Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1754
Peer-review started: August 21, 2023
First decision: September 29, 2023
Revised: October 11, 2023
Accepted: November 3, 2023
Article in press: November 3, 2023
Published online: December 15, 2023
Coronary artery disease (CAD) is a major cause of death worldwide, and India contributes to about one-fifth of total CAD deaths. The development of CAD has been linked to the accumulation of Nε-carboxymethyl-lysine (CML) in heart muscle, which correlates with fibrosis.
To assess the impact of CML and inflammatory markers on the biochemical and cardiovascular characteristics of CAD patients with and without diabetes.
We enrolled 200 consecutive CAD patients who were undergoing coronary angiography and categorized them into two groups based on their serum glycosylated hemoglobin (HbA1c) levels (group I: HbA1c ≥ 6.5; group II: HbA1c < 6.5). We analyzed the levels of lipoproteins, plasma HbA1c levels, CML, interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and nitric oxide.
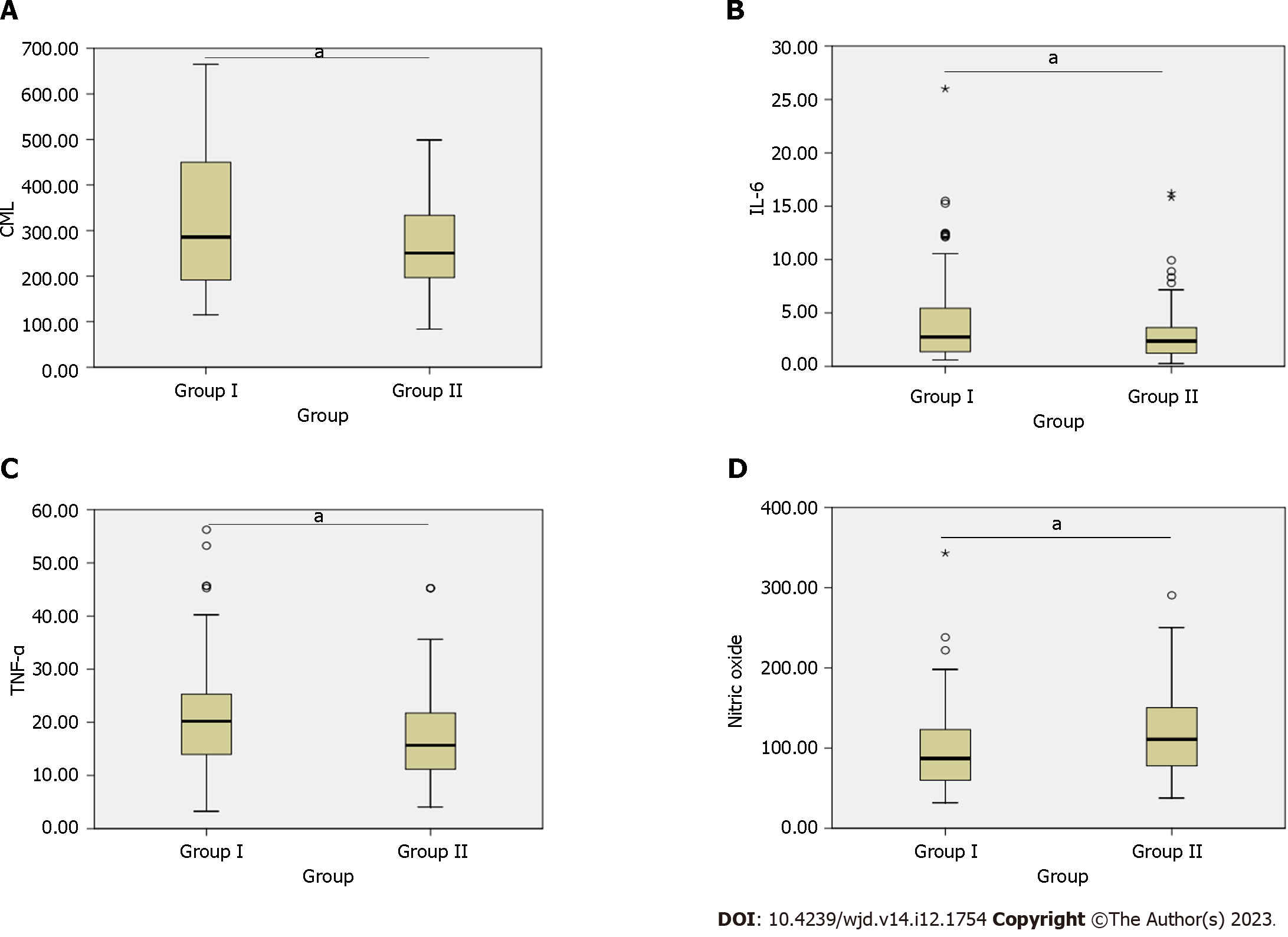
Group I (81 males and 19 females) patients had a mean age of 54.2 ± 10.2 years, with a mean diabetes duration of 4.9 ± 2.2 years. Group II (89 males and 11 females) patients had a mean age of 53.2 ± 10.3 years. Group I had more severe CAD, with a higher percentage of patients with single vessel disease and greater stenosis severity in the left anterior descending coronary artery compared to group II. Group I also exhibited a larger left atrium diameter. Group I patients exhibited significantly higher levels of CML, TNF-α, and IL-6 and lower levels of nitric oxide as compared with group II patients. Additionally, CML showed a significant positive correlation with IL-6 (r = 0.596, P = 0.001) and TNF-α (r = 0.337, P = 0.001) and a negative correlation with nitric oxide (r=-4.16, P = 0.001). Odds ratio analysis revealed that patients with CML in the third quartile (264.43-364.31 ng/mL) were significantly associated with diabetic CAD at unadjusted and adjusted levels with covariates.
CML and inflammatory markers may play a significant role in the development of CAD, particularly in diabetic individuals, and may serve as potential biomarkers for the prediction of CAD in both diabetic and non-diabetic patients.
Core Tip: Coronary artery disease (CAD) incidence is substantial in India.Its development is linked to the accumulation of Nε-carboxymethyl-lysine (CML). We assessed the impact of CML and inflammatory markers on biochemical and cardiovascular characteristics in diabetic and non-diabetic CAD patients. Diabetic patients exhibited elevated CML, tumor necrosis factor alpha, and interleukin 6 levels with reduced nitric oxide levels. CML levels displayed a significant correlation with interleukin 6, tumor necrosis factor alpha, and nitric oxide. The third quartile of CML was associated with diabetic CAD, suggesting its role as a biomarker in CAD prediction for diabetic and non-diabetic patients.
- Citation: Shrivastav D, Singh DD, Mir R, Mehra P, Mehta V, Dabla PK. Comparative analysis of Nε-carboxymethyl-lysine and inflammatory markers in diabetic and non-diabetic coronary artery disease patients. World J Diabetes 2023; 14(12): 1754-1765
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1754.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1754
Heart disease, specifically heart failure (HF) and coronary artery disease (CAD), is a major contributor to mortality in both developed and developing countries[1]. The World Health Organization states that the most common cause of death is cardiovascular disease (CVD), resulting in 17.9 million annual deaths. Subsequently, cancer, chronic respiratory ailments, and diabetes trail behind as causes of mortality[2]. In diabetic individuals with CAD, inadequate management of blood sugar levels is linked to both hospitalization and mortality[3]. Diabetes mellitus is a major risk factor for the cause and progression of atherosclerosis[4,5].
Some recent literature evidence suggests that advanced glycation end products (AGEs) play an important role in the acceleration of vascular disease[6]. AGEs are formed from the non-enzymatic reaction of sugars and proteins, leading to oxidative stress, inflammation, and endothelial dysfunction through various mechanisms[7]. In hyperglycemia, the accumulation of AGEs is thought to play a role in the onset of diabetic complications. AGE buildup can modify tissue structure, affecting its properties and making it more resistant to breaking down[8]. One of the major AGEs, Nε-carboxymethyl-lysine (CML) is formed by the non-enzymatic glycation and oxidation of monosaccharides (glucose) and proteins (lysine). The attachment of AGEs to receptor for AGEs (RAGE)may result in impaired cellular communication, protein structure and functional alterations, and mitochondrial malfunction, ultimately resulting in cellular demise. RAGE binding can also increase reactive oxygen species and stimulate inflammatory signaling through tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6). It also affects endothelial function by altering nitric oxide levels[9].
Subsequently, new evidence suggested that CML has made a major contribution to the development of CAD[10]. CML found in heart muscle shows a positive correlation with fibrosis and cardiac disease[11]and promotes hypertrophy, apoptosis, and myocardial fibrosis[12]. Elevated CML levels have been linked to poor collateralization in chronic total occlusion in diabetic CAD patients[13]. Along with CAD, CML is also significantly associated with many other diseases, like diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, and cancer[14].
In this study, we assessed the impact of CML in association with inflammatory markers on biochemical and cardiovascular characteristics in diabetic and non-diabetic CAD patients. We aimed to gain new insights while exploring the relationship between diabetes and CAD, which may open future prospects for therapeutic intervention in such patients.
This cross-sectional study was conducted at the Department of Biochemistry, G.B. Pant Institute of Postgraduate Medical Education and Research (GIPMER), New Delhi, India. We enrolled 200 age-matched and sex-matched, angiography-confirmed patients diagnosed with CAD from both OPD & IPD of Department of Cardiology. All patients signed an informed consent. The study was conducted in accordance with internationally accepted recommendations for clinical investigation (the Declaration of Helsinki of the World Medical Association, revised October 2013) with approval from the ethics committee of Maulana Azad Medical College and associated hospitals, New Delhi, India.
Venous blood (5 mL) was drawn under aseptic conditions from consented patients. Further, a 3-mL sample was transferred to an EDTA vial for glycosylated hemoglobin (HbA1c) and special chemistry analysis, and the remaining sample was transferred to a glucose vial for blood sugar analysis. Patients with HbA1c level ≥6.5% or having a previous diagnosis of diabetes were considered as diabetic CAD (group I), while patients with level <6.5% were categorized as non-diabetic CAD (group II). Group II patients with no prior history of diabetes and no history of anti-diabetic medication were classified as non-diabetic CAD. The serum levels of HbA1C were measured by a fully automated analyzer, whereas the CML, IL-6, TNF-α, and nitric oxide levels were determined by enzyme-linked immunosorbent assay methods.
Independent senior cardiologists utilized the angiographic data from the catheterization laboratory to calculate the severity of CAD using the Gensini scoring (GS) system. The left coronary artery was separated into left anterior descending (LAD), circumflex, and obtuse marginal branches, while the right coronary artery (RCA) was considered a single artery. The lesion score for each coronary segment was multiplied by a location-based factor, and then the scores were added together to calculate the GS.
The GS was determined by adding the scores from each coronary segment as follows: one point for 25% stenosis; two points for 26%-50% stenosis; four points for 51%-75% stenosis; eight points for 76%-90% stenosis; sixteen points for 91%-99% stenosis; and 32 points for total occlusion. The significance of the location of the lesion in the coronary circulation was also considered, with 5 points for the left main coronary artery, 2.5 points for the proximal LAD coronary artery and proximal left circumflex artery, 1.5 points for the mid-LAD coronary artery, 1 point for the RCA, the distal segment of the LAD coronary artery, the posterolateral artery, and the obtuse marginal artery, and 0.5 points for other segments[15].
A standard two-dimensional, M-mode, and Doppler echocardiography examination was conducted using the Philips EpiQ-7C echocardiography system. The examination measured various parameters including the dimension of the left atrium (LA) and the aortic root. The left ventricular ejection fraction (LVEF) was also calculated using Simpson’s method[16].
Patients over the age of 18 years who were confirmed with the diagnosis of CAD by resting electrocardiogram or coronary angiography with >50% stenosis were included in this study. Blood pressure was measured as an average of two readings recorded at least 5 min apart while the participants rested in a seated position. Hypertension was identified when the subject was either having a history of hypertension or a systolic blood pressure of ≥140 mmHg or a diastolic blood pressure of ≥90 mmHg. Patients with total cholesterol (TC) (>200 mg/dL), triglycerides (>150 mg/dL), high-density lipoprotein cholesterol (HDL-C) (<40 mg/dL), or low-density lipoprotein cholesterol (LDL-C) (>100 mg/dL) were defined as having dyslipidemia. Additionally, patients with renal or hepatic impairment as well as those who had undergone previous therapies such as coronary artery bypass graft surgery or percutaneous coronary intervention were excluded from the study.
The SPSS version 21 (IBM Corp., Chicago, IL, United States) was used to analyze the data. The mean and standard deviation and frequency and percentage were used to express quantitative and qualitative data, respectively. For quantitative data, an independent t-test was performed to compare two independent variables. The normality of the data was checked by the Kolmogorov-Smirnov test. Student’s t-test, analysis of variance, and Mann-Whitney U test were used to compare parametric and non-parametric variables. All statistical tests were carried out at a P < 0.05 significance level.
The mean age of group I was 54.2 ± 10.2 years, while the mean age for group II was 53.2 ± 10.3 years (P = 0.473). There was a male sex predominance with males constituting 81% in group I and 89% in group II. In group I, the duration of diabetes was 4.9 ± 2.2 years. Hypertension was more prevalent in group I (39%) than in group II (20%) (P = 0.001). The median systolic blood pressure was significantly higher in group I [125.50 mmHg; 95% confidence interval (CI): 118.0-140.0] compared to group II (120 mmHg; 95%CI: 114.0-129.5) (P = 0.001). In relation to medications, statin use was 79% in group I and 89% in group II. Beta-blockers were taken by 53 (53%) subjects in group I and 73 (73%) subjects in group II. Only 5 (5%) subjects in group II, compared to 17 (17%) in group I, were taking angiotensin converting enzyme (ACE) inhibitors (Table 1). The ACE inhibitor usage was lower as the drug history was taken just before the cardiac catheterization. Subsequently, patients were started on an ACE inhibitor once they were stable.
| Parameter | Group I, n = 100 | Group II, n = 100 | P value1 |
| Age | 54.2 ± 10.2 | 53.2 ± 10.3 | 0.4732 |
| Male:Female | 81 (81%): 19 (19%) | 89 (89%): 11 (11%) | 0.823 |
| Non-vegetarian diet | 80% | 60% | 0.0013 |
| Smoker | 65% | 50% | 0.0223 |
| Alcohol consumption | 26% | 25% | 0.5003 |
| Tobacco chewer | 49% | 39% | 0.1003 |
| Hypertensive | 39% | 20% | 0.0013 |
| Systolic blood pressure in mmHg as median | 125.5 (118.0-140.0) | 120.0 (114.0-129.5) | 0.0014 |
| Diastolic blood pressure in mmHg as median | 80.0 (72.0-84.0) | 80.0 (70.0-80.0) | 0.0894 |
| Medications | |||
| Statin | 79% | 89% | 0.0413 |
| Beta-blocker | 58% | 73% | 0.0183 |
| ACE inhibitor | 17% | 5% | 0.0053 |
Group I consisted of 57 patients with single vessel disease (SVD), 27 patients with double vessel disease, and 8 patients with triple vessel disease. However, group II had 35 patients with SVD, 36 patients with double vessel disease, and 11 patients with triple vessel disease. Eight patients in group I and fourteen patients in group II had normal angiograms (P = 0.016).
The mean and standard deviation of severity of stenosis in the LAD artery were observed as 90.51% ± 8.51%, in the left circumflex (LCX) artery as 90.91% ± 8.80%, and in the RCA as 90.32% ± 10.15% in group I. On the other hand, in group II, the mean and standard deviation of stenosis in the LAD were 87.85% ± 12.31%, in the LCX were 82.22% ± 22.33%, and in the RCA were 89.26% ± 12.90%. The GS was higher in group I, with a score of 26 (12–44) compared with group II with a score of 20 (12-40). Group I had a larger LA diameter of 2.93 ± 0.32 cm compared to 2.83 ± 0.39 cm in group II (P = 0.04). The aortic root diameter was slightly larger in group I at 2.15 ± 0.39 mm compared to 2.10 ± 0.40 mm in group II. Further, group I had a mean LVEF of 45.60% ± 12.04%, and group II had a mean EF of 46.70% ± 12.01%.
The patients were categorized based on their LVEF in Table 2. In group I, 38% of patients had preserved EF (LVEF ≥ 50%), 13% had mild EF reduction (LVEF 41%-49%), and 49% had reduced EF (LVEF < 40%). In group II, 43% of patients had preserved EF, 14% had mild EF reduction, and 43% had reduced EF. Anterior wall myocardial infarction was experienced by 39% of patients in group I and 39% of patients in group II, and inferior wall myocardial infarction was experienced by 26% of patients in group I and 21% of patients in group II.
| Parameter | Group I, n = 100 | Group II, n = 100 | P value1 |
| Angiography findings | |||
| Single vessel disease | 57 (57%) | 35 (35%) | 0.0162 |
| Double vessel disease | 27 (27%) | 36 (36%) | |
| Triple vessel disease | 8 (8%) | 15 (15%) | |
| Normal angiogram | 8 (8%) | 14 (14%) | |
| Stenosis in LAD as % | 90.51 ± 8.51 | 87.85 ± 12.31 | 0.053 |
| Stenosis in LCX as % | 90.91 ± 8.80 | 82.22 ± 22.33 | 0.233 |
| Stenosis in RCA as % | 90.32 ± 10.15 | 89.26 ± 12.90 | 0.733 |
| Gensini score | 26 (12-44) | 20 (12-40) | 0.473 |
| 2D echocardiography parameters | |||
| Left atrium diameter in cm | 2.93 ± 0.32 | 2.83 ± 0.39 | 0.013 |
| Aortic root diameter in mm | 2.15 ± 0.39 | 2.10 ± 0.40 | 0.273 |
| LVEF | 45.60 ± 12.04 | 46.70 ± 12.01 | 0.493 |
| Preserved ejection fraction, LVEF ≥ 50% | 38 (38%) | 43 (43%) | 0.692 |
| Mild ejection fraction, LVEF 41%-49% | 13 (13%) | 14 (14%) | |
| Reduced ejection fraction (LVEF < 40%) | 49 (49%) | 43 (43%) | |
| AWMI | 39 (39%) | 39 (39%) | 0.562 |
| IWMI | 26 (26%) | 21 (21%) | |
The TC, triglycerides levels, and very-LDL levels were found to be significantly higher in group I compared to group II (P = 0.006, P = 0.001, and P = 0.001, respectively). Further, both HbA1c and the blood sugar levels were found to be significantly higher in group I compared to group II (P = 0.001). The abovementioned intergroup comparison between biochemical parameters has been shown in Table 3.
| Biochemical parameters | Group I, median (25%-75% quartile) | Group II, median (25%-75% quartile) | P value1 |
| Total cholesterol in mg/dL | 143.50 (118.00-183.50) | 132.00 (100.25-163.75) | 0.0062 |
| Triglycerides in mg/dL | 150.00 (106.25-214.00) | 114.00 (75.00-148.75) | 0.0012 |
| HDL-C in mg/dL | 33.40 (27.33-38.98) | 34.55 (28.70-41.00) | 0.4492 |
| LDL-C in mg/dL | 78.00 (53.50-108.80) | 73.00 (52.00-92.75) | 0.2782 |
| VLDL-C in mg/dL | 29.00 (20.85-42.00) | 23.00 (15.00-30.00) | 0.0012 |
| Random blood sugar in mg/dL | 213.00 (131.50-275.75) | 113.00 (99.00-135.00) | 0.0012 |
| HbA1c as % | 8.09 (7.10-10.20) | 5.70 (5.40-5.98) | 0.0012 |
| Urea in mg/dL | 29.00 (23.00-39.60) | 28.55 (24.85-34.00) | 0.1772 |
| Creatinine in mg/dL | 0.90 (0.80-1.20) | 1.00 (0.80-1.10) | 0.8112 |
| Total bilirubin in mg/dL | 0.40 (0.30-0.70) | 0.50 (0.40-0.69) | 0.2602 |
| Total protein in gm/dL | 7.10 (6.80-7.60) | 7.10 (6.73-7.48) | 0.4412 |
| Albumin in gm/dL | 4.20 (4.00-4.40) | 4.28 (4.00-4.50) | 0.2812 |
| ALP in U/L | 108 (87.00-133.00) | 95.50 (84.25-110.00) | 0.0542 |
| SGOT in U/L | 26 (21.00-45.00) | 30.00 (22.00-47.50) | 0.2402 |
| SGPT in U/L | 28 (20.00-43.00) | 29.95 (22.00-49.00) | 0.1872 |
| Sodium in mEq/L | 136.00 (134.00-139.00) | 139.00 (136.00-141.00) | 0.0012 |
| Potassium in mEq/L | 4.60 (4.30-4.90) | 4.35 (4.10-4.80) | 0.0022 |
| CML in ng/mL | 264.43 (193.19- 364.34) | 250.68 (195.95-333.70) | 0.0312 |
| IL-6 in pg/mL | 2.75 (1.36-5.50) | 2.36 (1.23-3.60) | 0.0112 |
| TNF-α in pg/mL | 20.2 (13.65-25.32) | 15.67 (11.137-21.785) | 0.0062 |
| Nitric oxide in nmol/mL | 87.09 (59.84-124.37) | 110.86 (77.00-150.00) | 0.0022 |
The comparison of CML, IL-6, TNF-α, and nitric oxide between group I and group II (Figure 1) showed significant differences between the two groups: serum CML (264.43, 95%CI: 193.19-364.34 vs 250.68, 95%CI:195.95-333.70, P = 0.031), IL-6 (2.75, 95%CI: 1.36-5.50 vs 2.36, 95%CI: 1.23-3.60, P = 0.011), TNF-α (20.20, 95%CI: 13.65-25.32 vs 15.67, 95%CI: 11.14-21.79, P = 0.006), and nitric oxide (87.09, 95%CI: 59.84-124.37 vs 110.86, 95%CI: 77.00-150.00, P = 0.002).
Table 4 shows the lipid profile of individuals in group I and group II. In group I, 17% of individuals had high TC levels (> 200 mg/dL), whereas group II had a lower proportion of individuals with high TC levels (8%). The difference between the groups was significant with a P value of 0.043. In group I, 49% had high triglycerides levels (> 150 mg/dL), while 51% had normal levels (< 150 mg/dL). In group II, a significantly lower proportion of individuals had high triglyceride levels (24%), and a significantly higher proportion had normal levels (76%), with a P value of 0.001. A higher proportion of individuals in group I had low levels (< 40 mg/dL) of HDL (86%) compared to those with normal levels (> 40 mg/dL) (14%). In contrast, group II had a lower proportion of individuals with low HDL levels (73%) and a higher proportion with normal levels (27%) (P = 0.017). In group I, 70% of patients had normal LDL-C levels (< 100 mg/dL), while 30% had high levels (> 100 mg/dL). In group II, 80% of individuals had normal LDL-C levels and 20% had high levels, P = 0.094.
| Parameter | Group I | Group II | P value1 |
| High total cholesterol, > 200 mg/dL | 17 (17%) | 8 (8%) | 0.043 |
| Normal total cholesterol, < 200 mg/dL | 83 (83%) | 92 (92%) | |
| High triglycerides, > 150 mg/dL | 49 (49%) | 24 (24%) | 0.001 |
| Normal triglycerides, < 150 mg/dL | 51 (51%) | 76 (76%) | |
| Low HDL-C, < 40 mg/dL | 86 (86%) | 73 (73%) | 0.017 |
| Normal HDL-C, > 40 mg/dL | 14 (14%) | 27 (27%) | |
| High LDL-C, > 100 mg/dL | 70 (70%) | 80 (80%) | 0.094 |
| Normal LDL-C, < 100 mg/dL | 30 (30%) | 20 (20%) |
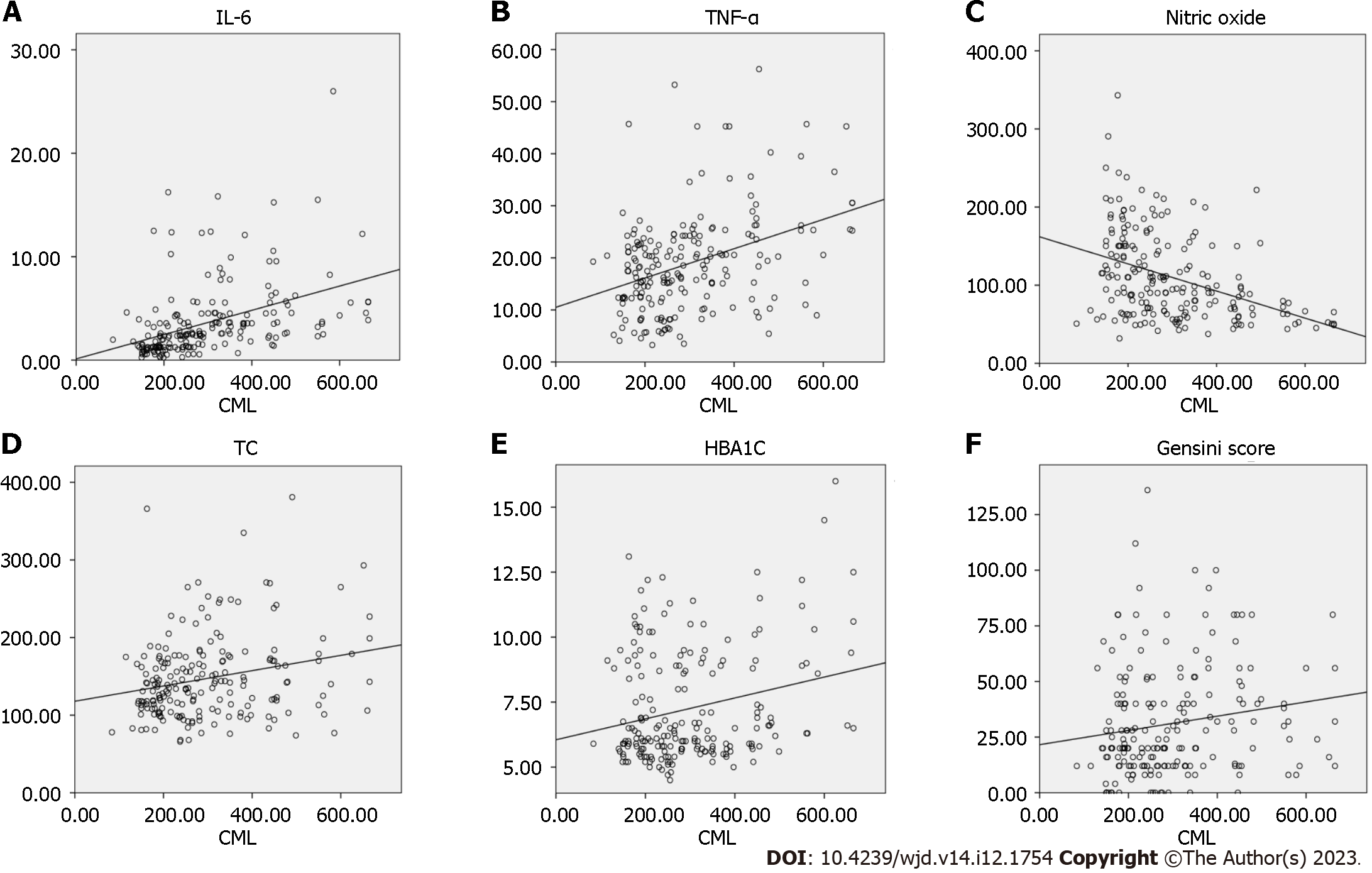
In the correlation analysis, CML exhibited significant positive correlations with IL-6 (r = 0.596), TNF-α (r = 0.337), TC(r = 0.21), HbA1c (r = 0.14), and the GS (r = 0.19) in the combined data from both group I and group II. The correlations of CML (group I vs group II), IL-6 (r = 0.502 vs r = 0.673), TNF-α (r = 0.256 vs r = 0.436), and nitric oxide (r = -0.484 vs r = -0.283) between the two groups were significant (Table 5). The linear regression analysis of CML revealed significant positive associations with IL-6 (r2 = 0.181, P = 0.001), TNF-α (r2 = 0.142, P = 0.001), TC (r2 = 0.056, P = 0.001), HbA1c (r2 = 0.057, P = 0.001), and the GS (r2 = 0.027, P = 0.02). Additionally, CML showed a significant negative association with nitric oxide (r2 = 0.163, P = 0.001) (Figure 2).
| Parameter | Subcategory | Combined (group I and group II) | Group I | Group II | |||
| Correlation coefficient | P value | Correlation coefficient | P value | Correlation coefficient | P value | ||
| CML | Gensini score | 0.193 | 0.006 | 0.056 | 0.577 | 0.353 | 0.001 |
| IL-6 | 0.596 | 0.001 | 0.502 | 0.001 | 0.673 | 0.001 | |
| TNF-α | 0.337 | 0.001 | 0.256 | 0.01 | 0.436 | 0.001 | |
| Nitric oxide | -0.416 | 0.001 | -0.484 | 0.001 | -0.283 | 0.004 | |
| TC | 0.216 | 0.002 | 0.25 | 0.01 | 0.109 | 0.281 | |
| Triglycerides | 0.156 | 0.027 | 0.087 | 0.389 | 0.169 | 0.093 | |
| HDL-C | -0.064 | 0.372 | -0.105 | 0.298 | 0.006 | 0.953 | |
| LDL-C | 0.251 | 0.001 | 0.289 | 0.003 | 0.151 | 0.134 | |
| VLDL-C | 0.131 | 0.065 | 0.045 | 0.654 | 0.176 | 0.081 | |
| Random blood sugar | -0.011 | 0.875 | -0.204 | 0.052 | 0.081 | 0.43 | |
| HbA1c | 0.14 | 0.048 | 0.006 | 0.951 | 0.044 | 0.66 | |
| Urea | -0.004 | 0.953 | -0.006 | 0.957 | -0.046 | 0.653 | |
| Creatinine | 0.059 | 0.405 | 0.129 | 0.202 | -0.047 | 0.646 | |
| Total bilirubin | 0.053 | 0.458 | 0.083 | 0.413 | 0.08 | 0.428 | |
| Total protein | 0.086 | 0.229 | 0.183 | 0.07 | -0.055 | 0.585 | |
| Albumin | 0.062 | 0.387 | 0.201 | 0.046 | -0.064 | 0.525 | |
| Alkaline phosphatase | 0.042 | 0.556 | 0.003 | 0.975 | 0.033 | 0.743 | |
| SGOT | -0.061 | 0.395 | -0.056 | 0.581 | -0.032 | 0.754 | |
| SGPT | 0.019 | 0.793 | 0.102 | 0.317 | -0.027 | 0.788 | |
| Sodium | 0.022 | 0.762 | 0.059 | 0.559 | 0.079 | 0.432 | |
| Potassium | 0.116 | 0.103 | 0.076 | 0.452 | 0.102 | 0.313 | |
| Duration of diabetes | - | - | 0.494 | 0.001 | - | - | |
The association between quartiles of CML and diabetic CAD was revealed by logistic regression analysis, while accounting for various covariates in separate models (Table 6). The first quartile of CML (83.73-193.18 ng/mL) served as the reference category. In the unadjusted model, the third quartile (264.43-364.31 ng/mL) had an odds ratio of 2.12 (95%CI: 1.17-3.85, P < 0.01). Following adjustments for non-vegetarian diet and hypertension (model 2), the odds ratio for the third quartile rose to 3.05 (95%CI: 1.31-7.06, P = 0.01). Furthermore, upon introducing further adjustments in Model 3, encompassing TC, triglycerides, LDL-C, IL-6, and TNF-α, the odds ratio for the third quartile became 3.32 (1.30-8.44, P = 0.01) while retaining its statistical significance.
| Risk model | CML quartile (range) (group I, n; group II, n) | Exp (B) | 95%CI (lower-upper) | Significance |
| Model 1: Unadjusted | CML first quartile (83.73-193.18 ng/mL) (group I, n =27; group II, n =23) | Ref | Ref | Ref |
| CML second quartile (193.19-264.42 ng/mL) (group I, n = 16; group II, n = 34) | 0.85 | 0.48-1.48 | 0.57 | |
| CML third quartile (264.43-364.31 ng/mL) (group I, n = 23; group II, n = 27) | 2.12 | 1.17-3.85 | 0.01 | |
| CML fourth quartile (364.32-665.00 ng/mL) (group I, n = 34; group II, n = 16) | 1.17 | 0.67-2.04 | 0.57 | |
| Model 2: Model 1 + age + sex + non-vegetarian diet + hypertension | CML first quartile (83.73-193.18 ng/mL) | Ref | Ref | Ref |
| CML second quartile (193.19-264.42 ng/mL) | 0.57 | 0.27-1.23 | 0.15 | |
| CML third quartile (264.43-364.31 ng/mL) | 3.05 | 1.31-7.06 | 0.01 | |
| CML fourth quartile (364.32-665.00 ng/mL) | 1.81 | 0.82-3.99 | 0.13 | |
| Model 3: Model 2+ total cholesterol + triglycerides + LDL-C + IL-6 + TNF-α | CML first quartile (83.73-193.18 ng/mL) | Ref | Ref | Ref |
| CML second quartile (193.19-264.42 ng/mL) | 0.84 | 0.35-2.02 | 0.70 | |
| CML third quartile (264.43-364.31 ng/mL) | 3.32 | 1.30-8.44 | 0.01 | |
| CML fourth quartile (364.32-665.00 ng/mL) | 2.49 | 1.03-6.04 | 0.04 |
CML is an AGE involved in the pathogenesis of CVD[17]. Recent studies have demonstrated that CML is linked to endothelial and cardiac dysfunction, left ventricular diastolic dysfunction, and an increase in carotid intima-media thickness, which is a subclinical marker of atherosclerosis in patients with type 2 diabetes[18]. In our cross-sectional study, we found an association between CML, inflammatory markers, and nitric oxide in both diabetic and non-diabetic CAD patients.
In our study, we observed that group I had a significantly higher frequency of risk factors including non-vegetarian diet intake, smoking, and hypertension. Further, we observed that group I had a higher number of individuals with SVD and a greater severity of stenosis in the LAD and LCX coronary arteries. However, in non-diabetic patients, the LAD was found to be the most affected[19]. Further, we observed that in group I, the diameter of the LA was significantly higher suggesting the chronicity of the disease. The incidence of anterior wall myocardial infarction was similar in both groups; the frequency of inferior wall myocardial infarction was higher in group I than in group II. The LVEF was decreased in both the groups. It has been reported previously that lower LVEF is common in diabetic CAD patients[20].
In the comparison of the biochemical profile, our study found that diabetic CAD patients exhibited significantly higher levels of TC, triglycerides, very-LDL, HbA1c, and potassium levels as well as significantly lower levels of HDL-C and serum sodium compared to non-diabetic CAD patients (Tables 2 and 3). Additionally, we observed that the serum levels of CML, TNF-α, and IL-6 were significantly higher, while the serum levels of nitric oxide were significantly lower in diabetic CAD patients. Similarly, Banach et al[21] suggested that dyslipidemia is a common occurrence among diabetic CAD patients and that individualized lipid-lowering therapy can effectively reduce associated complications and risks. Zhao et al[22](2023) suggested that patients with acute decompensated HF who had potassium levels outside the range of 3.50 to 4.00 mmol/L, lower levels of sodium, and hypochloremia had a worse short-term prognosis. There was also a positive correlation between the number of electrolyte imbalances and an adverse short-term prognosis among these patients[22]. Similarly, Ahmed et al[23] found that elevated CML levels have been linked to the development of ischemic heart disease in patients with type 2 diabetes. Koshino et al[24] suggested that increased levels of inflammatory markers (IL-6 and TNF-α) from their baseline increase the risk of CVD and are associated with long-term cardiovascular mortality and cardiovascular death. Similarly, Adela et al[25] found lower nitric oxide levels in subjects suffering from diabetes for more than 5 years.
Further, in the correlation analysis (Table 5), CML was overall positively correlated with the GS, IL-6, TNF-α, TC, LDL-C, and HbA1c and negatively correlated with nitric oxide and HDL-C. In group I, CML showed a positive correlation with IL-6, TNF-α, TC, and LDL-C, and a negative correlation with nitric oxide. Furthermore, in group II, CML showed a positive correlation with the GS, IL-6, and TNF-α and a negative correlation with nitric oxide.
Similarly, Kerkeni et al[26] suggested that the serum concentrations of AGEs (CML and pentosidine) were significantly elevated in patients with CAD. Furthermore, serum pentosidine levels are independently associated with the occurrence of CAD with odds of 1.52. Additionally, the optimal cutoff value for pentosidine to predict the presence of CAD was found to be 3.2 μmol/mol[26].
Gaens et al[27] suggested that CML upregulates RAGE-dependent inflammatory responses and increases serum IL-6 level and TNF-α, which are negatively associated with serum nitric oxide and a high body mass index. Further in logistic regression analysis we found the CML level (264.43-364.31 ng/mL) significantly increased the risk of diabetic CAD. Similarly, Semba et al[28] suggested that in non-diabetic subjects serum CML was associated with anemia (odds ratio 1.33, 95%CI: 1.03-1.72, P = 0.029) in a multivariate logistic regression model, adjusting for age, sex, race, smoking, coronary heart disease, HF, and renal insufficiency. Kralev et al[29] suggested that a cutoff value of CML > 9.5 AU/mg was associated with an odds ratio of acute myocardial infarction of 39.7.
In conclusion, this study provided evidence for the association of CML and inflammatory markers with CAD in diabetic and non-diabetic patients. The results suggested that CML, IL-6, and TNF-α may be potential biomarkers for the prediction of CAD in diabetic patients, while nitric oxide may be a potential biomarker for the prediction of CAD in non-diabetic patients. These findings have significant clinical implications for the early diagnosis and management of CAD, particularly in diabetic patients who are at higher risk for developing cardiovascular complications. Further research on a larger cohort is needed to validate these findings and explore the underlying mechanisms of CML and inflammatory markers in the development of CAD, which may be helpful developing therapeutic interventions further.
Coronary artery disease (CAD) is a widespread global health issue, responsible for a significant number of deaths. India bears a substantial burden, contributing to approximately one-fifth of CAD-related fatalities. The development of CAD has been closely linked to the accumulation of Nε-carboxymethyl-lysine (CML) in the heart muscle, a phenomenon associated with fibrosis. Understanding the role of CML in CAD development is crucial for combating this life-threatening condition.
This study is motivated by the need to shed light on the factors contributing to CAD, especially in the context of diabetes. CAD is a complex disease, and understanding its underlying mechanisms can help in early diagnosis and more effective management. Diabetes is a significant risk factor for CAD, and investigating the interplay between CML, inflammatory markers, and CAD in individuals with and without diabetes can provide valuable insights into its pathogenesis.
The primary objective of this research was to evaluate the impact of CML and inflammatory markers on the biochemical and cardiovascular characteristics of CAD patients, differentiating between diabetic and non-diabetes patients. The study aimed to identify potential links between CML, diabetes, and CAD and to assess if these factors could serve as predictive biomarkers.
To achieve these objectives, this study enrolled 200 consecutive CAD patients undergoing coronary angiography. The patients were categorized into two groups based on their serum glycosylated hemoglobin (HbA1c) levels, with diabetic CAD patients (group I) having HbA1c levels of ≥ 6.5 and non-diabetic CAD patients (group II) with HbA1c levels < 6.5. Various parameters, including lipoprotein levels, plasma HbA1c levels, CML, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and nitric oxide levels, were analyzed to assess the differences between the two groups.
The study revealed several significant findings. Group I, comprising 81 males and 19 females, had a mean age of 54.2 ± 10.2 years, with a mean diabetes duration of 4.9 ± 2.2 years. Group II, consisting of 89 males and 11 females, had a mean age of 53.2 ± 10.3 years. Group I exhibited more severe CAD, with a higher percentage of patients suffering from triple vessel disease and more severe stenosis in the left anterior descending coronary artery compared to group II. Group I patients also had a larger left atrium diameter. Significantly, group I patients displayed higher levels of CML, TNF-α, and IL-6 and lower levels of nitric oxide compared to group II patients. The study also demonstrated strong correlations between CML and inflammatory markers, with CML showing a significant positive correlation with IL-6 (r = 0.596, P = 0.001) and TNF-α (r = 0.337, P = 0.001) and a negative correlation with nitric oxide (r=-4.16, P = 0.001). Odds ratio analysis indicated that patients with CML in the third quartile (264.43-364.31 ng/mL) were significantly associated with diabetic CAD at both unadjusted and adjusted levels when considering various covariates.
CML and inflammatory markers, particularly IL-6 and TNF-α, may play a significant role in the development of CAD, especially in individuals with diabetes. These findings suggest that CML and inflammatory markers can serve as potential biomarkers for predicting CAD, not only in diabetic patients but also in non-diabetic individuals. Understanding the mechanisms linking CML and inflammation to CAD provides valuable insights for improved CAD diagnosis, risk assessment, and management, which can ultimately contribute to reducing the burden of this life-threatening disease.
Future studies should explore interventions targeting CML and inflammatory markers to mitigate CAD risk. Investigating therapeutic strategies and diagnostic tools based on these biomarkers can aid in early CAD detection and personalized treatment, potentially reducing CAD-related mortality rates globally.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lakusic N, Croatia; Long P, China S-Editor: Lin C L-Editor: Filipodia P-Editor: Chen YX
| 1. | Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72-115. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | World Health Organization. Noncommunicable diseases. Sep 16, 2023. [Cited 23 July 2023] Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. [Cited in This Article: ] |
| 3. | Chen Y, Zhang H, Hou X, Li X, Qian X, Feng X, Liu S, Shi N, Zhao W, Hu S, Zheng Z, Li G. Glycemic control and risk factors for in-hospital mortality and vascular complications after coronary artery bypass grafting in patients with and without preexisting diabetes. J Diabetes. 2021;13:232-242. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444-470. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Shrivastav D, Dabla PK, Singh DD, Mehta V. Type 2 diabetes mellitus and coronary artery stenosis: a risk pattern association study. Explor Med. 2023;4:336-42. [DOI] [Cited in This Article: ] |
| 6. | Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, Gugliucci A, Kapahi P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018;28:337-352. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Davis KE, Prasad C, Vijayagopal P, Juma S, Imrhan V. Advanced Glycation End Products, Inflammation, and Chronic Metabolic Diseases: Links in a Chain? Crit Rev Food Sci Nutr. 2016;56:989-998. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14:453. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Liman PB, Agustina R, Djuwita R, Umar J, Permadhi I, Helmizar, Hidayat A, Feskens EJM, Abdullah M. Dietary and Plasma Carboxymethyl Lysine and Tumor Necrosis Factor-α as Mediators of Body Mass Index and Waist Circumference among Women in Indonesia. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Lamprea-Montealegre JA, Arnold AM, McCLelland RL, Mukamal KJ, Djousse L, Biggs ML, Siscovick DS, Tracy RP, Beisswenger PJ, Psaty BM, Ix JH, Kizer JR. Plasma Levels of Advanced Glycation Endproducts and Risk of Cardiovascular Events: Findings From 2 Prospective Cohorts. J Am Heart Assoc. 2022;11:e024012. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Nogami M, Hoshi T, Toukairin Y, Arai T, Nishio T. Immunohistochemistry of advanced glycation end product N(ε)-(carboxymethyl)lysine in coronary arteries in relation to cardiac fibrosis and serum N-terminal-pro basic natriuretic peptide in forensic autopsy cases. BMC Res Notes. 2020;13:239. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Wang ZQ, Sun Z. Dietary N(ε)-(carboxymethyl) lysine affects cardiac glucose metabolism and myocardial remodeling in mice. World J Diabetes. 2022;13:972-985. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Li LY, Chen S, Li FF, Wu ZM, Shen Y, Ding FH, Wang XQ, Shen WF, Chen QJ, Dai Y, Lu L. High serum levels of N-epsilon-carboxymethyllysine are associated with poor coronary collateralization in type 2 diabetic patients with chronic total occlusion of coronary artery. BMC Cardiovasc Disord. 2022;22:282. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Nass N, Ignatov A, Andreas L, Weißenborn C, Kalinski T, Sel S. Accumulation of the advanced glycation end product carboxymethyl lysine in breast cancer is positively associated with estrogen receptor expression and unfavorable prognosis in estrogen receptor-negative cases. Histochem Cell Biol. 2017;147:625-634. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Wang KY, Zheng YY, Wu TT, Ma YT, Xie X. Predictive Value of Gensini Score in the Long-Term Outcomes of Patients With Coronary Artery Disease Who Underwent PCI. Front Cardiovasc Med. 2021;8:778615. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson's method. Heart. 2002;88:559-560. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc. 2009;57:1874-1880. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Ahmed KA, Muniandy S, Ismail IS. N(epsilon)-(Carboxymethyl)lysine and Coronary Atherosclerosis-Associated Low Density Lipoprotein Abnormalities in Type 2 Diabetes: Current Status. J Clin Biochem Nutr. 2009;44:14-27. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Chu ZG, Yang ZG, Dong ZH, Zhu ZY, Peng LQ, Shao H, He C, Deng W, Tang SS, Chen J. Characteristics of coronary artery disease in symptomatic type 2 diabetic patients: evaluation with CT angiography. Cardiovasc Diabetol. 2010;9:74. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Ehl NF, Kühne M, Brinkert M, Müller-Brand J, Zellweger MJ. Diabetes reduces left ventricular ejection fraction--irrespective of presence and extent of coronary artery disease. Eur J Endocrinol. 2011;165:945-951. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Banach M, Surma S, Reiner Z, Katsiki N, Penson PE, Fras Z, Sahebkar A, Paneni F, Rizzo M, Kastelein J. Personalized management of dyslipidemias in patients with diabetes-it is time for a new approach (2022). Cardiovasc Diabetol. 2022;21:263. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Zhao K, Zheng Q, Zhou J, Zhang Q, Gao X, Liu Y, Li S, Shan W, Liu L, Guo N, Tian H, Wei Q, Hu X, Cui Y, Geng X, Wang Q, Cui W. Associations between serum electrolyte and short-term outcomes in patients with acute decompensated heart failure. Ann Med. 2023;55:155-167. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | A Ahmed K, Muniandy S, S Ismail I. Role of N-(carboxymethyl)lysine in the development of ischemic heart disease in type 2 diabetes mellitus. J Clin Biochem Nutr. 2007;41:97-105. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Koshino A, Schechter M, Sen T, Vart P, Neuen BL, Neal B, Arnott C, Perkovic V, Ridker PM, Tuttle KR, Hansen MK, Heerspink HJL. Interleukin-6 and Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: New Insights From CANVAS. Diabetes Care. 2022;45:2644-2652. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Adela R, Nethi SK, Bagul PK, Barui AK, Mattapally S, Kuncha M, Patra CR, Reddy PN, Banerjee SK. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS One. 2015;10:e0125270. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Kerkeni M, Weiss IS, Jaisson S, Dandana A, Addad F, Gillery P, Hammami M. Increased serum concentrations of pentosidine are related to presence and severity of coronary artery disease. Thromb Res. 2014;134:633-638. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Gaens KH, Niessen PM, Rensen SS, Buurman WA, Greve JW, Driessen A, Wolfs MG, Hofker MH, Bloemen JG, Dejong CH, Stehouwer CD, Schalkwijk CG. Endogenous formation of Nε-(carboxymethyl)lysine is increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J Hepatol. 2012;56:647-655. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Semba RD, Patel KV, Sun K, Guralnik JM, Ershler WB, Longo DL, Ferrucci L. Association between serum carboxymethyl-lysine, a dominant advanced glycation end product, and anemia in adults: the Baltimore longitudinal study of aging. J Am Geriatr Soc. 2008;56:2145-2147. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Kralev S, Zimmerer E, Brueckmann M, Lang S, Kälsch T, Rippert A, Lin J, Borggrefe M, Hammes HP, Süselbeck T. Elevation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in patients presenting with acute myocardial infarction. Clin Chem Lab Med. 2009;47:446-451. [PubMed] [DOI] [Cited in This Article: ] |