Copyright
©The Author(s) 2021.
World J Diabetes. May 15, 2021; 12(5): 603-615
Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.603
Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.603
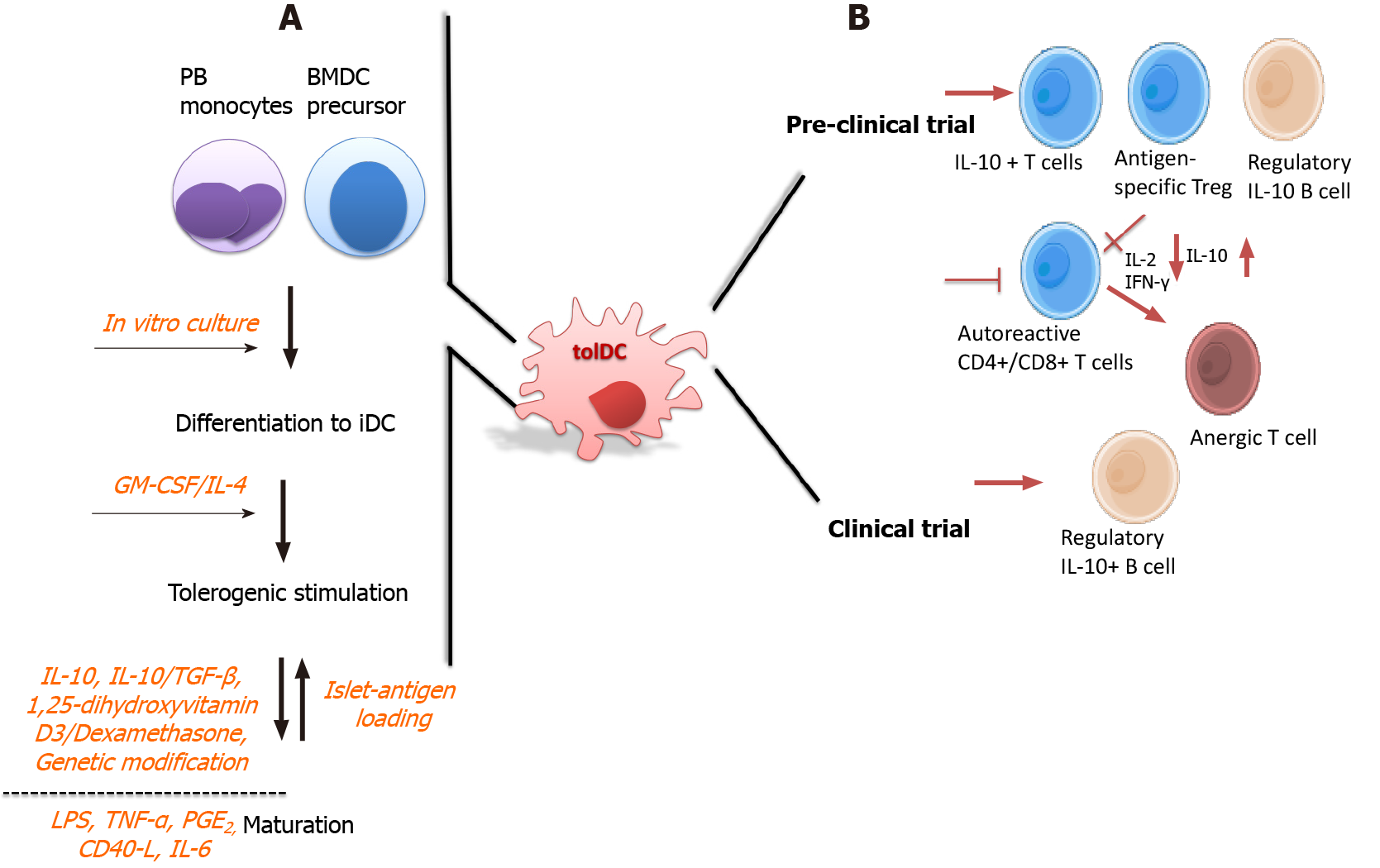
Figure 2 Tolerogenic dendritic cells in type 1 diabetes therapy: manufacturing and tolerogenic mechanisms described in preclinical and clinical trials.
A: Tolerogenic dendritic cells (tolDCs) are alternatively generated from peripheral blood monocytes, or bone marrow precursors, which are subjected in culture with sequentially stimulation processes. Immature DC differentiation is firstly generated with growth factors, which in turn, owing to their plasticity, are subjected to tolerogenic stimulation with immunomodulatory agents to obtain tolDCs. Besides, some protocols perform the manufacturing with additional maturation stimuli, such as lipopolysaccharide or tumor necrosis factor-α previous to or after the tolerogenic stimulus to obtain stable tolDCs; B: According to their regulatory mechanism, tolDCs may induce an increased frequency of interleukin (IL)-10-expressing T cells and expand the antigen-specific regulatory T cell population, which show optimal suppressive activity; further, tolDCs reduce the activation and proliferation of autoreactive naïve and memory CD4+ and CD8+ T cells, otherwise becoming anergic. Additionally, the regulatory roles of tolDCs also reach B cells, since a high level of regulatory B cells expressing IL-10 are expanded by tolDCs, which are associated to a protective role in type 1 diabetes, being the only described immunoregulatory mechanism obtained from a clinical trial. BMDC: Bone marrow-derived dendritic cell; GM-CSF: Granulocyte-macrophage colony-stimulating factor; IFN: Interferon; PGE2: Prostaglandin E2; PB: Peripheral blood; IL: Interleukin; tolDC: Tolerogenic dendritic cell; TGF: Transforming growth factor; LPS: Lipopolysaccharide.
- Citation: Ríos-Ríos WJ, Sosa-Luis SA, Torres-Aguilar H. Current advances in using tolerogenic dendritic cells as a therapeutic alternative in the treatment of type 1 diabetes. World J Diabetes 2021; 12(5): 603-615
- URL: https://www.wjgnet.com/1948-9358/full/v12/i5/603.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i5.603