Copyright
©The Author(s) 2022.
World J Diabetes. Oct 15, 2022; 13(10): 877-887
Published online Oct 15, 2022. doi: 10.4239/wjd.v13.i10.877
Published online Oct 15, 2022. doi: 10.4239/wjd.v13.i10.877
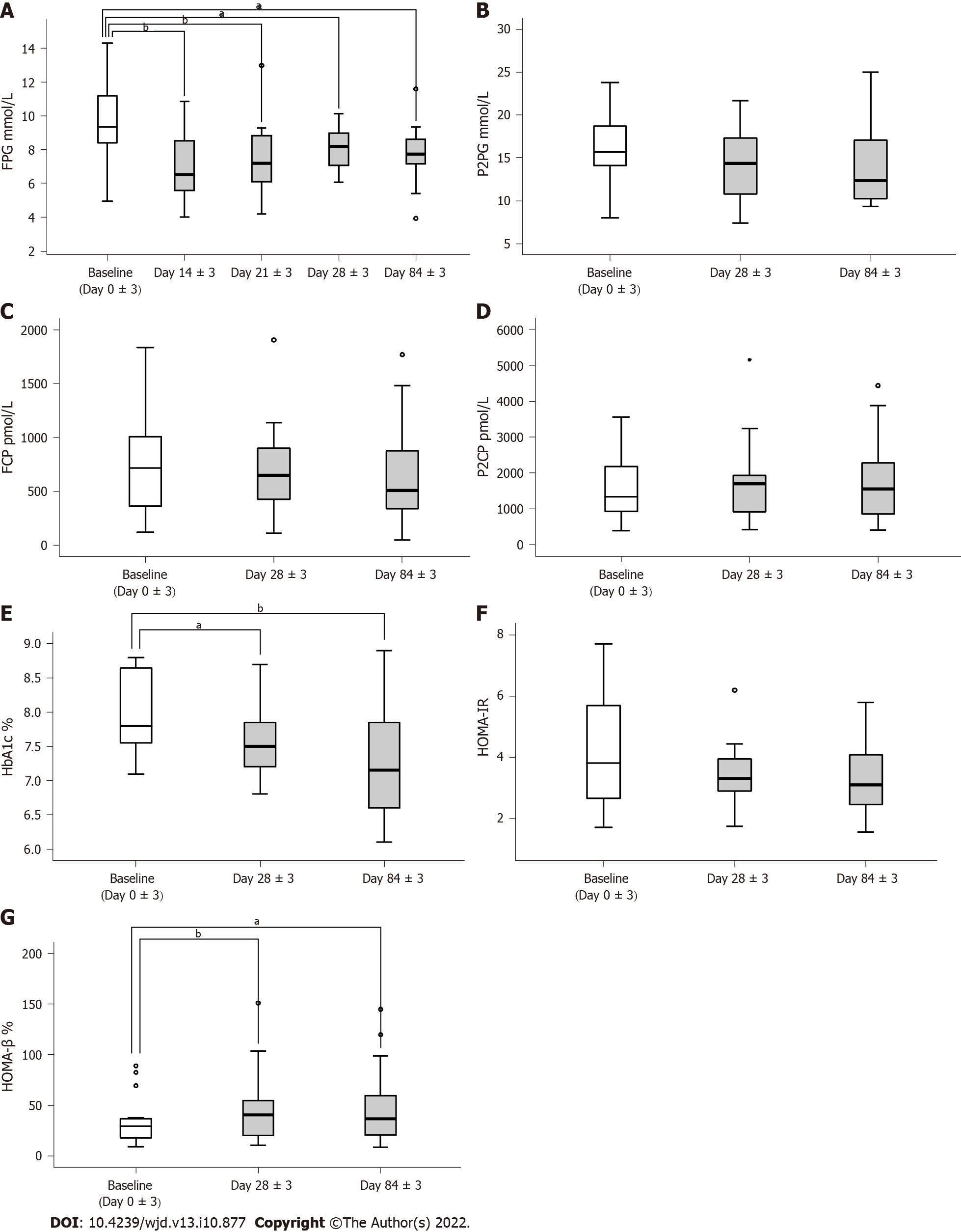
Figure 2 Assessment of the effectiveness of human umbilical cord blood-mesenchymal stem cell treatment.
A: Fasting plasma glucose tested on day 0 ± 3, day 14 ± 3, day 21 ± 3, day 28 ± 3, and day 84 ± 3; B-D: 2-h postprandial blood glucose, fasting C-peptide, and 2-h postprandial C-peptide tested on day 0 ± 3, day 28 ± 3, and day 84 ± 3; E: Glycosylated hemoglobin A1c levels tested on day 0 ± 3, day 28 ± 3, and day 84 ± 3; F and G: Homeostasis model assessment of insulin resistance and homeostasis model assessment of β-cell function calculated on day 0 ± 3, day 28 ± 3, and day 84 ± 3. aP < 0.05, bP < 0.01. FPG: Fasting plasma glucose; P2BG: 2-h postprandial blood glucose; FCP: Fasting C-peptide; P2CP: 2-h postprandial C-peptide; HbA1c: Glycosylated hemoglobin A1c; HOMA-IR: Homeostasis model assessment of insulin resistance; HOMA-β: Homeostasis model assessment of β-cell function.
- Citation: Lian XF, Lu DH, Liu HL, Liu YJ, Han XQ, Yang Y, Lin Y, Zeng QX, Huang ZJ, Xie F, Huang CH, Wu HM, Long AM, Deng LP, Zhang F. Effectiveness and safety of human umbilical cord-mesenchymal stem cells for treating type 2 diabetes mellitus. World J Diabetes 2022; 13(10): 877-887
- URL: https://www.wjgnet.com/1948-9358/full/v13/i10/877.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i10.877