Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1539
Peer-review started: January 29, 2021
First decision: June 16, 2021
Revised: June 21, 2021
Accepted: August 5, 2021
Article in press: August 5, 2021
Published online: September 15, 2021
Management of diabetic foot ulcers is the biggest challenge to the clinician, as conventional antibiotic therapies and local wound care have their own limitations. They are not effective for control of infections and promotion of healing because of cytotoxic effects. In view of cytotoxicity of routinely used topical antiseptic agents, this article focuses on the search of an ideal topical antiseptic agent that is safe and effective in controlling infectious agents and also in promoting the healing process. This review focuses on the use of various acids such as citric, acetic, hyaluronic, and hypochlorous acids as topical agents in diabetic foot infections. This article also focuses on the different roles of acids in the treatment of diabetic foot infections.
Core Tip: Diabetic foot ulcer is the most serious complication of diabetes mellitus. The biggest challenge is to find an ideal topical antiseptic agent that is safe and effective in controlling infectious agents and promoting the healing process. This article focuses on the use of acids as topical agents to control diabetic foot infections, with special emphasis on the different roles of citric, acetic, hyaluronic, and hypochlorous acids in the effective management of diabetic foot ulcers.
- Citation: Nagoba B, Gavkare A, Rayate A, Mumbre S, Rao A, Warad B, Nanaware N, Jamadar N. Role of an acidic environment in the treatment of diabetic foot infections: A review. World J Diabetes 2021; 12(9): 1539-1549
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1539.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1539
Diabetes mellitus is a global public health problem. The global diabetes prevalence in 2019 was estimated to be 9.3%and is expected to rise to 10.2% by 2030 and 10.9% by 2045[1].Development of foot ulcers is one of the most serious complications of diabetes mellitus. The annual risk of foot ulceration in diabetic patients is 2% whereas the lifetime risk is 12%-25%, which increases further in the presence of peripheral neuropathy[2-6]. Intrinsic factors such as loss of sensation because of peripheral neuropathy, vascular insufficiency because of microvascular disease, and impaired immune response along with mechanical factors such as increased plantar pressure associated with foot deformity and calluses, local trauma, and infection are the important risk factors[3,7,8].
Infection is the most common sequela of diabetic foot ulceration, and once established, it becomes progressively severe and more difficult to treat. An infected foot ulcer is the most common cause of diabetes-related hospital admission, and if not treated well in time, it is the most common cause leading to lower extremity amputa
Foot infections in diabetics are most commonly caused by bacteria. Both aerobes and anaerobes have been shown to cause infection[12-17]. Fungi are also known to be associated with foot infections in diabetics[18,19]. Polymicrobial etiology has been reported to be more common than monomicrobial infection. Bacteriological analysis shows a predominance of both Gram-positive and Gram-negative bacteria. Among the Gram-positive aerobic bacteria, Staphylococcus aureus (S. aureus) is the most common bacterium. Coagulase-negative Staphylococci and Streptococci are the other bacterial pathogens isolated from foot infections. Among the Gram-negative aerobes, Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli), Klebsiella spp., Proteus spp., Citrobacter spp., Acinetobacter spp., etc. are common isolates. Among anaerobic bacteria, Peptostreptococci, Clostridia and Peptococci are the common Gram-positive isolates and Bacteroides spp., Prevotella spp., Fusobacterium spp., etc. are common Gram-negative anaerobic isolates. Among fungi, Candida spp., C. albicans in particular, has been reported to be the most common[12-19].
A critical part of the management of diabetic foot ulcers is to treat the infection to reduce microbial load quantitatively to a level that can be resolved by the host immune system. As most of the etiological agents associated with diabetic foot infections are known to form biofilms and are resistant to multiple antimicrobial agents, they are difficult to eliminate from the infection site. It has been observed that biofilm formation is associated with increased virulence and delayed wound healing[20]. Biofilms release a variety of toxic components during the taxis of neutrophils and discourage the process of phagocytosis. In chronic wounds, formation of biofilms discourages wound healing by increasing the inflammatory response[21].
The ideal management of diabetic foot infections should positively and potentially reduce the incidence of infection-related morbidities, duration of hospital stay, the cost of treatment, and most importantly, reduce limb amputations[22]. Compared with other wounds, diabetic foot ulcers are more prone to infection, and infection is one of the most important factors that delay wound healing. Hence, good wound care for control of infection is critical for successful wound healing[23], but successful treatment of diabetic foot infections is the biggest challenge for the following reasons: (1) Parenterally or orally administered antimicrobial agents have been shown not to reach adequate levels in chronic granulation tissue and have no effect on growing bacterial populations in granulating wounds[24]. Biofilm formation by infecting agents in diabetic foot ulcers makes wound healing and infections difficult to resolve by hampering local access of antimicrobial agents and because diabetes hampers the immune system[25]. Biofilm formation not only helps to prevent phagocytosis but also helps to increase the resistance of infecting agents to antimicrobial agents[26,27]. In a previous study, we found that in spite of in vitro susceptibility of infecting agent isolated from patients to antimicrobial agents, administration of the antimicrobial agent to patients did not result in successful outcomes. The result indicates that systemic antimicrobial therapy may not have practical and potential value in such cases, making local wound care the backbone of treatment[28]. And (2) Many topical antiseptic agents are used for wound care in diabetics. Some are good in controlling infections but their cytotoxic effect on the cells involved in the wound healing process and other cells like dermal and epidermal cells limit their use. Available experimental data show that majority of the agents retard healing by interfering with the normal process and can be harmful rather than useful. Studies show that these agents should be avoided, especially in the treatment of diabetic foot ulcers[29-32].
Hydrogen peroxide is the most commonly used antiseptic for washing diabetic foot ulcers, but is toxic to newly formed epithelium[33], because it kills fibroblasts, which have an important role in healing and epithelialization. In addition, it may also destroy normal cells surrounding the wound[34]. Povidone-iodine (betadine) is another commonly used antiseptic agent, but because it is also cytotoxic to fibroblasts and other cells involved in wound healing, it fails to promote good wound healing. Most studies show that it impairs wound healing and reduces wound strength[30,35-37]. Sodium hypochlorite (Dakin’s solution) has also been reported to be toxic to fibroblasts and keratinocytes and has been found to delay the process of epithelialization and neovascularization. It has also been reported to retard collagen synthesis and inhibit migration of neutrophils in a wound bed[38-42]. Silver nitrate has also been reported to slow down the process of epithelialization and may delay wound healing[30,38]. Many other antiseptic agents such as iodine, alcohol, chlorhexidine, mafenide acetate, silver compounds, and benzalkonium chloride, etc. have been reported to retard wound healing[31,38,43-45]. In view of these observations, the treatment of diabetic foot ulcers has always been a big challenge to the clinician as conventional therapies (antibiotic therapy and local wound care) have limitations. Infection is the most common and most important reason for nonhealing/poor healing of diabetic foot ulcers. Infecting organisms are most difficult to eliminate from the infection site. Infections of diabetic foot ulcers need special attention, and if not controlled well in time, may become limb threatening and sometimes life threatening by progressing to osteomyelitis or gangrene, which can lead to septicemia, amputation and death. The biggest challenge is the search of an ideal topical antiseptic agent that is safe and effective in controlling/eradicating infectious agents from the infection site and as well as promoting/hastening the healing process.
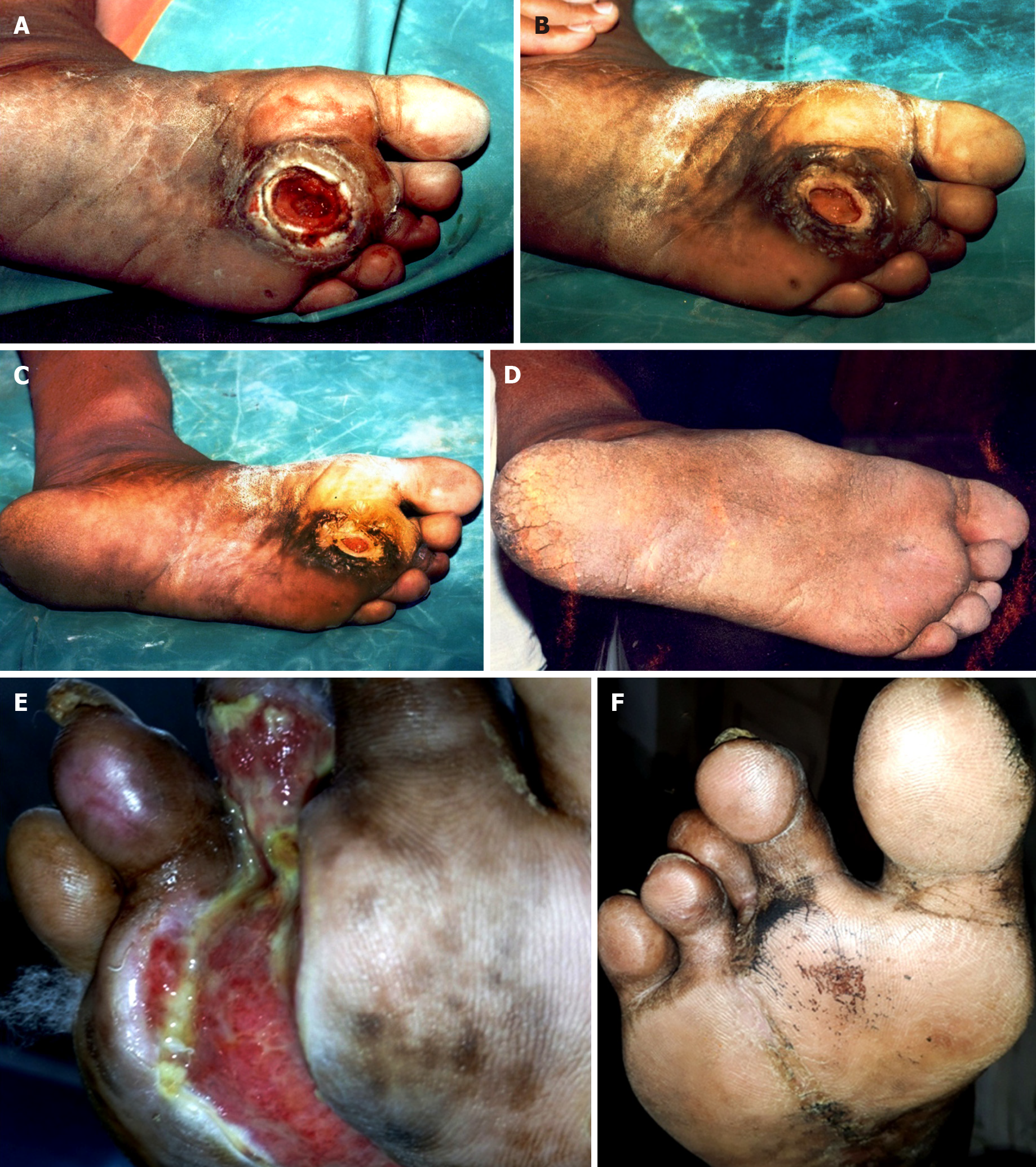
Various acids, such as citric, acetic, hyaluronic, and hypochlorous (HOCl) acids are topical agents used in the treatment of diabetic foot infections. Citric acid (2%-3%) has been used to treat a variety of infected wounds and ulcers such as necrotizing fasciitis, lepromatous ulcers, burns infections, surgical site infections, post-operative wounds in HIV/AIDS patients, traumatic wounds, diabetic foot ulcers, and many others[46-55]. In our initial study, citric acid was successfully used to treat diabetic foot ulcers infected with multiple antibiotic-resistant strains of P. aeruginosa[52]. Later on, citric acid was found to be effective against S. aureus also. Considering its activity against S. aureus, a case report published in 2000 described the successful treatment of a diabetic foot infected with multiple antibiotic-resistant P. aeruginosa and S. aureus and not responding to conventional antibiotic therapy and local wound care by application of a 2% citric acid solution[53]. A subsequent study reported the activity of citric acid against multiple antibiotic-resistant E. coli (MAREC). The in vitro sensitivity of E. coli to citric acid was reported in 2008, with successful use of 3% citric acid gel to treat diabetic foot ulcers infected with MAREC, with complete elimination of MAREC from infected sites and successful healing following 29-42 applications of citric acid[54]. A study published in 2010, found that citric acid was effective against almost all aerobic bacterial pathogens commonly associated with diabetic foot ulcers, i.e. S. aureus, P. aeruginosa, E. coli, Klebsiella spp., S. albus, Citrobacter spp., Streptococci and Proteus vulgaris. That study reported that citric acid was found effective in control of diabetic foot infections and successful management of Wagner grades I and II ulcers, and even Wagner grade III ulcers without deep osteomyelitis. The success rate was more than 94% in Wagner grade I and II ulcers, and 86.21% in Wagner grade III ulcers[13]. A recent case study described the treatment of a 70-year-old man with a diabetic leg ulcer that developed at the operative site 2 years after coronary artery bypass graft surgery. The ulcer was infected with methicillin-resistant S. aureus (MRSA) and had not responded to conventional treatment for months. It was successfully treated by application of 3% citric acid once daily for 30 d[55]. These studies of infected diabetic foot ulcers did not report any adverse effects, which shows that citric acid was found to be a safe and most effective topical antimicrobial agent for the treatment of diabetic foot ulcers. Citric acid has been found effective in chronic plantar ulcers in diabetic individuals with uncontrolled blood sugar levels and infected with multiple antibiotic-resistant bacteria not responding to conventional therapies for months (See Figure 1).
Acetic acid has been used for the treatment of skin and soft tissue infections and burn wound infections caused by P. aeruginosa[56-58]. It is rarely used in the treatment of diabetic foot infections and infections caused by other microbial agents. Agrawal et al[59] reported that acetic acid controlled the overgrowth of many common isolates in addition to P. aeruginosa, including Streptococcus, S. aureus, Proteus mirabilis, Citrobacter spp., C. albicans, Aspergillus niger, A. fumigatus and Cryptococcus neoformans. In a previous study, 52-year-old man with diabetic foot ulcer infected with P. aeruginosa was successfully treated with 3% acetic acid. Application of acetic acid once daily for 12 d successfully eliminated P. aeruginosa and resulted in successful healing of the ulcer[60]. Agrawal et al[59] reported remarkable improvement in raw areas in 7-14 d. They noted similar results even in cases with exposed tendons and crush injuries in diabetic patients, and even in infections caused by antibiotic-resistant strains. Fejfarová et al[61]also reported favorable outcomes of reduced ulcer dimensions using 1% acetic acid in diabetic foot ulcers, but the difference was not statistically significant.
Hyaluronic acid has been used in the management of diabetic foot ulcers in previous studies[62-65]. Lee et al[62] reported higher mean percentages of wound area reduction, wound depth reduction, and increase of healthy granulation tissue in the experimental group than in the control group indicating the potential of hyaluronic acid dressings to accelerate diabetic wound healing. A meta-analysis by Chen et al[63] found that hyaluronic acid was beneficial in treating diabetic foot ulcers by increasing the rate of wound healing, evidence that further supports the use of hyaluronic acid in the treatment of diabetic foot ulcers. In a study conducted by Lee et al[64], hyaluronic acid treatment achieved a significantly higher complete healing rate(84.6%) than was observed in the control group(41.6%). Healing was faster in the hyaluronic acid group and had a shorter mean duration of achieving a 50% ulcer size reduction without any adverse events, indicating that hyaluronic acid was safe and effective in treating diabetic foot ulcers. A study by Hwang et al[65] also concluded that hyaluronic acid dressing without additional substances was a safe and effective treatment for diabetic foot ulcers.
HOCl is another option for acid treatment of infected diabetic foot ulcers. HOCl has been reported to be effective against Candida spp., Proteus spp., Klebsiella spp., Pseudomonas spp. and MRSA. It was found comparatively more effective than hydrogen peroxide and povidone-iodine as a potent antimicrobial agent as well as being a better wound healing agent in diabetic foot ulcers, as evidenced by the formation of healthy granulation tissue and a significant reduction in number of organisms on quantitative culture. HOCl has also been reported to soften the wound surface eschar, clean and remove necrotic tissue and biofilms from diabetic foot infections. When compared with hydrogen peroxide and povidone-iodine, HOCl was found to cause a significant reduction in the quantity of exudate, had broad spectrum antimicrobial activity against a variety of microbes, and caused significant reductions in bacterial count and bacterial burden. HOCl appears to be a potent topical antiseptic to treat diabetic foot ulcers. It effectively controls the bioburden without impeding the process of wound healing[66-70].
The various acids used to treat diabetic foot infections are known to have different roles in controlling infections caused by a variety of microbes and in promoting healing by participating in different stages in the healing of diabetic foot ulcers. Apart from the specific roles of different acids, the acidic environment created by all acids helps in the following ways. (1) Antimicrobial property: Application of acids to the wound surface creates an acidic environment. A pH of < 6.0 at the wound surface makes it an environment unsuitable for the growth and multiplication of most pathogenic bacteria, which require an optimum pH of 7. Acids thus have antimicrobial property that helps in rapid cleaning of infected surfaces[71]. (2) Inhibition of enzyme activity: The acidic environment inhibits the activity of proteolytic enzymes such as elastase and plasmin produced by various bacteria and by wound itself. The proteases are highly active in alkaline conditions. The acidic environment slows/inhibits their activity and formation of their end products, which are toxic to the healing process[71,72]. (3) Increase in oxygenation: The acidic environ
Citric acid is known to have key roles in the process of wound healing. As shown in Table 1, it helps in the management of a variety of infected wounds, including diabetic foot ulcers, in a number of ways.
| Order of efficacy | Name of acid | Roles |
| 1 | Citric acid | Antibacterial activity[13,51]; Decrease in pH-preventing growth and multiplication[13,76,79]; Fibroblastic growth, neovascularization and epithelialization[13,51,76,77]; Notable clinical changes[76,77,79] |
| 2 | Acetic acid | Mainly antipseudomonal activity[55-57]; Anti biofilm activity[81-83] |
| 3 | Hyaluronic acid | Reduces inflammatory response[22,84,85]; Increases angiogenesis and promotes granulation[22,84,85]; Proliferation of keratin cells[22,84,85]; Contributes to scarring[22,84,85]; Scavenger of free radicals and tissue degrading enzymes[85,86]; Controls tissue hydration[87] |
| 4 | Hypochlorous acid | Antimicrobial activity[70]; Wound debridement[70]; Anti-biofilm activity[70]; Promotes granulation[66] |
Antibacterial activity: Citric acid is inhibitory to all bacterial pathogens commonly associated with diabetic foot ulcers. However, it has not been found effective against fungal pathogens. MICs (minimum inhibitory concentrations) in the range of 500-2500 µg/mL against different bacterial isolates from diabetic foot ulcers and nondiabetic traumatic wounds have been reported. P. aeruginosa (MIC 500-1000 µg/mL) has been found to be the most susceptible and Klebsiella spp. (MIC 2000-2500 µg/mL) least susceptible to citric acid[13,51].
Decrease in the wound surface pH: Decrease in pH has an important role in wound healing. It is a biochemical indicator of wound healing processes and can be used to monitor the progression of wound healing. Wounds treated with citric acid show a pH ranging from 4 to 6, which is not suitable for the growth and multiplication of most bacterial pathogens that cause infection, and thus helps in effective elimination of bacteria from the infection site, leading to rapid cleaning up of infected surfaces. In addition, lowering the pH of wound surfaces also helps to reduce bacterial toxicity (e.g., endotoxins and metalloproteinases), altering protease activity, etc. Microbiological evaluation after application of citric acid shows significant reductions in bacterial counts, or no growth, suggesting that citric acid effectively controls the infection[13,76-79].
Fibroblastic growth, neovascularization, and epithelialization: Histopathological studies show that application of citric acid boosts fibroblastic growth and promotes neovascularization, which increases the microcirculation of nutrients and improves oxygenation. This enhances epithelialization and increases the migration of epithelial cells from the surrounding skin. Epithelialization in turn acts as stimulus for the deposition of ground substance and formation of healthy granulation tissue, thereby leading to faster wound healing[13,51,76]. A significant increase in granulation tissue compared with control treatment has been reported after application of citric acid[77].
Notable clinical changes: Application of citric acid results in significant reduction in wound size, early reduction in the amount of discharge and sloughing, and reduces hospital stay[76,77,79]. Significant reductions in common signs of inflammation such as edema, wound discharge, and erythema were noted in a study by Tandon et al[77].
In most of the studies, acetic acid has been reported to be inhibitory to P. aeruginosa only[56-58]. However, Agrawal et al[59] found that it has antibacterial activity against bacterial pathogens commonly associated with wound infections, and antifungal activity as well. An in vitro study by Lineaweaver et al[80] showed that 0.25% acetic acid was toxic to fibroblasts, slowed wound epithelialization, and limited neutrophil function. It is well tolerated in vivo and gives superior results in the treatment of wounds infected with P. aeruginosa[57]. Bjarnsholt et al[81] reported that acetic acid lowered the pH and was effective in removing biofilms. It kills planktonic bacteria as well as helps eradicate bacteria growing in biofilms. Halstead et al[82] found that acetic acid was active against drug-resistant and biofilm-producing bacteria. In a study by Bjarnsholt et al[81], acetic acid was found effective against planktonic cells as well as biofilms of P. aeruginosa and S. aureus, and was found to have potential clinical use as a topical agent to eradicate biofilms in chronic infections caused by P. aeruginosa.
Hyaluronic acid is known to have a key role in every phase of wound healing. During the inflammatory phase, it binds to fibrinogen to initiate the clotting pathway, allows inflammatory cell migration, creates edema to allow cell infiltration and inhibits migration of neutrophils to reduce inflammatory response. During the granulation phase, it promotes cell mitosis and increases cell migration and angiogenesis. During re-epithelialization, it is associated with the proliferation of keratin cells and facilitates their migration. During the remodeling phase, it contributes to normal and pathological scarring[22,83,84]. Hyaluronic acid also serves as a scavenger of free radicals and tissue degrading enzymes that cause prolonged inflammation in chronic wounds[84,85]. It has been also reported to have important role in controlling tissue hydration[86].
HOCl has antibacterial as well as antifungal activity. It kills pathogens without causing cytotoxicity to keratinocytes or fibroblasts. The killing of pathogens promotes the natural healing process. It has been reported to be an effective wound cleaning and debriding agent by softening the wound surface eschar and removing necrotic tissue and biofilms from infected diabetic foot ulcers[70]. It significantly reduces the number of microbes in wounds and promotes rapid formation of healthy granulation tissue[66].
The results of various studies show that conventional antibiotic treatment and local wound care with routinely used topical antiseptic agents have limitations. In view of cytotoxic effects on the cells involved in the process of wound healing, various acids are better options to treat diabetic foot infections. Citric acid, hyaluronic acid, and HOCl in a decreasing order of efficacy, and to a lesser extent acetic acid can be used as better alternatives to control infection and promote the healing of diabetic foot ulcers.
Authors wish to thank Mr. Jogdand V and Mr. Badne D from Department of Medical Education for their assistance in preparation of manuscript. Thanks are also due to Mr. Ghante D, Mr. Borgaonkar P and Mr. Kothiwale N from Department of Art and Photography, MIMSR Medical College, Latur, India for photographs of clinically treated diabetic foot ulcers.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He Z, Korkmaz P S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5345] [Cited by in F6Publishing: 4630] [Article Influence: 926.0] [Reference Citation Analysis (8)] |
| 2. | World Health Organization. Global report on diabetes. France: 2016. [cited 20 January 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/9789241565257. [Cited in This Article: ] |
| 3. | Kavitha KV, Tiwari S, Purandare VB, Khedkar S, Bhosale SS, Unnikrishnan AG. Choice of wound care in diabetic foot ulcer: A practical approach. World J Diabetes. 2014;5:546-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 83] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 4. | Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1841] [Cited by in F6Publishing: 1670] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 5. | International Diabetes Federation. IDF Diabetes Atlas.9th ed. 2019. [cited 2 January 2021]. In: International Diabetes Federation [Internet].Available from: https://www.diabetesatlas.org. [Cited in This Article: ] |
| 6. | International Diabetes Federation. Clinical Practice Recommendation on the Diabetic Foot: A guide for health care professionals. 2017:1-70 [cited 18 January 2021]. In: International Diabetes Federation [Internet]. Available from: https://www.idf.org/component/attachments/?task=download&id=1152. [Cited in This Article: ] |
| 7. | Bakker K, Apelqvist J, Schaper NC; International Working Group on Diabetic Foot Editorial Board. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28 Suppl 1:225-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 8. | Farahani RM, Kloth LC. The hypothesis of 'biophysical matrix contraction': wound contraction revisited. Int Wound J. 2008;5:477-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1532] [Cited by in F6Publishing: 1427] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 10. | Richard JL, Sotto A, Lavigne JP. New insights in diabetic foot infection. World J Diabetes. 2011;2:24-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 80] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Viswanathan V, Rao VN. Managing diabetic foot infection in India. Int J Low Extrem Wounds. 2013;12:158-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Jaju K, Pichare A, Davane M, Nagoba B. Profile and Antibiotic Susceptibility of Bacterial Pathogens Associated With Diabetic Foot Ulcers From a Rural Area. Wounds. 2019;31:158-162. [PubMed] [Cited in This Article: ] |
| 13. | Nagoba BS, Gandhi RC, Wadher BJ, Rao A, Hartalkar AR, Selkar SP. A simple and effective approach for the treatment of diabetic foot ulcers with different Wagner grades. Int Wound J. 2010;7:153-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC, Chaudhry R. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care. 2006;29:1727-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Viswanathan V, Jasmine JJ, Snehalatha C, Ramachandran A. Prevalence of pathogens in diabetic foot infection in South Indian type 2 diabetic patients. J Assoc Physicians India. 2002;50:1013-1016. [PubMed] [Cited in This Article: ] |
| 16. | Gerding DN. Foot infections in diabetic patients: the role of anaerobes. Clin Infect Dis. 1995;20 Suppl 2:S283-S288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 105] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Johnson S, Lebahn F, Peterson LR, Gerding DN. Use of an anaerobic collection and transport swab device to recover anaerobic bacteria from infected foot ulcers in diabetics. Clin Infect Dis. 1995;20 Suppl 2:S289-S290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Raja NS. Microbiology of diabetic foot infections in a teaching hospital in Malaysia: a retrospective study of 194 cases. J Microbiol Immunol Infect. 2007;40:39-44. [PubMed] [Cited in This Article: ] |
| 19. | Bansal E, Garg A, Bhatia S, Attri AK, Chander J. Spectrum of microbial flora in diabetic foot ulcers. Indian J Pathol Microbiol. 2008;51:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Banu A, Noorul Hassan MM, Rajkumar J, Srinivasa S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas Med J. 2015;8:280-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Damir A. Why Diabetic foot ulcers do not heal. J Int Med Sci Acad. 2011;24:205. [Cited in This Article: ] |
| 22. | Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, Tan JS; Infectious Diseases Society of America. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 572] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 23. | Niezgoda JA, Sordi PJ, Hermans MH. Evaluation of Vashe Wound Therapy in the clinical management of patients with chronic wounds. Adv Skin Wound Care. 2010;23:352-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. A comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg. 1986;121:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 112] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2563] [Cited by in F6Publishing: 2353] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 26. | Najafi RB, Samani SM, Pishva N, Moheimani F. Formulation and Clinical Evaluation of Povidone-Iodine Ophthalmic Drop. Iran J Pharm Res. 2003;2:157-160. [DOI] [Cited in This Article: ] |
| 27. | Simmons TJ, Hashim D, Vajtai R, Ajayan PM. Large area-aligned arrays from direct deposition of single-wall carbon nanotube inks. J Am Chem Soc. 2007;129:10088-10089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Nagoba B, Kolhe S, Wadher B. Antibiotic Treatment Failure in Chronic Nosocomial Wound Infections. Eur J Gen Med. 2009;6:60. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Brånemark PI, Albrektsson B, Lindström J, Lundborg G. Local tissue effects of wound disinfectants. Acta ChirScand Suppl. 1966;357:166-176. [PubMed] [Cited in This Article: ] |
| 30. | Kramer SA. Effect of povidone-iodine on wound healing: a review. J VascNurs. 1999;17:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Pathare NA. Infections and diabetes mellitus. J Diabet Asso Ind. 1998;38:4-6. [Cited in This Article: ] |
| 32. | Robson MC, Edstrom LE, Krizek TJ, Groskin MG. The efficacy of systemic antibiotics in the treatment of granulating wounds. J Surg Res. 1974;16:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 83] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Wilson JR, Mills JG, Prather ID, Dimitrijevich SD. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv Skin Wound Care. 2005;18:373-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, Hwong W, Barati E, Belisle B, Celeri C, Robson MC. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6:e5. [PubMed] [Cited in This Article: ] |
| 35. | Agarwal R, Khan A, Aggarwal AN, Gupta D. Efficacy & safety of iodopovidone pleurodesis: a systematic review & meta-analysis. Indian J Med Res. 2012;135:297-304. [PubMed] [Cited in This Article: ] |
| 36. | Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015;CD003949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Cooper ML, Laxer JA, Hansbrough JF. The cytotoxic effects of commonly used topical antimicrobial agents on human fibroblasts and keratinocytes. J Trauma. 1991;31:775-82; discussion 782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 117] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Dumville JC, Lipsky BA, Hoey C, Cruciani M, Fiscon M, Xia J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2017;6:CD011038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Miller JM, Creazzo J, Witt C. Wound healing: an introductory clinical approach. Contemp Pediatr Physic. 1992;1:38-42. [Cited in This Article: ] |
| 40. | Dakin HD. THE ANTISEPTIC ACTION OF HYPOCHLORITES: The Ancient History of the "New Antiseptic.". Br Med J. 1915;2:809-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Kozol RA, Gillies C, Elgebaly SA. Effects of sodium hypochlorite (Dakin's solution) on cells of the wound module. Arch Surg. 1988;123:420-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 62] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Kjolseth D, Frank JM, Barker JH, Anderson GL, Rosenthal AI, Acland RD, Schuschke D, Campbell FR, Tobin GR, Weiner LJ. Comparison of the effects of commonly used wound agents on epithelialization and neovascularization. J Am Coll Surg. 1994;179:305-312. [PubMed] [Cited in This Article: ] |
| 43. | Bellinger CG, Conway H. Effects of silver nitrate and sulfamylon on epithelial regeneration. PlastReconstr Surg. 1970;45:582-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Lineaweaver W, McMorris S, Soucy D, Howard R. Cellular and bacterial toxicities of topical antimicrobials. PlastReconstr Surg. 1985;75:394-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 142] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, Flores-Morales V, Martinez-Fierro ML. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics (Basel). 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 57] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 46. | Nagoba BS, Gandhi RC, Wadher BJ, Gandhi SP, Selkar SP. Citric acid treatment of necrotizing fasciitis: a report of two cases. Int Wound J. 2010;7:536-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Nagoba BS, Wadher BJ, Rao A, Selkar SP, Gandhi RC. Treatment of lepromatous ulcers using citric acid as a sole antimicrobial agent. Int Wound J. 2012;9:553-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Nagoba BS, Gandhi RC, Hartalkar AR, Wadher BJ, Selkar SP. Simple, effective and affordable approach for the treatment of burns infections. Burns. 2010;36:1242-1247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Nagoba B, Raju R, Wadher B, Gandhi R, Rao AK, Selkar S, Hartalkar A. Citric acid treatment of surgical site infections: a prospective open study. Wound Pract Res. 2011;19:82-87. [Cited in This Article: ] |
| 50. | Nagoba B, Patil Dawale C, Raju R, Wadher B, Chidrawar S, Selkar S, Suryawanshi N. Citric acid treatment of post operative wound infections in HIV/AIDS patients. J Tissue Viability. 2014;23:24-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Nagoba B, Gandhi R, Wadher B, Rao A, Selkar S. Simple and effective approach for the treatment of traumatic wounds in non-diabetic patients: a prospective open study. Int Wound J. 2013;10:585-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Nagoba BS, Deshmukh SR, Wadher BJ, Mahabaleshwar L, Gandhi RC, Kulkarni PB, Mane VA, Deshmukh JS. Treatment of superficial pseudomonal infections with citric acid: an effective and economical approach. J Hosp Infect. 1998;40:155-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Nagoba BS, Kulkarni PB, Wadher BJ, Kulkarni UP, Mahabaleshwar L. Citric acid treatment of diabetic foot: a simple and effective approach. J Assoc Physicians India. 2000;48:739-741. [PubMed] [Cited in This Article: ] |
| 54. | Nagoba BS, Wadher BJ, Rao AK, Kore GD, Gomashe AV, Ingle AB. A simple and effective approach for the treatment of chronic wound infections caused by multiple antibiotic resistant Escherichia coli. J Hosp Infect. 2008;69:177-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Nagoba BS, Rawal C, Davane M. Citric acid treatment of diabetic leg ulcer infected with methicillin resistant Staphylococcus aureus. J Wound Care. 2021;In press. [Cited in This Article: ] |
| 56. | Sloss JM, Cumberland N, Milner SM. Acetic acid used for the elimination of Pseudomonas aeruginosa from burn and soft tissue wounds. J R Army Med Corps. 1993;139:49-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Nagoba BS, Deshmukh SR, Wadher BJ, Patil SB. Acetic acid treatment of pseudomonal postoperative wound infection. J Hosp Infect. 1997;36:243-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Nagoba BS, Selkar SP, Wadher BJ, Gandhi RC. Acetic acid treatment of pseudomonal wound infections--a review. J Infect Public Health. 2013;6:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Agrawal KS, Sarda AV, Shrotriya R, Bachhav M, Puri V, Nataraj G. Acetic acid dressings: Finding the Holy Grail for infected wound management. Indian J Plast Surg. 2017;50:273-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Nagoba B, Wadher B, Kulkarni P, Kolhe S. Acetic acid treatment of pseudomonal wound infections. Eur J Gen Med. 2008;5:104-106. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Fejfarová V, Tibenská H, Niklová J, Bém R, Dubský M, Wosková V, Němcová A, Jirkovská A, Jude E, Lánská V. Benefits of Acidifying Agents in Local Therapy of Diabetic Foot Ulcers Infected by Pseudomonassp: A Pilot Study. Int J Low Extrem Wounds. 2019;18:262-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Lee Y, Yun TK, Han S. Effect of Hyaluronic Acid Dressing on Diabetic Ulcer Healing -A Pilot Study Effect of Hyaluronic Acid Dressing on Diabetic Ulcer Healing - A Pilot Study. J Korean Wound Manag Soc. 2014;10:67-74. [Cited in This Article: ] |
| 63. | Chen CP, Hung W, Lin SH. Effectiveness of hyaluronic acid for treating diabetic foot: a systematic review and meta-analysis. Dermatol Ther. 2014;27:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Lee M, Han SH, Choi WJ, Chung KH, Lee JW. Hyaluronic acid dressing (Healoderm) in the treatment of diabetic foot ulcer: A prospective, randomized, placebo-controlled, single-center study. Wound Repair Regen. 2016;24:581-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Hwang Y, Park YJ, Shim D-W, Lee M, Park KH, Suh JW, Han SH, Choi WJ, Lee JW. Hyaluronic Acid Dressing in the Treatment of Diabetic Foot Ulcer: A Prospective, Randomized, Placebo-Controlled, Single-Center Study. Foot Ankle Orthop. 2016;1:2473011416S0007. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 66. | Ragab II, Kamal A. The Effectiveness of Hypochlorous Acid Solution on Healing of Infected Diabetic Foot Ulcers. J Educ Pract. 2017;8:58-71. [Cited in This Article: ] |
| 67. | Payne WG, Wright TE, Ko F, Wheeler C, Wang X, Robson MC. Bacterial Degradation of Growth Factors. J Appl Res. 2003;3:35-40. [Cited in This Article: ] |
| 68. | Chopra I. Efflux-based antibiotic resistance mechanisms: the evidence for increasing prevalence. J Antimicrob Chemother. 1992;30:737-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Chopra I, Johnson SC, Bennett PM. Inhibition of Providencia stuartii cell envelope enzymes by chlorhexidine. J Antimicrob Chemother. 1987;19:743-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Mekkawy MM, Kamal A. A Randomized Clinical Trial: The Efficacy of Hypochlorous Acid on Septic Traumatic Wound. J Educ Pract. 2014;5:89-101. [Cited in This Article: ] |
| 71. | Nagoba B, Suryawanshi N, Wadher B, Selkar S. Acidic Environment and Wound Healing: A Review. Wounds. 2015;27:5-11. [Cited in This Article: ] |
| 72. | Greener B, Hughes AA, Bannister NP, Douglass J. Proteases and pH in chronic wounds. J Wound Care. 2005;14:59-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Hunt TK, Hopf HW. Wound healing and wound infection. What surgeons and anesthesiologists can do. Surg Clin North Am. 1997;77:587-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Hunt TK, Beckert S. Therapeutical and practical aspects of oxygen in wound healing. In: Lee BY. The Wound Management Manual. New York: McGraw-Hill Professional, 2004: 44-54. [Cited in This Article: ] |
| 75. | Gethin G. The significance of surface pH in chronic wounds. Wounds UK. 2007;3:52-56. [Cited in This Article: ] |
| 76. | Nagoba BS, Gandhi RC, Wadher BJ, Potekar RM, Kolhe SM. Microbiological, histopathological and clinical changes in chronic infected wounds after citric acid treatment. J Med Microbiol. 2008;57:681-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Tandon S, Singh B, Kapoor S, Mangal S. Comparison of Effect of pH Modulation on Wound Healing with Topical Application of Citric Acid Versus Superoxide Ions. Niger J Surg. 2020;26:122-126. [PubMed] [Cited in This Article: ] |
| 78. | Bennison LR, Miller CN, Summers RJ, Minnis AMB, Sussman G, Mcguiness W, Bennison LR. The pH of wounds during healing and infection: a descriptive literature review. Wound Pract Res. 2017;25:63-69. [Cited in This Article: ] |
| 79. | Prabhu V, Prasadi S, Shivani A, Gore A, Pawar V. Does wound pH modulation with 3% citric acid solution dressing help in wound healing: A pilot study. Saudi Surg J. 2014;2:38-46. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Lineaweaver W, Howard R, Soucy D, McMorris S, Freeman J, Crain C, Robertson J, Rumley T. Topical antimicrobial toxicity. Arch Surg. 1985;120:267-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 278] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Bjarnsholt T, Alhede M, Jensen PØ, Nielsen AK, Johansen HK, Homøe P, Høiby N, Givskov M, Kirketerp-Møller K. Antibiofilm Properties of Acetic Acid. Adv Wound Care (New Rochelle). 2015;4:363-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Halstead FD, Rauf M, Moiemen NS, Bamford A, Wearn CM, Fraise AP, Lund PA, Oppenheim BA, Webber MA. The Antibacterial Activity of Acetic Acid against Biofilm-Producing Pathogens of Relevance to Burns Patients. PLoS One. 2015;10:e0136190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 83. | Frenkel JS. The role of hyaluronan in wound healing. Int Wound J. 2014;11:159-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 798] [Cited by in F6Publishing: 732] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 85. | Kvam BJ, Fragonas E, Degrassi A, Kvam C, Matulova M, Pollesello P, Zanetti F, Vittur F. Oxygen-derived free radical (ODFR) action on hyaluronan (HA), on two HA ester derivatives, and on the metabolism of articular chondrocytes. Exp Cell Res. 1995;218:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Laurent TC, Laurent UB, Fraser JR. The structure and function of hyaluronan: An overview. Immunol Cell Biol. 1996;74:A1-A7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 333] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 87. | Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 875] [Cited by in F6Publishing: 764] [Article Influence: 42.4] [Reference Citation Analysis (0)] |