis pedis pulsations on both sides were normal.
The proband’s HbA1c levels were 9.1%, FBG levels were 6.8 mmol/L, PBG levels were 21.8 mmol/L, FCP levels were 135.4 pmol/L, PCP levels were 600.1 pmol/L, Scr levels were 79 umol/L, eGFR levels were 70.18 mL/min/(1.73 m²), 24 h urine protein was 0.531 g, and lactic acid levels were 0.6mmol/L.
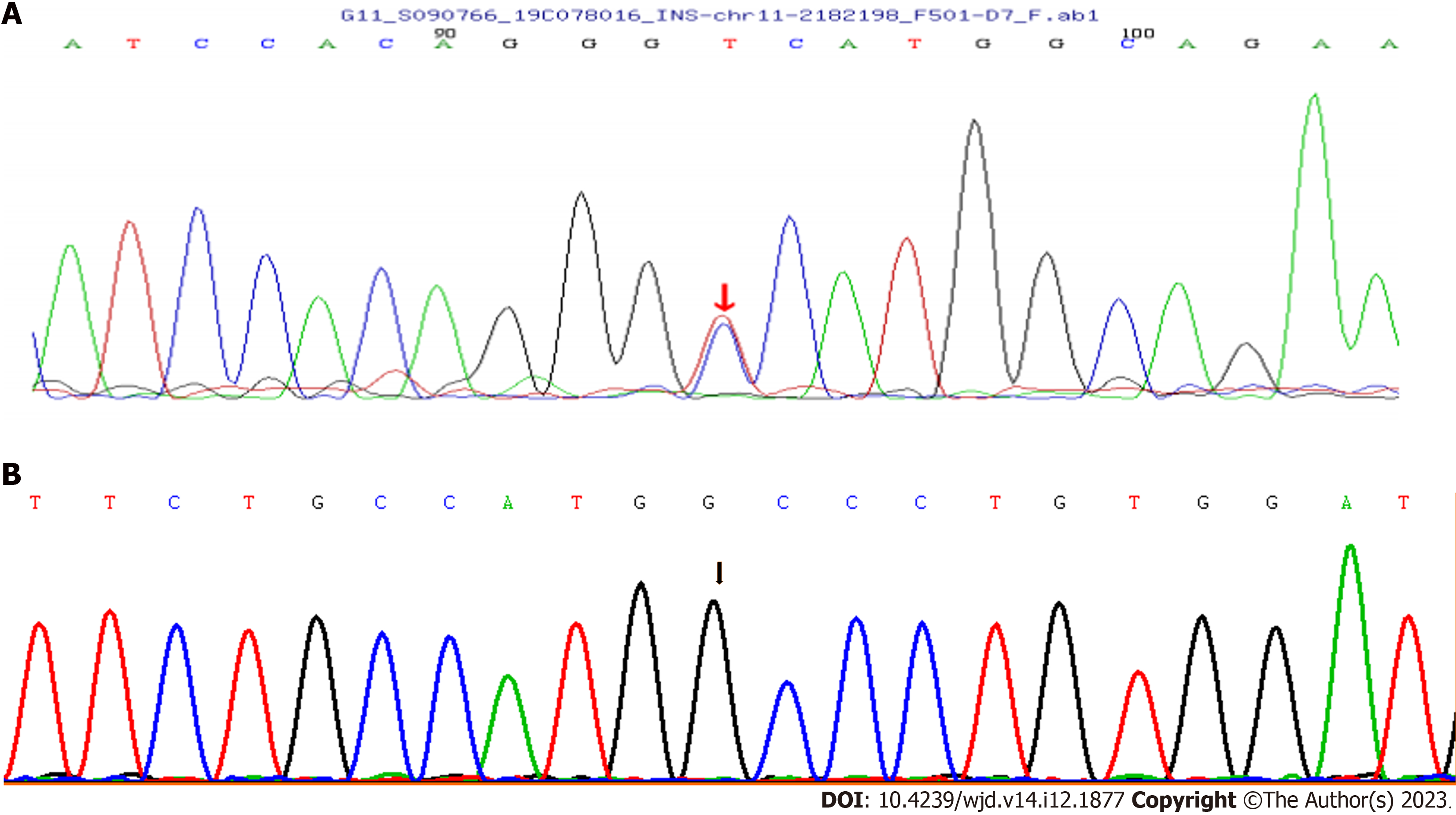
The proband and her sister have a heterozygous mutation (c.4G>A) in exon 2 of INS on chromosome 11, leading to the amino acid replacement p.Ala2Thr (A2T). Her father did not carry the mutation (Figure 1), and neither did her daughter. Due to early death, the mother did not undergo genetic testing (Figure 2). The proband’s human leukocyte antigen (HLA) genotype was also evaluated, and no HLA gene variations linked to T1DM were found (Table 1). The proband and her family members did not grant consent for genetic testing of mitochondrial gene mutations.
| Gene | Allele1 | Allele2 |
| HLA-DRB1 | DRB1 09:01 | DRB1 09:01 |
| HLA-DQA1 | DQA1 03:03 | DQA1 03:03 |
| HLA-DQB1 | DQB1 03:02 | DQB1 03:03 |
| HLA-A | A 02:01 | A 24:02 |
| HLA-B | B 51:01 | B 51:01 |
| HLA-C | C 01:02 | C 01:02 |
The findings of the fundus examination were normal.
Combined with the genetic sequencing results, the proband was eventually diagnosed as MODY10, with the presence of DKD (G2A3 stage), DPN, and diabetic macroangiopathy.
Subsequently, she was prescribed metformin (0.5 g before dinner) and four daily insulin injections (4 U, 4 U, 3 U insulin aspart before three meals and 14 U insulin degludec before bedtime, 0.52 U/kg/d).
The patient’s blood glucose levels were tracked using a continuous glucose monitoring system. During the 9-d review period, she spent 42% of her time within 3.9-10 mmol/L, 50% of her time between 10.1-13.9 mmol/L, and 8% of her time within 3.1-3.8 mmol/L.
MODY is a type of diabetes that is caused by a single gene mutation and inherited in an autosomal dominant manner[1]. To date, at least 14 types of MODY have been identified (Table 2). The clinical features and treatment regimens of MODY patients vary not only by subtypes, but also within the same subtype[8,9]. Due to a limited number of reports on MODY10, less is known about this subtype.
| Subtype | Gene mutation | Prevalence | Clinical feature | Treatment |
| MODY1 | HNF4A | Common | One-half of patients are neonatal macrosomia; blood sugar control deteriorates gradually as the disease advances; low levels of apolipoproteins and triglycerides; without insulin resistance or β cell autoimmunity | Medication-free in the early stage; sensitive to sulfonylureas |
| MODY2 | GCK | Common | Slight elevation in fasting blood glucose and glycated hemoglobin levels; usually asymptomatic | Typically does not require medication |
| MODY3 | HNF1A | Common | Renal glucose threshold is decreased; low levels of hs-CRP; without insulin resistance or β cell autoimmunity; similar to MODY1 | Sensitive to sulfonylureas |
| MODY4 | PDX1/IPF1 | Rare | Overweight/obesity in some patients; commonly occurs post-puberty; postprandial blood sugar usually rises significantly | Mostly treated with insulin |
| MODY5 | HNF1B | Uncommon | Often combined with genitourinary malformations, hepatic dysfunction, renal dysfunction, renal cysts, hyperuricemia, exocrine pancreas insufficiency; onset occurs typically during adolescence or early adulthood. | Early insulin therapy may be required |
| MODY6 | NEUROD1 | Rare | Phenotype is different. Overweight/obesity, intellectual disabilities and brain abnormalities occur in some patients | Significant variations in treatment regimens |
| MODY7 | KLF11 | Extremely rare | Mild hyperglycemia, hyperlipidemia | Insulin |
| MODY8 | CEL | Extremely rare | Impaired endocrine and exocrine pancreatic function | Insulin |
| MODY9 | PAX4 | Extremely rare | Progressive hyperglycemia; ketoacidosis may occur | Mostly treated with insulin |
| MODY10 | INS | Rare | Earlier onset of diabetes, an increased risk of diabetic microvascular complication; degree of islet dysfunction varies | Significant variations in treatment regimens |
| MODY11 | BLK | Extremely rare | Overweight/obesity in some patients | Most patients require insulin, but some may be treated with diet or oral hypoglycemic agents |
| MODY12 | ABCC8 | Rare | Common in neonatal diabetes, symptoms are similar to MODY1 and 3 | Sensitive to sulfonylureas |
| MODY13 | KCNJ11 | Extremely rare | Common in neonatal diabetes, some patients develop diabetes from the second decade of life onwards | Sensitive to sulfonylureas |
| MODY14 | APPL1 | Extremely rare | Overweight/obesity in some patients | Significant variations in treatment regimens |
Genetic testing of the proband and her sister revealed an A2T mutation in INS, indicating that MODY10 should be considered. However, the patient tested positive for islet antibodies, necessitating differentiation from T1DM. Subsequent HLA gene testing conclusively excluded this possibility. Indeed, islet-related antibody positivity is not exclusive to T1DM. In a study by Urbanová et al[10] consisting of 28 MODY patients from the Czech Republic, seven individuals were found to be positive for GADA or islet antigen 2 antibody. Although it was not clear why these patients were positive, the existence of islet autoantibodies seems to be correlated with later onset and worsening glycemic control[10]. Despite this, the proband, her mother, and her diabetic sibling all suffered from diabetes and hearing impairment, prompting consideration of mitochondrial diabetes. However, the patient’s lactate levels were normal, and a progressive decline in islet function was not observed. In addition, clinical features of mitochondrial diabetes, such as stroke, skeletal muscle impairment, or retinopathy, were not observed[11]. Furthermore, the offspring of the proband and her sister remained healthy. Considering these factors, the likelihood of mitochondrial diabetes was low. Multiple studies have reported that hearing impairment occurs in many non-mitochondrial diabetic patients, as well as within the MODY patient[12]. Hyperglycemia, microvascular complications, and mitochondrial damage are probably the main reasons for hearing loss in individuals with diabetes[12].
Based on the available literature, individuals with MODY10 tended to have an earlier onset of diabetes, with an average age of onset at 13.7 years, and were non-obese[13]. They were usually negative for islet antibodies and exhibited an increased risk of diabetic microvascular complications[5,8]. Due to differences in mutation sites, individuals with MODY10 exhibited varying degrees of islet dysfunction and required individualized treatment regimens[4,5,14,15]. Treatment options included diet and exercise, oral hypoglycemic agents, and insulin, with the highest insulin usage rate among them. Although patients can be treated with diet or oral hypoglycemic agents at diagnosis, they become insulin-independent as the condition progresses. In some cases, high doses of insulin supplementation might be necessary[5,8].
In our study, the proband and her sister were diagnosed with MODY10 and their mother was strongly suspected of having the disease. The clinical features of these three persons were consistent with some previous studies, but not all. Specifically, all three persons were non-obese and received different treatment regimens. Diabetic microangiopathy appeared to be more common than macroangiopathy. However, there were also some differences. Firstly, all the individuals in our study had a later age of onset, at least later than the common age of onset of MODY10[13]. Secondly, the proband was positive for islet antibodies.
A2T refers to the substitution of alanine by threonine in the signal peptide, which causes a change in protein secondary structure (α-helix to β-sheet)[7]. Such conformational changes may affect the cleavage of preproinsulin, which is subsequently retained in the endoplasmic reticulum, resulting in endoplasmic reticulum stress, and eventually leads to reduced production of insulin[7].
Apart from this report, there have been two articles consisting of 10 participants that have presented with clinical characteristics for A2T mutation carriers (Table 3)[6,7]. Combined with our research, we found that the A2T mutation does not always result in diabetes mellitus, as evidenced by Yan et al[6] study, which found that one person had impaired glucose tolerance. Diabetic patients who carry A2T mutations typically experience a later onset of diabetes, have a normal BMI, and no islet antibodies. Most patients maintain stable blood glucose levels by using oral drugs. A minority of patients are medicine-free and insulin-independent, but some may require a high dose of insulin, as was the case with the proband and her sister in our study.
| Our study | Zhang et al[7] | Yan et al[6] | ||||||||||
| No. | 1 | 2 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 5 | 6 |
| Age (yr) | 53 | 48 | 25 | 46 | 42 | 69 | / | 47 | 66 | 58 | 34 | 62 |
| Sex | Female | Female | Male | Female | Male | Male | Male | Male | Male | Female | Male | Male |
| Onset age of diabetes (yr) | 27 | Around 40 | 22 | 39 | 33 | 50 | 31 | 47 | 66 | 54 | 34 | 57 |
| BMI (kg/m2) | 20.24 | 23.5 | 21.7 | 23.9 | 21 | 24.2 | / | 24.54 | 24.21 | 28.94 | 23 | 23.1 |
| HbA1c (%) | 9.1 | / | 7.6 | 6.8 | 7.7 | 9.8 | / | 5.6 | 7.6 | 7.7 | 10.9 | 7.2 |
| FBG (mmol/L) | 6.8 | / | 9.3 | 7.8 | 8.3 | 9.6 | 16 | 5.65 | 8.98 | 9.44 | 5.53 | 8.34 |
| PBG (mmol/L) | 21.8 | / | 11.9 | 12.7 | 15.2 | 17.8 | / | 5.02 | 18.82 | 19.99 | 17.69 | 16.85 |
| FINS (pmol/L) | / | / | 51.54 | 61.30 | 57.11 | 84.28 | / | 48.84 | 26.52 | 277.56 | 56.04 | 85.8 |
| PINS (pmol/L) | / | / | 206.16 | 190.84 | 134.42 | 314.12 | / | 507.3 | 52.62 | 562.62 | 121.38 | 478.26 |
| FCP (pmol/L) | 135.4 | / | / | / | / | / | / | / | / | / | / | / |
| PCP (pmol/L) | 600.1 | / | / | / | / | / | / | / | / | / | / | / |
| GADA | + | / | - | - | - | - | - | - | - | - | - | - |
| IA-2A | / | / | - | - | - | - | - | - | - | - | - | - |
| Diagnosis | DM | DM | DM | DM | DM | DM | DM | IGT | DM | DM | DM | DM |
| Complications | DKD, DPN, macroangiopathy | None | / | / | / | / | / | / | / | / | / | / |
| Therapy | OHA + Insulin | Insulin | OHA→Insulin | OHA | OHA | OHA | OHA | - | OHA | - | OHA | OHA |
Herein, we offer a comprehensive summary of the clinical characteristics observed in individuals with MODY10 carrying A2T mutations. Furthermore, we present an atypical MODY10 case resulting from the A2T mutation. The patient exhibited positive islet autoantibodies, as well as demonstrated significant familial inheritance and hearing impairment, which increased the potential for misdiagnosis. We stress that not all patients adhere to the conventional presentation, highlighting the importance of increased vigilance and careful consideration to prevent cases from being overlooked or misdiagnosed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beg MMA, Kyrgyzstan; Wani I, India S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34:1878-1884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Pihoker C, Gilliam LK, Ellard S, Dabelea D, Davis C, Dolan LM, Greenbaum CJ, Imperatore G, Lawrence JM, Marcovina SM, Mayer-Davis E, Rodriguez BL, Steck AK, Williams DE, Hattersley AT; SEARCH for Diabetes in Youth Study Group. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab. 2013;98:4055-4062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 3. | Flannick J, Johansson S, Njølstad PR. Common and rare forms of diabetes mellitus: towards a continuum of diabetes subtypes. Nat Rev Endocrinol. 2016;12:394-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Molven A, Ringdal M, Nordbø AM, Raeder H, Støy J, Lipkind GM, Steiner DF, Philipson LH, Bergmann I, Aarskog D, Undlien DE, Joner G, Søvik O; Norwegian Childhood Diabetes Study Group, Bell GI, Njølstad PR. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57:1131-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Meur G, Simon A, Harun N, Virally M, Dechaume A, Bonnefond A, Fetita S, Tarasov AI, Guillausseau PJ, Boesgaard TW, Pedersen O, Hansen T, Polak M, Gautier JF, Froguel P, Rutter GA, Vaxillaire M. Insulin gene mutations resulting in early-onset diabetes: marked differences in clinical presentation, metabolic status, and pathogenic effect through endoplasmic reticulum retention. Diabetes. 2010;59:653-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Yan J, Jiang F, Zhang R, Xu T, Zhou Z, Ren W, Peng D, Liu Y, Hu C, Jia W. Whole-exome sequencing identifies a novel INS mutation causative of maturity-onset diabetes of the young 10. J Mol Cell Biol. 2017;9:376-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Zhang J, Liu Y, Li M, Ge X, Wang Y, Huang X, Yang D, Zhang R, Chen Y, Lu M, Yin J, Song M, Wang F, Jiang M, Liu L. Identification of Ala2Thr mutation in insulin gene from a Chinese MODY10 family. Mol Cell Biochem. 2020;470:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Dusatkova L, Dusatkova P, Vosahlo J, Vesela K, Cinek O, Lebl J, Pruhova S. Frameshift mutations in the insulin gene leading to prolonged molecule of insulin in two families with Maturity-Onset Diabetes of the Young. Eur J Med Genet. 2015;58:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Garin I, Perez de Nanclares G, Gastaldo E, Harries LW, Rubio-Cabezas O, Castaño L. Permanent neonatal diabetes caused by creation of an ectopic splice site within the INS gene. PLoS One. 2012;7:e29205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Urbanová J, Rypáčková B, Procházková Z, Kučera P, Cerná M, Anděl M, Heneberg P. Positivity for islet cell autoantibodies in patients with monogenic diabetes is associated with later diabetes onset and higher HbA1c level. Diabet Med. 2014;31:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Maassen JA, 'T Hart LM, Van Essen E, Heine RJ, Nijpels G, Jahangir Tafrechi RS, Raap AK, Janssen GM, Lemkes HH. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53 Suppl 1:S103-S109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Samocha-Bonet D, Wu B, Ryugo DK. Diabetes mellitus and hearing loss: A review. Ageing Res Rev. 2021;71:101423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Aarthy R, Aston-Mourney K, Mikocka-Walus A, Radha V, Amutha A, Anjana RM, Unnikrishnan R, Mohan V. Clinical features, complications and treatment of rarer forms of maturity-onset diabetes of the young (MODY) - A review. J Diabetes Complications. 2021;35:107640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Piccini B, Artuso R, Lenzi L, Guasti M, Braccesi G, Barni F, Casalini E, Giglio S, Toni S. Clinical and molecular characterization of a novel INS mutation identified in patients with MODY phenotype. Eur J Med Genet. 2016;59:590-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Tosur M, Soler-Alfonso C, Chan KM, Khayat MM, Jhangiani SN, Meng Q, Refaey A, Muzny D, Gibbs RA, Murdock DR, Posey JE, Balasubramanyam A, Redondo MJ, Sabo A. Exome sequencing in children with clinically suspected maturity-onset diabetes of the young. Pediatr Diabetes. 2021;22:960-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |