Published online Apr 15, 2021. doi: 10.4239/wjd.v12.i4.383
Peer-review started: January 25, 2021
First decision: February 25, 2021
Revised: February 27, 2021
Accepted: March 13, 2021
Article in press: March 13, 2021
Published online: April 15, 2021
Diabetes mellitus (DM) significantly increases the risk of heart disease, and DM-related healthcare expenditure is predominantly for the management of cardiovascular complications. Diabetic heart disease is a conglomeration of coronary artery disease (CAD), cardiac autonomic neuropathy (CAN), and diabetic cardiomyopathy (DCM). The Framingham study clearly showed a 2 to 4-fold excess risk of CAD in patients with DM. Pathogenic mechanisms, clinical presentation, and management options for DM-associated CAD are somewhat different from CAD among nondiabetics. Higher prevalence at a lower age and more aggressive disease in DM-associated CAD make diabetic individuals more vulnerable to premature death. Although common among diabetic individuals, CAN and DCM are often under-recognised and undiagnosed cardiac complica
Core Tip: Cardiovascular disease is the major cause of morbidity and mortality among patients with diabetes mellitus (DM). Three distinct and common clinical entities viz., coronary artery disease (CAD), cardiac autonomic neuropathy (CAN) and diabetic cardiomyopathy (DCM) collectively form diabetic heart disease. The pathophy
- Citation: Rajbhandari J, Fernandez CJ, Agarwal M, Yeap BXY, Pappachan JM. Diabetic heart disease: A clinical update. World J Diabetes 2021; 12(4): 383-406
- URL: https://www.wjgnet.com/1948-9358/full/v12/i4/383.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i4.383
The current prevalence of diabetes mellitus (DM) is 463 million, which is equivalent to 9.3% of the world population. The global pandemic of diabetes is expected to raise this figure to 578 million (10.2%) by the year 2030 and 700 million (10.9%) by 2045[1]. This pandemic is associated with serious economic implications. Nearly 10% of the global health expenditure is spent on diabetes care, which is equal to United States $760 billion in 2019, and this is expected to reach United States $845 billion by the year 2045[2]. DM is the eighth leading cause of death and the third leading cause of years lost with disability[3]. Diabetes greatly increases the risk of cardiovascular diseases including ischaemic heart disease, stroke, and heart failure (HF). These cardiovascular complications contribute to a majority of diabetes-associated morbidity and mortality. Moreover, the healthcare cost related to diabetes management is predominantly contributed to by the expenditure for treatment of these complications. Therefore, its prevention is of paramount importance to reduce morbidity, mortality and healthcare costs[2].
Cardiac disease that develops as a direct consequence of DM in patients with type 1 DM (T1DM) or type 2 DM (T2DM) is known as diabetic heart disease. Diabetic heart disease is a conglomeration of coronary artery disease (CAD), cardiac autonomic neuropathy (CAN), and diabetic cardiomyopathy (DCM), and these diseases are characterized by molecular, structural, and functional changes in the myocardium[4]. CAD is the often-recognised cardiac complication of diabetes. On the contrary, CAN and DCM although common, are often undiagnosed complications with devastating consequences, including increased mortality. This review aims to critically appraise the available literature related to cardiac complications of DM, to empower clinicians to manage DM more judiciously and scientifically.
T1DM is associated with a twofold rise in all-cause mortality and a threefold rise in cardiovascular mortality, compared to the general population, even with optimal glycaemic control with glycosylated haemoglobin (HbA1c) of ≤ 6.9%/52 mmol/mol. On the contrary, T1DM patients with poor glycaemic control (HbA1c > 9.7%/83 mmol/mol) have an eightfold higher risk of all-cause mortality and a tenfold higher risk of cardiovascular mortality[5]. Among patients with T1DM, acute metabolic complications including hypoglycaemia and diabetic ketoacidosis are the leading causes of all-cause mortality in subjects under 30 years, whereas cardiovascular diseases (CVD) are the predominant causes of all-cause mortality in those over 30 years of age[6]. Overall, CVD contributes to nearly 44% of the all-cause mortality in T1DM subjects at any age[7].
In a recent observational study among adults with T1DM, the level of glycaemic control, hypertension, dyslipidaemia, and diabetic nephropathy were significantly associated with the risk of CVD in addition to body mass index, increasing age, and duration of diabetes[8]. T1DM subjects without nephropathy have a minimal increase in all-cause mortality, indicating that nephropathy is the main driver for CVD and all-cause mortality[9]. Apart from nephropathy, other microvascular complications of DM such as retinopathy and peripheral polyneuropathy are associated with adverse cardiovascular outcomes and all-cause mortality in T1DM[10].
In subjects with T2DM, nearly 52% of all-cause mortality is contributed by CVD[7]. In general, T2DM is associated with a twofold rise in all-cause mortality and a threefold rise in cardiovascular mortality. The relative risks are higher in women and subjects under 55 years of age, as compared to men and those above 55 years of age[11]. Similarly, the relative risks are also higher with poor glycaemic control and renal disease characterized by albuminuria and deterioration in estimated glomerular filtration rate. T2DM patients under 55 years of age with HbA1c of ≤ 6.9% (52 mmol/mol) have a twofold rise in all-cause mortality and cardiovascular mortality. Even with a modest rise of HbA1c to the range of 7.0%-7.8% (53-62 mmol/mol) there is a twofold rise in all-cause mortality and 2.5-fold rise in cardiovascular mortality; and when it is in the range of 7.9%-8.7% (63-72 mmol/mol), there is a 2.5-fold rise in all-cause mortality and a fourfold rise in cardiovascular mortality. With HbA1c in the range of 8.8%-9.6% (73-82 mmol/mol), there is a threefold rise in all-cause mortality and a fourfold rise in cardiovascular mortality; and in uncontrolled diabetes with HbA1c > 9.7% (83 mmol/mol), there is a fourfold rise in all-cause mortality and a fivefold rise in cardiovascular mortality (Swedish National Diabetes Register)[12]. This suggests adverse cardiovascular and all-cause mortality risks in relation to poorer glycaemic control in T2DM subjects.
Optimal glycaemic control and control of cardiovascular risk factors are associated with the attenuation of all-cause mortality and cardiovascular mortality in T2DM. A recent observational study showed a modest but significant reduction in mortality with improved diabetes care (only 16% excess all-cause mortality and 18% excess cardiovascular mortality compared to the figures mentioned above for those with poor control)[13]. Among patients with T2DM, men show a higher absolute risk of first-time cardiovascular complications like myocardial infarction (MI), stroke, cardiovascular mortality, and HF (major adverse cardiovascular events including HF), whereas T2DM women exhibit a higher relative risk of first-time cardiovascular complications. This could be explained by the fact that diabetes attenuates the protective effect of oestrogen on atherosclerosis. Other possibilities include differences in the cardiovascular risk burden between sexes, sex differences in accessing healthcare resources for primary prevention, poorer glycaemic control, or treatment adherence in women. However, the sex difference has no impact on recurrent cardiovascular events[14].
Diabetes mellitus was considered a ‘CAD equivalent’ when earlier studies showed that patients with diabetes without prior MI have a risk of death from CAD equal to that of patients without diabetes, but with prior MI[15]. However, subsequent studies and a meta-analysis have proven that ‘CAD equivalent’ is an overestimation, and there is a 43% lesser risk of developing CAD in subjects with diabetes without prior MI compared to those without diabetes but with prior MI[16]. A small coronary angiographic study showed that the cardiovascular complications that occur in T2DM patients depend on angiographic status rather than diabetes status, meaning that in the absence of obstructive CAD on angiography, there is little difference in the incidence of cardiovascular events among patients with or without diabetes[17].
A population-based study from Denmark stratified 93866 patients who underwent coronary angiography based on the presence or absence of diabetes and obstructive CAD. It was observed that among patients without significant CAD, those with or without diabetes have equivalent all-cause mortality, cardiovascular mortality, and MI[18]. The study also observed that among patients without significant CAD, those with diabetes were more often on prophylactic therapy with aspirin, statin, and antihypertensive agents as compared to those without diabetes. Thus, for patients with diabetes, prophylactic therapy could reduce the risk for MI and mortality equivalent to that of a person without diabetes.
The Framingham study observed that diabetes is associated with a 2-4 times greater risk for MI and 4-6 times greater risk for HF[19]. Cardiovascular complications including CAD and stroke are the causes of death in nearly 75% of patients with T2DM in developing countries[20]. The INTERHEART study supported the association between diabetes and MI on a global platform. With the implementation of appropriate primary prevention strategies, the risk for first-time cardiovascular complications has come down significantly. Similarly, with effective revascularisation techniques and secondary prevention strategies, the risk for recurrent cardiovascular events has significantly reduced[21].
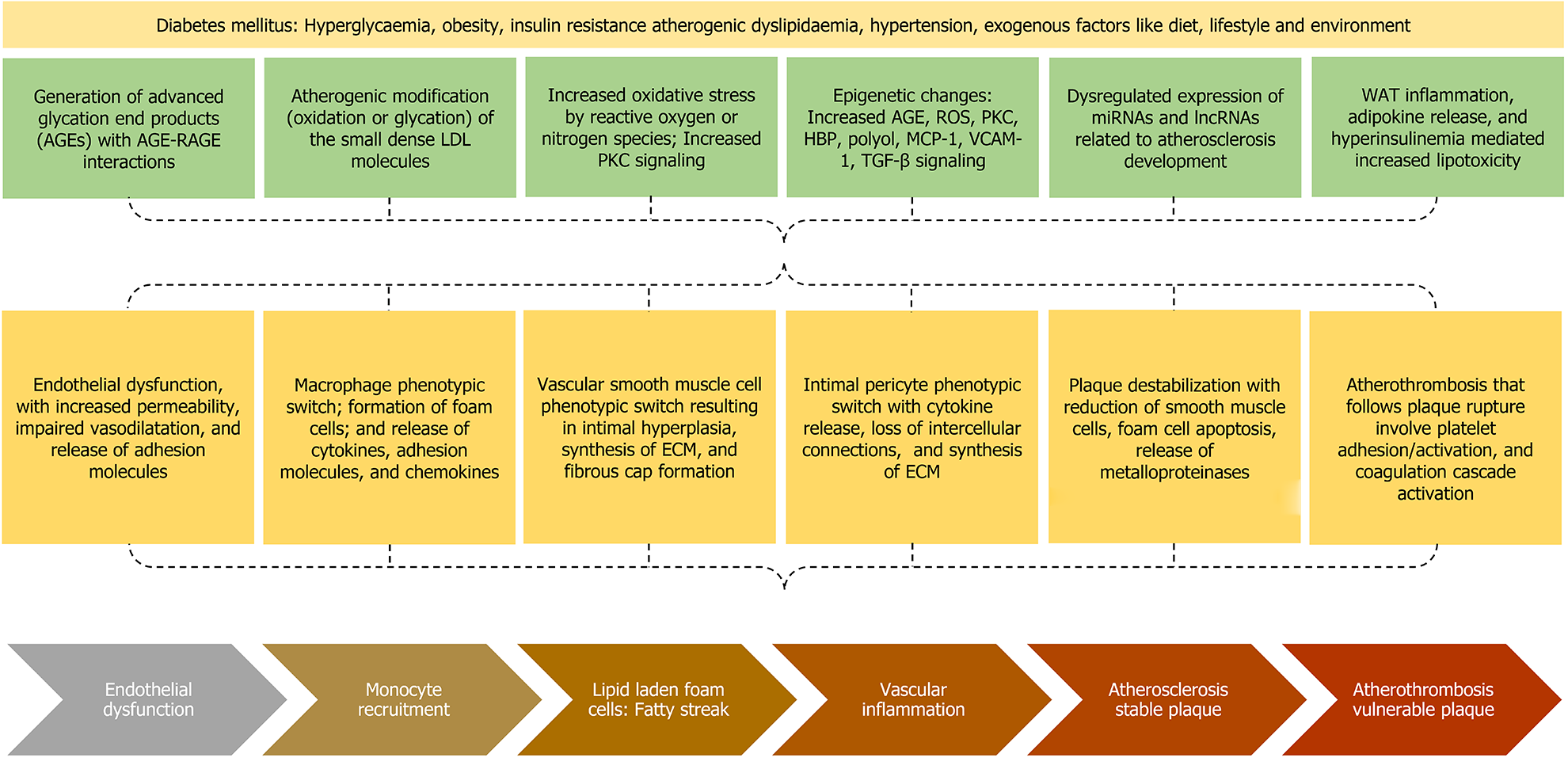
The phenomenon of persistent hyperglycaemia associated with increased cardiovascular disease is known as “metabolic memory” or “legacy effect”. There are several extremely complex mechanisms involved in mediating this phenomenon (Figure 1). Advanced glycation end products (AGEs) are generated by nonenzymatic glycation of proteins, lipids, or lipoproteins. The triggers for AGEs generation are hyperglycaemia, hypoxia, ischaemia, or reperfusion[22]. AGE-Receptors for AGE (RAGE) interaction exerts pro-inflammatory effects, generates reactive oxygen species (ROS), expresses adhesion molecules in the endothelium including vascular cell adhesion molecule 1 (VCAM-1) and intercellular cell adhesion molecule 1 (ICAM-1), promotes entry of monocytes into the subendothelium, decreases vasodilation by decreasing nitric oxide (NO), enhances vasoconstriction by increasing endothelin-1, enhances macrophage phagocytosis by expressing the scavenger receptors (SR) on the surface of macrophages including cluster of differentiation-36 (CD36) and SR class A1[23,24].
Another mechanism by which diabetes increases the cardiovascular risk is the atherogenic modification of low-density lipoprotein (LDL), in which the LDL molecule is first desialylated to form small dense LDL. This is followed by oxidation or glycation of small dense LDL, which favours the interaction with subendothelial proteoglycan, enhancing the retention time of LDL, the LDL phagocytosis by the macrophages to form the lipid-laden foam cells, and the release of proinflammatory cytokines including tumour necrosis factor-alpha (TNF-α), interleukin 1-beta (IL1-β), IL-6, and matrix metalloproteinase (MMP) by the foam cells[23]. This in turn augments the atherogenic potential of LDL in subjects with DM, even at the normal levels for a nondiabetic individual.
Diabetes mellitus is associated with increased oxidative stress due to either increased production of ROS/reactive nitrogen species (RNS) or decreased clearance of ROS/RNS. Various triggers for ROS/RNS are direct effects from hyperglycaemia (excessive activation of mitochondrial electron transport chain), or indirect effects from AGEs, cytokines, upregulation of polyol pathway, upregulation of hexosamine biosynthetic pathway [O-linked β-N-acetylglucosamine (O-GlcNAc)], enhanced protein kinase C (PKC) signalling, oxidised small dense LDL, hyperinsulinaemia (decreased phosphatidylinositol-(3,4,5)-trisphosphate/protein kinase B pathway and increased mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase pathway), and platelet-activating factor (PAF)[25-27]. The enhanced oxidative stress is associated with endothelial dysfunction, which is characterised by increased vascular permeability and impaired vasodilation, the latter mediated by decreased NO, increased endothelin 1, and increased angiotensin II levels. Moreover, there is increased risk for leukocyte/platelet adhesion, thrombosis, and inflammation due to release of adhesion molecules including ICAM-1 and VCAM-1. The hyperleptinaemia and hypoadiponectinaemia are also associated with endothelial dysfunction and transmigration of LDL particles[28].
The enhanced PKC signalling results either from oxidative stress or the direct effect of hyperglycaemia. The enhanced PKC signalling results in increased cytokine production, increased extracellular matrix (ECM) production, and endothelial dysfunction characterised by decreased NO production, impaired vasodilation, and increased permeability[23]. Hyperglycaemia can result in heritable modifications in gene expression without changing the DNA sequences[29]. These epigenetic modifica
The macrophage phenotypic switch from anti-inflammatory M2 to pro-inflammatory M1 phenotype results in the release of various cytokines (TNF-α, IL1-β, IL-6, and MMP), chemokines (MCP-1), and ICAM-1 release, which are mediated by increased NF-kB and activator protein-1 (AP-1) signalling. Various triggers for the macrophage switch are AGEs, polyols, O-GlcNAc, PKC, lipotoxicity, oxidative stress, mitochondrial dysfunction, and epigenetic modifications[30]. The VSMC phenotypic switch is associated with proliferation, intimal migration, and dedifferentiation into several phenotypes including synthetic, calcific, adipogenic, and macrophagic phenotypes. These changes result in intimal hyperplasia, synthesis of ECM, development of fibrous cap, and deposits of microcalcification within the intimal wall, all of which are thought to be mediated via TGF-β signalling[30,31].
The intimal pericyte phenotypic switch results in accumulation of lipid in pericytes through phagocytosis, secretion of pro-inflammatory cytokines, loss of intercellular connections, and increased production of ECM components[32]. Plaque destabilization, a process converting a stable plaque into a thrombosis-prone plaque, is characterised by foam cell apoptosis producing a large necrotic core, reduction of VSMCs in the fibrous cap, and release of metalloproteinase enzymes that thin down the fibrous cap resulting in the development of a vulnerable plaque[33]. Plaque rupture is followed by the development of atherothrombosis which involves platelet adhesion, platelet activation, and activation of coagulation cascade by tissue factor, plasminogen activator inhibitor-1 (PAI-1), and fibrinogen that is favoured in the presence of lowered antithrombin activity[34].
CAD tends to be a more complex disease in patients with DM. It is characterized by diffuse, calcified, rapidly progressing disease with multivessel involvement, that often requires coronary revascularization in addition to optimal medical therapy[20]. Moreover, both culprit and non-culprit lesions in patients with DM exhibit more vulnerable features (lipid-rich core, macrophage accumulation, and thin fibrous cap), indicating pan-vascular plaque instability[35]. Diabetic patients constitute one quarter of all cases undergoing coronary revascularisation procedures such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Diabetic patients with multivessel disease respond superiorly to CABG compared to PCI. However, CABG is not feasible in many diabetic patients due to the presence of complex atherosclerotic lesions and associated comorbidities. With technological advancement and newer devices like drug-eluting stents, PCI can be used in complex lesions such as those found in diabetic patients. However, the revascularisation outcomes are still worse in patients with diabetes compared to those without[36]. Among patients with CAD undergoing revascularisation with drug eluting stents, the presence of diabetes is associated with nearly double the rate of MI, definite stent thrombosis and cardiovascular mortality[37]. Moreover, patients with diabetes have 86% higher rates of in-stent restenosis[38]. Among patients admitted with acute coronary syndrome treated by CABG, the presence of diabetes increased the long-term mortality by 1.6-fold, though the 30 d mortality remained equal between those with or without diabetes[39].
In patients with CAD, presence of diabetes is associated with a twelvefold higher risk of future adverse cardiac events including mortality or non-fatal MI compared to its absence[40]. In coronary angiographic studies, the angiographical significance exhibits a poor correlation with haemodynamic (ischaemic) significance. Therefore, fractional flow reserve (FFR) is used as the gold-standard invasive technique to diagnose the coronary artery stenoses of sufficient hemodynamic severity to induce myocardial ischaemia. Myocardial perfusion imaging (MPI) with single-photon emission computed tomography is a non-invasive method to detect coronary artery stenoses of sufficient hemodynamic severity to induce myocardial ischaemia[41]. The invasive FFR or noninvasive MPI studies exhibiting an absence of ischaemia do not provide the same degree of confidence in patients with diabetes compared to patients without diabetes. Therefore, deferring revascularization based upon the absence of ischaemia (known as ischaemia-driven revascularisation strategy) does not appear to be safe in patients with diabetes as compared to those without.
There are various possible explanations for this phenomenon, including a high prevalence of microvascular dysfunction, rapid atherosclerosis progression, high atherosclerotic disease burden, and high-risk plaque composition (bigger necrotic core and larger calcium content) among the diabetic population[41]. In patients with DM, various risk factors related to atherosclerotic plaque progression include hypertension, male sex, mean plaque burden > 75% at baseline, and glycaemic variability[42]. The atherosclerotic burden can be measured invasively with the gold standard intravascular ultrasound or noninvasively with the coronary computed tomography angiography and coronary artery calcium (CAC) score. Patients with DM and metabolic syndrome have high CAC score, and in these subjects, the CAC score is a strong predictor of cardiovascular disease and mortality[43,44].
Though most patients with CAD present with angina, it may be absent in 20%-30% of patients with diabetes[45]. Baseline investigation with electrocardiogram (ECG) is of little value, but stress tests using either treadmill or dobutamine stress echocardiogram should be considered for diagnostic workup in suitable patients[46]. Similarly, myocardial perfusion scans can be used which have 88% sensitivity and 84% overall specificity[47]. In T2DM patients, numerous biomarkers could allude to an increased risk of CAD. These markers can be classified into established biomarkers related to lipid regulation (LDL, high density lipoprotein, and very low density lipoproteins) and novel biomarkers including biomarkers related to inflammation (fibrinogen or high-sensitivity C-reactive protein), lipid-associated biomarkers [Lipoprotein associated PA2 or Lipoprotein (a)] and organ-specific biomarkers (hs-troponin, Cystatin C or NTproBNP)[48]. However, no single biomarker has been found to be completely reliable. Hence, using multiple biomarkers is the way forward in reaching a timely diagnosis of CAD[49,50].
Small dense LDL particles are more atherogenic due to their specific characteristics, which include lower binding affinity to LDL receptors, higher penetration into the subendothelial layer, longer half-life, and lower resistance to oxidative stress[49]. Glycated apolipoprotein B can be used as a surrogate marker of subclinical atherosclerosis[51]. Higher lipoprotein (a) levels are associated with poor CVD outcomes in patients with DM and is a predictor of recurrent CVD events[52]. The Triglyceride Glucose index (TyG) calculated using fasting glucose and triglyceride levels can be used as a surrogate marker of insulin resistance and subclinical atherosclerosis, to predict cardiovascular events and to detect asymptomatic coronary artery stenosis in patients with T2DM[53]. Moreover, TyG can be used as a marker to predict the progression of coronary atherosclerosis[54].
In 2007, the New England Journal of Medicine published a meta-analysis that demonstrated an increased risk of MI and other cardiovascular events with the use of antidiabetic drug rosiglitazone. Thereafter, the United States Food and Drug Administration (FDA) made it mandatory to undertake cardiovascular outcome studies for all antidiabetic medications before receiving final approval. Cardiovascular outcome trials of various antidiabetic drugs currently available are given in Table 1. The cardiovascular outcome trials have uncovered unexpected benefits of cardiovascular protection with some of the new classes of agents, such as the glucagonlike peptide-1 receptor agonists (GLP-1 RAs) and the sodium-glucose cotransporter-2 (SGLT-2) inhibitors[55].
| Drug | Trial name | Primary outcome | HR (95%CI) |
| Metformina | UKPDS legacy data[131] | MI | 0.67 (0.51-0.89) |
| Gliclazide MR | ADVANCE[132] | CV death, MI, stroke | 0.94 (0.84-1.06) |
| Pioglitazonea | Meta-analysis[133] | CV death, MI, stroke | 0.83 (0.72-0.97) |
| Rosiglitazone | RECORD[134] | CV event or CV death | 0.99 (0.85-1.16) |
| Acarbose (Chinese population) | ACE[135] | CV death, MI, stroke, UA, HF hospitalisation | 0.98 (0.86-1.11) |
| Nateglinide | NAVIGATOR[136] | CV death, MI, stroke, HF hospitalisation | 0.94 (0.82-1.09) |
| Saxagliptin | SAVOR-TIMI[137] | CV death, MI, ischaemic stroke | 1.01 (0.88-1.15) |
| Alogliptin | EXAMINE[138] | CV death, MI, stroke, UA, and HF | 0.98 (0.86-1.12) |
| Sitagliptin | TECOS[139] | CV death, MI, stroke, UA hospitalisation | 0.98 (0.89-1.09) |
| Linagliptin vs glimepiride | CAROLINA[140] | CV death, MI, stroke | 0.98 (0.84-1.14) |
| Empagliflozina | EMPA-REG[141] | CV death, MI, stroke | 0.86 (0.74-0.99) |
| Canagliflozina | CANVAS[142] | CV death, MI, stroke | 0.86 (0.75-0.97) |
| Dapagliflozin | DECLARE[143] | CV death, MI, ischaemic stroke | 0.93 (0.84-1.03) |
| Ertugliflozin | VERTIS-CV[144] | CV death, MI, stroke | 0.97 (0.85-1.11) |
| Lixisenatide | ELIXA[145] | CV death, MI, stroke, UA | 1.02 (0.89-1.17) |
| Liraglutidea | LEADER[146] | CV death, MI, stroke | 0.87 (0.78-0.97) |
| Semaglutidea | SUSTAIN-6[147] | CV death, MI, stroke | 0.74 (0.58-0.95) |
| Exenatide | EXSCEL[148] | CV death, MI, stroke | 0.91 (0.83-1.0) |
| Dulaglutidea | REWIND[149] | CV death, MI, stroke | 0.88 (0.79-0.99) |
| Albiglutidea | HARMONY[150] | CV death, MI, stroke | 0.78 (0.68-0.90) |
Statin therapy is associated with reduced risk of development and progression of CAD, with a consequent reduction of cardiovascular events and mortality. In a meta-analysis of 18686 subjects, during a mean follow-up of 4.3 years, the all-cause mortality was reduced by 9% in patients with diabetes (relative risk or RR 0.91, 95%CI: 0.82-1.01; P = 0.02), whereas the same was reduced by 13% in those without diabetes (0.87, 95%CI: 0.82-0.92; P < 0.0001), for each one mmol/L reduction in LDL cholesterol[56]. The major cardiovascular adverse events were lowered by 21% in those with diabetes (0.79, 95%CI: 0.72-0.86; P < 0.0001) and without diabetes (0.79, 95%CI: 0.76-0.82; P < 0.0001). Moreover, there was a reduction in MI or coronary death (0.78, 95%CI: 0.69-0.87; P < 0.0001), coronary revascularisation (0.75, 95%CI: 0.64-0.88; P < 0.0001), and stroke (0.79, 95%CI: 0.67-0.93; P = 0.0002) in patients with diabetes[56].
Weight loss interventions (bariatric surgery), proprotein convertase subtilisin-kexin type 9 inhibitors, novel molecules that could block the actions of RAGE signalling, RNA therapeutics that target miRNAs and long noncoding RNAs, and drugs that target distinct components of the immune or inflammatory response hold promise as new modalities of treatment for prevention of cardiovascular disease in patients with DM[55].
Diabetic autonomic neuropathy can be classified into CAN, sudomotor neuropathy, gastrointestinal autonomic neuropathy, and urogenital autonomic neuropathy[57]. CAN is associated with impairment of autonomic control of the cardiovascular system and is a major cause of silent cardiovascular events in patients without overt cardiac disease[58]. Though the prevalence of CAN is highly variable, it is estimated to affect at least 20% of unselected patients and up to 65% of those with increasing diabetes duration and age[59]. It is increasingly observed in patients with prediabetes and metabolic syndrome, with a reported prevalence of up to 11% and 24%, respec
The autonomic nervous system involvement in CAN occurs in an ascending length-dependent manner. Therefore, the vagus nerve, which is the longest parasympathetic nerve, is involved early resulting in parasympathetic denervation and sympathetic predominance. Patients at this early stage will be asymptomatic (sub-clinical stage) and the diagnosis can only be made based on heart rate variability (HRV), baroreflex sensitivity tests or increased left ventricular torsion on cardiac imaging[61]. Towards the end stage of disease, sympathetic denervation results in unresponsiveness of heart rate and blood pressure to sleep, exercise, stress, or chemical stimulation such as adenosine. At this stage, patients become symptomatic with light-headedness, weakness, palpitations, fainting and syncope on standing. Other features that can be associated with CAN include reduced exercise tolerance, silent myocardial ischaemia, intraoperative complications (hypotension, bradycardia, and the need for vasopressor support), and foot complications including foot ulcers due to associated sudomotor dysfunction, Charcot neuroarthropathy and lower limb amputations[62]. Poor response of heart rate and blood pressure during exercise in turn fail to increase cardiac output accordingly, leading to poor exercise tolerance.
The signs associated with CAN include tachycardia, orthostatic hypotension, reverse dipping, and non-dipping on ambulatory blood pressure monitoring[59]. Orthostatic hypotension indicates an advanced stage of CAN and suggests a poor prognosis with higher mortality in diabetic patients with CAN[63]. Orthostatic hypotension is mainly attributable to the damage to the efferent sympathetic vasomotor fibres, particularly in the splanchnic vasculature. Other contributing factors are reduced cardiac output, postprandial blood pooling, insulin-induced hypotension and volume depletion due to diuretics[64]. Patients can sometimes present with postural orthostatic tachycardia syndrome, which is characterised by the presence of orthostatic symptoms occurring on standing, an increase in heart rate of ≥ 30 beats/minute when moving from a recumbent to a standing position that lasts more than 30 seconds and the absence of orthostatic hypotension (more than 20 mmHg drop in systolic blood pressure)[65].
Reverse dipping (reversal of normal physiological drop in blood pressure at night) and non-dipping are commonly found in diabetic patients with CAN, due to sympathetic predominance during the night, leading to the development of nocturnal hypertension and left ventricular hypertrophy[66]. Patients with CAN are susceptible to silent myocardial ischaemia and/or infarction. There is a prolongation of subjective anginal threshold, and there is a delayed and diminished appreciation of ischaemic pain, associated with early ECG changes prior to the onset of ischaemic symptoms[62]. Asymptomatic ischaemia can induce lethal arrhythmias, which is especially important in patients with CAN who have prolonged QT interval[67].
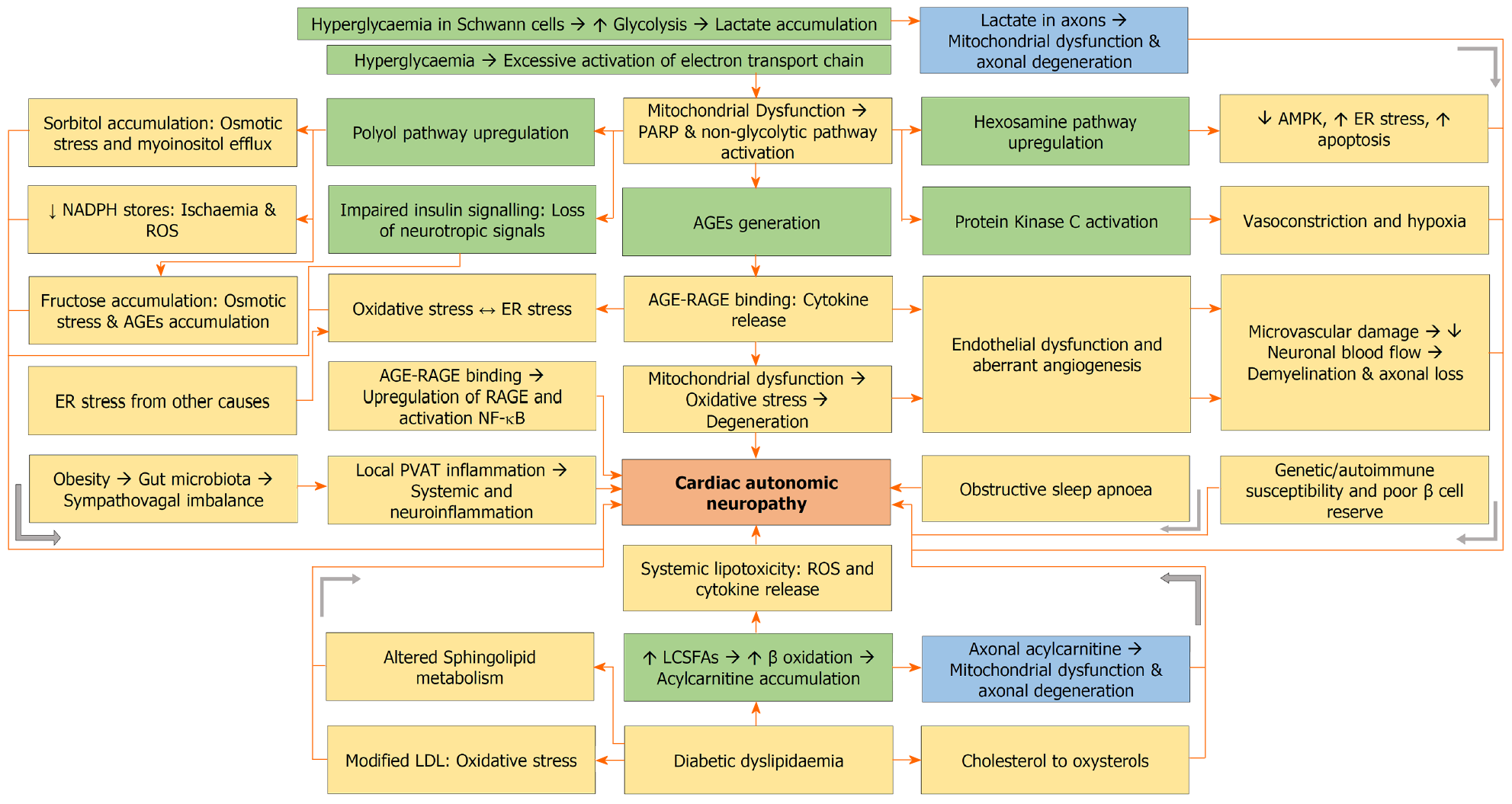
Pathophysiology of CAN is the same as any other form of diabetic neuropathy and is contributed by hyperglycaemia and dyslipidaemia (Figure 2). Hyperglycaemia is the main driver of diabetic neuropathy in T1DM, whereas dyslipidaemia is the main driver in T2DM patients[68]. Diabetes is associated with high substrate load of glucose and free fatty acids. In the presence of hyperglycaemia, glucose enters the Schwann cells through glucose transporter 3 (GLUT3). Excess glucose undergoes glycolysis, and pyruvate exceeds the capacity of tricarboxylic acid (TCA) cycle, resulting in a shift to anaerobic metabolism and accumulation of lactate. Lactate is shuttled from Schwann cells into axons, resulting in mitochondrial dysfunction and axonal degeneration[69].
Moreover, hyperglycaemia results in excessive activation of the electron transport chain, leading to mitochondrial dysfunction, ROS generation, oxidative stress, DNA damage, and activation of poly adenosine diphosphate ribose polymerase. The latter, in turn, inhibits glyceraldehyde-3-phosphate dehydrogenase resulting in accumulation of glycolytic metabolites, with upregulation of polyol, hexosamine, and diacylglycerol (DAG) and PKC pathways, as well as generation of AGEs[70-76]. The AGE-RAGE interactions, oxidative stress, endoplasmic reticulum (ER) stress and upregulated non-glycolytic pathways result in endothelial dysfunction characterized by impaired vasodilation mediated by decreased NO bioavailability, increased endothelin-1, increased PAI-1 and aberrant angiogenesis. Angiogenesis is increased in diabetic nephropathy and retinopathy, whereas it is decreased in diabetic neuropathy[70]. Microvascular damage decreases neuronal blood flow resulting in demyelination, axonal loss, decreased myelinated fibre density, and reduced nerve conduction velocity[76].
Common mechanisms for the excessive ROS generation in patients with hyperglycaemia include excessive activation of the electron transport chain, AGE-RAGE interaction, pro-inflammatory cytokines, upregulated non-glycolytic pathways, and high protein folding load. Crosstalk exists between oxidative stress and ER stress[70]. AGE accumulation and hexosamine/polyol pathway upregulation are associated with a rise in misfolded or unfolded proteins and, therefore, ER stress. Oxidative stress increases the misfolding of proteins with worsening ER stress. Similarly, misfolded proteins result in adenosine triphosphate depletion, thereby increasing oxidative stress[70,75].
A high substrate load of long chain saturated fatty acids including palmitate and stearate is associated with increased β oxidation to form acetyl CoA. When the capacity of the TCA cycle is exceeded, toxic acylcarnitine accumulates inside Schwann cells, which is then shuttled into axons, resulting in mitochondrial dysfunction and axonal degeneration[68]. In addition, oxidation of cholesterol into oxysterols in neuronal cells results in neuronal injury and apoptosis[77,78]. Furthermore, altered sphingolipid metabolism with the generation of neurotoxic deoxysphingolipids can be another mechanism for nerve damage in T2DM patients with diabetic neuropathy[79,80].
Lifestyle factors like high-calorie diets are associated with alterations in gut microbiota and the emergence of metabolic endotoxemia. The metabolic endotoxaemia activates the Toll-like receptors 4 to create a sympatho-vagal imbalance characterised by an increased proinflammatory sympathetic outflow and a decreased anti-inflammatory parasympathetic outflow, culminating in perivascular adipose tissue inflammation, systemic inflammation, neuronal inflammation and CAN[58]. Obstructive sleep apnoea is associated with chronic intermittent hypoxia, increased oxidative stress, and microvascular dysfunction[59]. Other factors implicated in the pathogenesis of CAN include genetic and epigenetic changes, autoimmune autonomic ganglionopathy, and low C-peptide levels indicating a poor pancreatic β cell reserve[62].
The CAN Subcommittee of Toronto Consensus Panel on Diabetic Neuropathy recommends that T2DM patients should be screened for CAN at the time of diagnosis, and T1DM patients should be screened for CAN within 5 years of their diagnosis. The committee also recommends that the screening should be done as a part of perioperative risk assessment in patients with CAD[81]. American Diabetes Association (ADA) recommends screening for CAN in patients with microvascular complications, neuropathic complications, and hypoglycaemia unawareness[82]. CAN should be suspected in diabetic and prediabetic patients when they present with resting tachycardia, postural tachycardia, reduced HRV, bradycardia, impaired exercise tolerance, orthostatic hypotension, supine hypertension, or intra-operative or post-operative cardiovascular instability[57].
The gold standard for diagnosis of CAN is Cardiovascular Autonomic Reflex Testing (CARTs), which consists of tests of parasympathetic function, including HRV to deep breathing, heart rate response to standing, and heart rate response to Valsalva manoeuvre; tests of sympathetic adrenergic function including beat-to-beat blood pressure response to Valsalva manoeuvre, and the systolic and diastolic blood pressure changes in response to tilt table or active standing; and tests of sympathetic cholinergic function including quantitative sudomotor axon reflex test, thermoregu
Even from the subclinical stage, CAN is independently associated with an increased risk for cardiac arrhythmias, silent myocardial ischaemia, major adverse cardiovascular events, myocardial dysfunction, sudden cardiac death (from either silent myocardial ischaemia or QT interval prolongation), all-cause mortality, and cardiovascular mortality[60]. In patients with diabetes, the 5-year mortality from the time of diagnosis of CAN is 16%-50%[83]. The risk for cardiovascular disease is increased proportionately to the stage of CAN, the faster the progression, the greater the cardiovascular disease risk[84].
Patients with CAN are at high risk of developing HF with preserved ejection fraction (HFpEF), an entity that has a significantly high mortality risk. Subclinical CAN with parasympathetic denervation and sympathetic predominance results in high myocardial catecholamine levels, high myocardial oxygen demand, left ventricular hypertrophy, left ventricular remodelling, myocardial apoptosis and fibrosis, all of which manifest as HFpEF[85]. Moreover, CAN may contribute to the progression of atherosclerosis through many mechanisms including a rise in heart rate and blood pressure, trophic changes in the arterial wall, arterial stiffness, and a pro-inflammatory state associated with autonomic neuropathy[86].
As it is associated with adverse cardiovascular consequences, interventions to prevent or reverse CAN should be implemented, as parasympathetic denervation may be reversible if diagnosed soon after onset[87]. ADA suggests optimising glycaemic control in T1DM patients, multifactorial interventions targeting glycaemia and other cardiovascular risk factors in T2DM patients, and lifestyle modifications in patients with prediabetes with or without metabolic syndrome for the prevention of CAN[82]. Though some drugs developed on the basis of pathophysiological mechanisms have shown promise in the preclinical trials, but all failed in obtaining approval. Therefore, there are currently no FDA-approved disease-modifying drugs for the management of CAN. Symptomatic patients with orthostatic hypotension can be treated with either fludrocortisone or midodrine, and those with postprandial hypotension can be treated with octreotide, using the same principles in the management of neurogenic orthostatic hypotension[88].
Elderly subjects with diabetes have a high incidence of HF compared to those without diabetes (39% vs 23%), and those without HF at baseline have a relative risk of 1.3 for developing HF after 43 months of observation[89]. Diabetes increases the prevalence of HF by threefold in patients less than 75 years of age and by twofold in patients more than 75 years of age[90]. The prevalence of HF in subjects with diabetes is 12%-57%, whereas the prevalence of diabetes in subjects with HF is 4.3%-28%[90]. Moreover, HF including HFpEF and HF with reduced ejection fraction (HFrEF) often coexist with T2DM in nearly 30%-40% of cases, with the HFpEF being commoner than HFrEF[90,91]. Both T1DM and T2DM are associated with increased risk for HF in women compared to men, with T1DM associated with 47% greater risk and T2DM associate with 9% greater risk[92]. Hyperglycaemia in patients with DM promotes the development of HF. Each 1% increment in HbA1c is associated with a 30% increase in HF in patients with T1DM and 8% increase in HF in patients with T2DM[93,94]. HF in subjects with diabetes is associated with increased rates of hospitalisation, cardiovascular mortality, and all-cause mortality, with the greatest risk for patients with HFpEF, compared to patients with HFrEF[95,96].
HF in patients with DM could arise from a combination of DCM, ischaemic cardiomyopathy, CAN, and hypertensive cardiomyopathy. The pathogenic mechanisms that are known to cause DCM and CAN, namely, hyperglycaemia, insulin resistance, and hyperinsulinaemia are also risk factors for the development of ischaemic heart disease and hypertension among patients with DM[25].
DCM is defined as a condition of cardiac dysfunction encompassing myocardial metabolic, structural, and functional changes in the absence of other cardiac risk factors, such as CAD, hypertensive heart disease, and significant valvular disease, in individuals with T1DM or T2DM[97,98]. In the early stages, DCM remains asymptomatic and the only sign is an increase in left ventricular mass (hypertrophy), which is independent of hypertension and body weight, and results from dysfunctional cardiac remodelling. In the next stage, myocardial fibrosis develops, leading to diastolic dysfunction, which is the most common manifestation of DCM. The diastolic dysfunction can be asymptomatic during the early stages or can present with HFpEF. A small number of people with DCM progress to develop overt systolic dysfunction presenting as HFrEF[99].
Left ventricular diastolic dysfunction develops early during diabetes and is detected in up to 75% of T2DM patients[90]. A recent study estimated the prevalence of left ventricular diastolic dysfunction among diabetic patients in the hospital population and the general population and observed a prevalence of 48% in the former and 35% in the latter group[100]. The same team estimated the prevalence of left ventricular systolic dysfunction in the hospital population and general population separately as 18% and 2%, respectively[101]. The degree of glucose dysregulation was proportional to the severity of diastolic dysfunction, risk of incident HF and cardiovascular mortality in patients with T2DM. Nearly 50% of HF patients with T2DM have HFpEF, especially the older, female, hypertensive patients. Accurate diagnosis of HFpEF is often difficult as symptoms are often mild, and hence misdiagnosis is common. Though CAD is the major cause of HFrEF in patients with T2DM, other causes like DCM should be considered in the differential diagnosis[90].
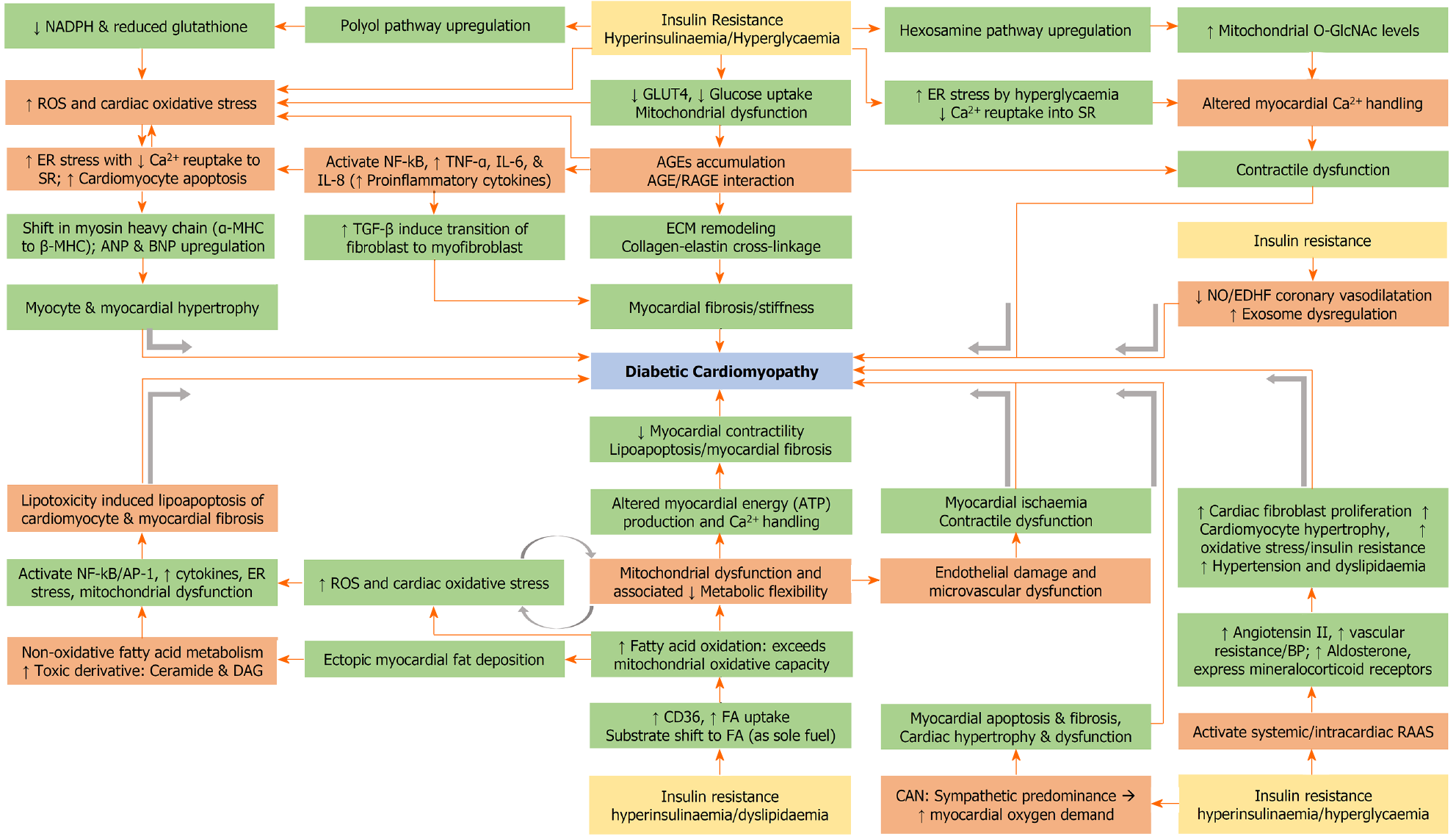
Under normal conditions, the cardiac energy demand is met by fatty acid oxidation, with a small contribution from glucose. However, under stressful situations, the cardiomyocytes rely on increased contributions from glucose[102]. The ability to use a variety of fuels to generate energy is known as metabolic substrate flexibility[99]. In adult cardiomyocytes, glucose enters the cell via GLUT4, whereas fatty acid enters via fatty acid translocase (FAT or CD36)[103]. Insulin resistance is associated with decreased GLUT4-mediated glucose uptake and increased CD36-mediated fatty acid uptake into the cardiomyocytes, with sole reliance on fatty acid for fuel. The glucose metabolism that accompanies hyperinsulinaemia and chronic hyperglycaemia is associated with an increased generation of ROS and oxidative stress. The oxidative stress will divert glucose metabolism from its usual glycolytic pathway to alternative pathways including the polyol pathway, resulting in the generation of AGEs, or the hexosamine biosynthetic pathway (HBP) resulting in the generation of O-GlcNAc[104].
The ROS and AGE (following interaction with receptors for AGE or RAGE) trigger activation of NF-kB, thereby inducing inflammation mediated by TNF-α, IL-6, IL-8, and MCP-1 and myocardial fibrosis mediated by TGF-β and MMP[103,105]. The AGE-RAGE interactions result in crosslink between collagen and elastin as part of ECM remodelling, which in turn leads to increased myocardial stiffness and impaired relaxation[104]. Moreover, ROS triggers ER stress in cardiomyocytes resulting in their apoptosis, and impaired myocardial calcium handling leading to cardiac dysfunction[104]. The chronic activation of HBP with the generation of O-GlcNAc also results in impaired myocardial calcium handling[106]. Following apoptosis, the viable cardiomyocytes undergo compensatory hypertrophy, which is associated with a shift in myosin heavy chain (MHC) from α-MHC to β-MHC and upregulation of atrial natriuretic peptide and brain natriuretic peptide (BNP)[107].
In patients with insulin resistance, increased fatty acid uptake is associated with increased oxidative and non-oxidative fatty acid metabolism, with the latter resulting in the generation of toxic intermediates including ceramide and DAG[108]. Once the mitochondrial oxidative capacity is exceeded, this results in mitochondrial dysfunction, generation of ROS, oxidative stress, ER stress, generation of inflammatory cytokines, and lipotoxicity mediated apoptosis (lipoapoptosis)[104]. Hyperglycaemia is associated with inappropriate activation of the renin-angiotensin-aldosterone system (RAAS) with a rise in angiotensin II and aldosterone and increased expression of mineralocorticoid receptors, increasing the oxidative stress, cardiac fibroblast proliferation, and cardiomyocyte hypertrophy[109]. Coronary endothelial dysfunction, in the form of impaired NO and endothelium-derived hyperpolarising factor mediated vasodilation, leads to microvascular dysfunction, myocardial ischaemia and contractile dysfunction.
Exosomes are extracellular vesicles that are released by cardiovascular system-related cells, including cardiomyocytes, endothelial cells, fibroblasts, smooth muscle cells, platelets, leukocytes, monocytes, and macrophages[110]. These exosomes are mediators of intercellular communication, and contain a variety of biological components like miRNAs, proteins, and lipids. Exosomal dysregulation is a mechanism for the pathogenesis of DCM. The exosomes released by cardiomyocytes to endothelial cells inhibit proliferation, migration, and tube formation of endothelial cells, leading to microvascular dysfunction. The exosomes released by endothelial cells to cardiomyocytes cause inhibition of cardiomyocyte autophagy, and promotion of cardiomyocyte apoptosis. Moreover, the exosomes released by fibroblasts to cardiomyocytes serve as a mediator of cardiomyocyte hypertrophy[110].
The key components in the pathogenesis are myocardial fibrosis, left ventricular remodelling, and cardiac dysfunction[111]. Activation of proinflammatory cytokines, production of ROS, dysfunction of mitochondria and ER in cardiac tissue are the key operating mechanisms that contribute towards cardiac remodelling, fibrosis, and diastolic dysfunction. These are triggered due to the combined effects of enhanced deposition of AGEs and lipotoxic metabolites, activation of RAAS, altered calcium homeostasis and abnormal insulin signalling pathways through activation of the mammalian target of rapamycin (mTOR)-S6 kinase 1 pathway leading to abnormal intracellular glucose transport in cardiomyocytes[112]. Figure 3 illustrates the complex pathophysiological mechanisms involved in the development of DCM.
Currently, there are no specific clinical features, structural/functional changes, or biomarkers for the diagnosis of DCM. In the very early stages, there are only substructural changes in the cardiomyocytes, and the diagnosis is only possible through very sensitive methods including longitudinal myocardial strain alterations (a measure of tissue deformation), strain rate and myocardial tissue velocity, which are indicators of early adverse left ventricular remodelling[113]. In the middle stages of DCM, when cardiomyocyte hypertrophy and fibrosis develop with associated structural changes like left ventricular hypertrophy and increased muscle mass, non-invasive imaging technologies including echocardiography and gadolinium-enhanced cardiac magnetic resonance imaging (MRI) will be able to detect diastolic and/or systolic dysfunction. In advanced stages of DCM, there will be worsening of fibrosis and development of microvascular changes, and this stage will be often accompanied by overt HF, ischaemic heart disease and hypertension[113].
Transthoracic echocardiography can be used for evaluation of structural changes (2D echo for left ventricular mass), and functional changes (trans-mitral doppler for diastolic dysfunction and tissue doppler imaging for diastolic and systolic dysfunction) in patients with DCM[114], even though the diastolic dysfunction observed in patients with T2DM is worsened when hypertension and obesity coexist[115]. The ratio between early passive trans-mitral inflow velocity (E) and velocity at the medial mitral annulus (e’) can be used as a measure of left ventricular filling pressure[116]. Abnormal E/e’ is correlated with the development of HF and increased mortality, independent of other risk factors such as hypertension and CAD[117]. Gadolinium-enhanced cardiac MRI is much more accurate than echocardiography for the evaluation of structural changes (myocardial fibrosis, steatosis, and left ventricular mass), functional changes (for diastolic and systolic function: late gadolinium enhancement), and metabolic changes (Magnetic Resonance spectroscopy for myocardial triglyceride content and high-energy phosphate metabolism)[118]. Positron emission tomography, which could diagnose DCM at much earlier stages, can be used to assess myocardial metabolic abnormalities. However, this is still a research tool as it is costly, time-consuming, and needs expertise for accurate interpretation of the results[103].
Although diastolic dysfunction diagnosed invasively through cardiac catheterisation (left ventricular end-diastolic pressure > 16 mmHg or mean pulmonary capillary wedge pressure > 12 mmHg) is the most definitive evidence of diastolic HF, it is rarely necessary for the diagnosis of DCM, due to the availability of various highly sensitive and specific noninvasive tests[119]. However, coronary angiography has an added benefit of detecting CAD, including microvascular CAD. Various biomarkers are undergoing investigation as markers of structural changes (matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase, for myocardial fibrosis) and functional changes (mi-RNA for contractile function, procollagen 3 N-terminal peptide and troponin for LV dysfunction, and BNP for LV diastolic and systolic function)[114].
Glycaemic control alone is insufficient to prevent the development of DCM, indicating the need for targeted therapeutic strategies. Some of the newer antidiabetic drugs such as the GLP-1 RAs and the SGLT-2 inhibitors have exhibited direct protective effects on the myocardial tissue[120]. Therapeutic approaches including RNA-based therapy (a form of gene therapy), drugs targeting the mitochondrial oxidative stress, and metabolic modulator drugs including trimetazidine, ranolazine, perhexiline, alpha lipoic acid, resveratrol, luteolin, riboflavin, sodium ferulate, cyclovirobuxine, and epigallocatechin-3-gallate are currently being investigated for the prevention and treatment of DCM[121-123].
Precision medicine is a rapidly advancing field of medicine. It is based on the principle of identification of the underlying molecular pathophysiological mechanisms of disease and the design of specific therapeutic interventions against these mechanisms[124]. The messenger RNA is the coding RNA, whereas the various regulatory RNAs which are not translated into proteins are known as non-coding RNAs. The different regulatory non-coding RNAs include small interfering RNAs (siRNAs), miRNAs, lncRNAs, and circular RNAs. The siRNAs and miRNAs are 20-22 nucleotides in length, and these are involved in the post-transcriptional regulation of gene expression. The lncRNAs that are > 200 nucleotides in length are involved in the regulation of transcription, splicing and in the regulation of siRNAs and miRNAs[125].
RNA sequencing technologies have identified that various non-coding RNAs have a role in the pathogenesis of cardiovascular disease. These non-coding RNAs can be used as treatment targets. RNA therapeutics, a form of gene therapy, has received widespread application to target the RNA molecules and regulate gene expression and protein production. The non-coding RNAs that are associated with cardiovascular disease can be packaged into viral vectors including adenovirus, lentivirus, or adeno-associated virus, and can be delivered into the target cells to mediate therapeutic benefits by regulating the expression of the target gene[126]. Inhibition of endogenous miRNA and lncRNA can be achieved by administering antisense oligonucleotides that are complementary to their sequences[125]. Smaller non-coding RNAs like miRNAs and siRNAs can also be delivered into the cells using various chemical vehicles like lipid mixtures (lipofection), lipid nanoparticles, or dendrimers; or using various biological vehicles including minicells or exosomes[127].
RNA therapeutics delivered using viral vectors have certain advantages such as the ease of generating vectors, highly efficient transduction, and long-term stable gene expression, whereas the non-viral delivery methods like oligonucleotide-based therapies have advantages like ease of dosage control, low immunogenicity and zero risk of genomic integration. The exosome-mediated and nanoparticle-mediated delivery methods might further improve the efficiency and accuracy of RNA-based therapeutics[126]. However, gene expression might still be uncontrollable with the abovementioned methods. Recently, a delivery system based on probiotic bacteria has been developed, which is more efficient in reaching a high level of gene expression and is more controllable as host bacteria can be manipulated[128]. In summary, manipulation of non-coding RNAs by silencing the hazardous non-coding RNAs or administering beneficial on-coding RNAs could reduce cardiovascular disease in patients with DM[129]. Moreover, RNA binding proteins are important in post-transcriptional gene expression. Many RNA binding proteins and RNA binding protein-regulated RNA networks are disrupted in patients with DM and diabetic complications. Thus, RNA binding proteins provide another therapeutic option for the prevention of cardiovascular disease in patients with DM[130].
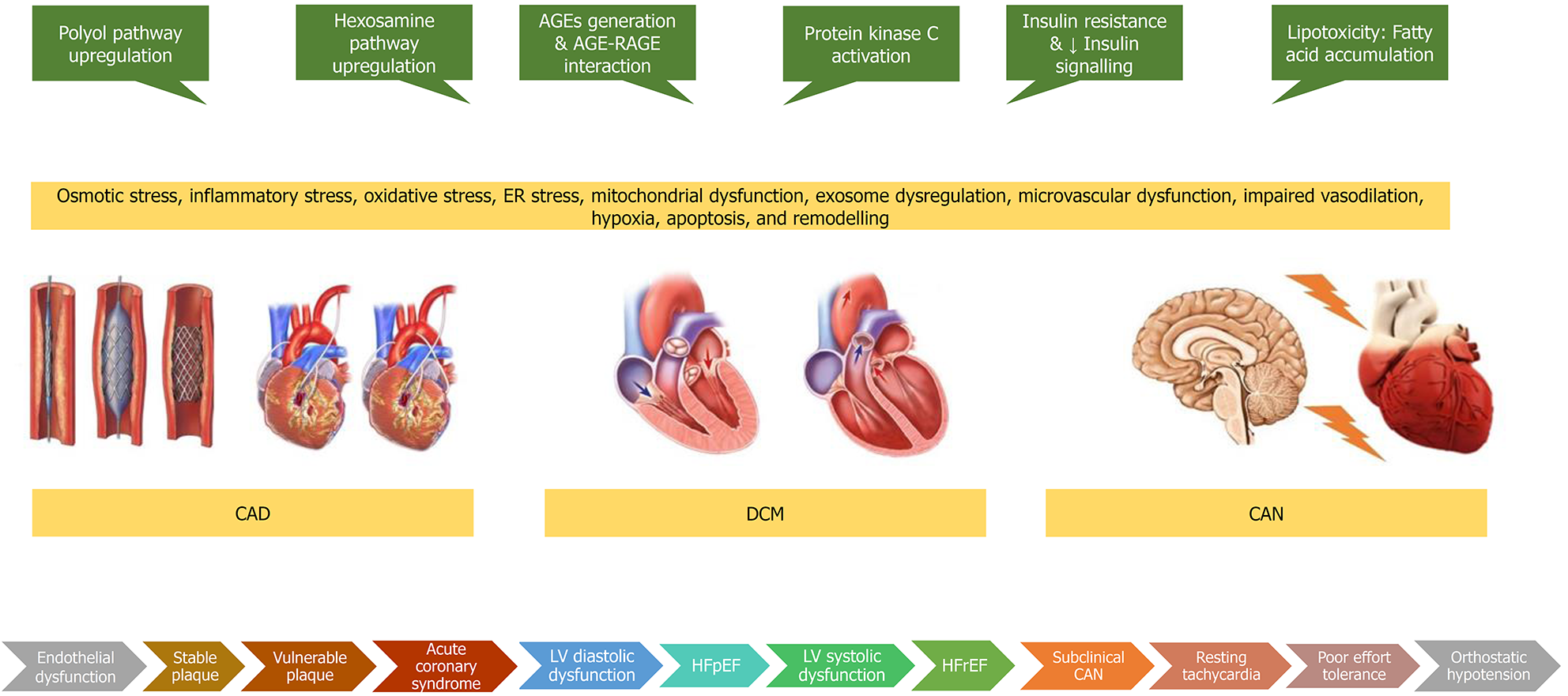
Diabetic heart disease is a conglomeration of CAD, CAN, and DCM as shown in Figure 4. CAD associated with diabetes tends to be a more complex disease and is characterised by diffuse, calcified, rapidly progressing disease, with more vulnerable features and multivessel involvement that often requires coronary revascularization in addition to optimal medical therapy. However, revascularisation outcomes are still worse in patients with diabetes compared to those without diabetes. CAN should be suspected in diabetic and prediabetic patients when they present with resting tachycardia, postural tachycardia, reduced HRV, bradycardia, impaired exercise tolerance, orthostatic hypotension, supine hypertension, or intra-operative or post-operative cardiovascular instability. CAN is independently associated with an increased risk of cardiac arrhythmias, silent myocardial ischaemia, major adverse cardiovascular events, myocardial dysfunction, sudden cardiac death, all-cause mortality, and cardiovascular mortality. DCM is characterised by cardiac dysfunction encompassing metabolic, structural, and functional myocardial changes developing in individuals with DM, in the absence of CAD, hypertensive heart disease, and significant valvular disease. Diastolic dysfunction may be asymptomatic during the early stages or can present with HFpEF. A small number of people progress to develop overt systolic dysfunction presenting as HFrEF. Glycaemic control alone is insufficient to prevent the development of diabetic heart disease. Control of traditional risk factors such as dylipidaemia, hypertension, and smoking are important, along with the appropriate use of various preventive medications such as statins, antiplatelet agents and RAAS modifiers. Some newer antidiabetic drugs such as the GLP-1 RAs and the SGLT-2 inhibitors have exhibited a direct cardioprotective effect. Many drugs based on the pathophysiological mechanisms including RNA therapeutics are under development.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu J S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | International Diabetes Federation. IDF Diabetes Atlas, 9th edition (Online), 2019. [Cited November 2, 2020]. Available from: https://www.diabetesatlas.org/en/. [Cited in This Article: ] |
| 2. | Nathanson D, Sabale U, Eriksson JW, Nyström T, Norhammar A, Olsson U, Bodegård J. Healthcare Cost Development in a Type 2 Diabetes Patient Population on Glucose-Lowering Drug Treatment: A Nationwide Observational Study 2006-2014. Pharmacoecon Open. 2018;2:393-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | US Burden of Disease Collaborators, Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A, Khan AR, Ahmadi A, Ferrari AJ, Kasaeian A, Werdecker A, Carter A, Zipkin B, Sartorius B, Serdar B, Sykes BL, Troeger C, Fitzmaurice C, Rehm CD, Santomauro D, Kim D, Colombara D, Schwebel DC, Tsoi D, Kolte D, Nsoesie E, Nichols E, Oren E, Charlson FJ, Patton GC, Roth GA, Hosgood HD, Whiteford HA, Kyu H, Erskine HE, Huang H, Martopullo I, Singh JA, Nachega JB, Sanabria JR, Abbas K, Ong K, Tabb K, Krohn KJ, Cornaby L, Degenhardt L, Moses M, Farvid M, Griswold M, Criqui M, Bell M, Nguyen M, Wallin M, Mirarefin M, Qorbani M, Younis M, Fullman N, Liu P, Briant P, Gona P, Havmoller R, Leung R, Kimokoti R, Bazargan-Hejazi S, Hay SI, Yadgir S, Biryukov S, Vollset SE, Alam T, Frank T, Farid T, Miller T, Vos T, Bärnighausen T, Gebrehiwot TT, Yano Y, Al-Aly Z, Mehari A, Handal A, Kandel A, Anderson B, Biroscak B, Mozaffarian D, Dorsey ER, Ding EL, Park EK, Wagner G, Hu G, Chen H, Sunshine JE, Khubchandani J, Leasher J, Leung J, Salomon J, Unutzer J, Cahill L, Cooper L, Horino M, Brauer M, Breitborde N, Hotez P, Topor-Madry R, Soneji S, Stranges S, James S, Amrock S, Jayaraman S, Patel T, Akinyemiju T, Skirbekk V, Kinfu Y, Bhutta Z, Jonas JB, Murray CJL. The State of US Health, 1990-2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA. 2018;319:1444-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 851] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 4. | Rawal S, Manning P, Katare R. Cardiovascular microRNAs: as modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc Diabetol. 2014;13:44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Lind M, Svensson AM, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2015;372:880-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 6. | Gagnum V, Stene LC, Jenssen TG, Berteussen LM, Sandvik L, Joner G, Njølstad PR, Skrivarhaug T. Causes of death in childhood-onset Type 1 diabetes: long-term follow-up. Diabet Med. 2017;34:56-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44 Suppl 2:S14-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 767] [Cited by in F6Publishing: 753] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 8. | Shah VN, Bailey R, Wu M, Foster NC, Pop-Busui R, Katz M, Crandall J, Bacha F, Nadeau K, Libman I, Hiers P, Mizokami-Stout K, DiMeglio LA, Sherr J, Pratley R, Agarwal S, Snell-Bergeon J, Cengiz E, Polsky S, Mehta SN. Risk Factors for Cardiovascular Disease (CVD) in Adults with Type 1 Diabetes: Findings from Prospective Real-life T1D Exchange Registry. J Clin Endocrinol Metab. 2020;105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Jørgensen ME, Almdal TP, Carstensen B. Time trends in mortality rates in type 1 diabetes from 2002 to 2011. Diabetologia. 2013;56:2401-2404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Garofolo M, Gualdani E, Giannarelli R, Aragona M, Campi F, Lucchesi D, Daniele G, Miccoli R, Francesconi P, Del Prato S, Penno G. Microvascular complications burden (nephropathy, retinopathy and peripheral polyneuropathy) affects risk of major vascular events and all-cause mortality in type 1 diabetes: a 10-year follow-up study. Cardiovasc Diabetol. 2019;18:159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Taylor KS, Heneghan CJ, Farmer AJ, Fuller AM, Adler AI, Aronson JK, Stevens RJ. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care. 2013;36:2366-2371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373:1720-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 656] [Cited by in F6Publishing: 664] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 13. | Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho K, Wilson PWF, Phillips LS. Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J Am Heart Assoc. 2019;8:e011295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 231] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 14. | Malmborg M, Schmiegelow MDS, Nørgaard CH, Munch A, Gerds T, Schou M, Kistorp C, Torp-Pedersen C, Hlatky MA, Gislason G. Does type 2 diabetes confer higher relative rates of cardiovascular events in women compared with men? Eur Heart J. 2020;41:1346-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4846] [Cited by in F6Publishing: 4384] [Article Influence: 168.6] [Reference Citation Analysis (1)] |
| 16. | Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Diabet Med. 2009;26:142-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Saely CH, Aczel S, Marte T, Langer P, Drexel H. Cardiovascular complications in Type 2 diabetes mellitus depend on the coronary angiographic state rather than on the diabetic state. Diabetologia. 2004;47:145-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Olesen KKW, Madsen M, Egholm G, Thim T, Jensen LO, Raungaard B, Bøtker HE, Sørensen HT, Maeng M. Patients With Diabetes Without Significant Angiographic Coronary Artery Disease Have the Same Risk of Myocardial Infarction as Patients Without Diabetes in a Real-World Population Receiving Appropriate Prophylactic Treatment. Diabetes Care. 2017;40:1103-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035-2038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 1308] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 20. | Naito R, Kasai T. Coronary artery disease in type 2 diabetes mellitus: Recent treatment strategies and future perspectives. World J Cardiol. 2015;7:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 21. | Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7301] [Cited by in F6Publishing: 7019] [Article Influence: 351.0] [Reference Citation Analysis (1)] |
| 22. | Schmidt AM. Diabetes Mellitus and Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2019;39:558-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 23. | Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 382] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 24. | Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a Therapeutic Target in Atherosclerosis. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 25. | Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133:2459-2502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 679] [Cited by in F6Publishing: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 26. | Ferrannini G, Norhammar A, Gyberg V, Mellbin L, Rydén L. Is Coronary Artery Disease Inevitable in Type 2 Diabetes? Diabetes Care. 2020;43:2001-2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Byon CH, Kim SW. Regulatory Effects of O-GlcNAcylation in Vascular Smooth Muscle Cells on Diabetic Vasculopathy. J Lipid Atheroscler. 2020;9:243-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Freitas Lima LC, Braga VA, do Socorro de França Silva M, Cruz JC, Sousa Santos SH, de Oliveira Monteiro MM, Balarini CM. Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol. 2015;6:304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front Endocrinol (Lausanne). 2018;9:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 30. | Low EL, Baker AH, Bradshaw AC. TGFβ, smooth muscle cells and coronary artery disease: a review. Cell Signal. 2019;53:90-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 32. | Ivanova EA, Bobryshev YV, Orekhov AN. Intimal pericytes as the second line of immune defence in atherosclerosis. World J Cardiol. 2015;7:583-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Hafiane A. Vulnerable Plaque, Characteristics, Detection, and Potential Therapies. J Cardiovasc Dev Dis. 2019;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276:618-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 35. | Sugiyama T, Yamamoto E, Bryniarski K, Xing L, Fracassi F, Lee H, Jang IK. Coronary Plaque Characteristics in Patients With Diabetes Mellitus Who Presented With Acute Coronary Syndromes. J Am Heart Assoc. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49:643-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Jensen LO, Thayssen P, Junker A, Maeng M, Tilsted HH, Kaltoft A, Hansen KN, Christiansen EH, Kristensen SD, Ravkilde J, Madsen M, Sørensen HT, Thuesen L, Lassen JF. Comparison of outcomes in patients with vs without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV trial). Am J Cardiol. 2012;110:1585-1591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Cassese S, Byrne RA, Schulz S, Hoppman P, Kreutzer J, Feuchtenberger A, Ibrahim T, Ott I, Fusaro M, Schunkert H, Laugwitz KL, Kastrati A. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J. 2015;36:94-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Ram E, Sternik L, Klempfner R, Iakobishvili Z, Fisman EZ, Tenenbaum A, Zuroff E, Peled Y, Raanani E. Type 2 diabetes mellitus increases the mortality risk after acute coronary syndrome treated with coronary artery bypass surgery. Cardiovasc Diabetol. 2020;19:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Iskander S, Iskandrian AE. Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol. 1998;32:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 278] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 41. | Kennedy MW, Fabris E, Suryapranata H, Kedhi E. Is ischemia the only factor predicting cardiovascular outcomes in all diabetes mellitus patients? Cardiovasc Diabetol. 2017;16:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Li S, Tang X, Luo Y, Wu B, Huang Z, Li Z, Peng L, Ling Y, Zhu J, Zhong J, Liu J, Chen Y. Impact of long-term glucose variability on coronary atherosclerosis progression in patients with type 2 diabetes: a 2.3 year follow-up study. Cardiovasc Diabetol. 2020;19:146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Tehrani DM, Malik S, Wong ND. Coronary artery calcium screening in persons with metabolic syndrome and diabetes: implications for prevention. Metab Syndr Relat Disord. 2013;11:143-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Raffield LM, Cox AJ, Criqui MH, Hsu FC, Terry JG, Xu J, Freedman BI, Carr JJ, Bowden DW. Associations of coronary artery calcified plaque density with mortality in type 2 diabetes: the Diabetes Heart Study. Cardiovasc Diabetol. 2018;17:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Klein L, Gheorghiade M. Management of the patient with diabetes mellitus and myocardial infarction: clinical trials update. Am J Med. 2004;116 Suppl 5A:47S-63S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Elhendy A, Tsutsui JM, O'Leary EL, Xie F, McGrain AC, Porter TR. Noninvasive diagnosis of coronary artery disease in patients with diabetes by dobutamine stress real-time myocardial contrast perfusion imaging. Diabetes Care. 2005;28:1662-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Kumar R, Patel CD, Marwah A, Gupta R, Sharma S, Malhotra A. Detection of coronary artery disease by stress thallium scintigraphy in diabetic patients. Nucl Med Commun. 2001;22:287-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Rusnak J, Fastner C, Behnes M, Mashayekhi K, Borggrefe M, Akin I. Biomarkers in Stable Coronary Artery Disease. Curr Pharm Biotechnol. 2017;18:456-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Patsouras A, Farmaki P, Garmpi A, Damaskos C, Garmpis N, Mantas D, Diamantis E. Screening and Risk Assessment of Coronary Artery Disease in Patients With Type 2 Diabetes: An Updated Review. In Vivo. 2019;33:1039-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | McCarthy CP, McEvoy JW, Januzzi JL Jr. Biomarkers in stable coronary artery disease. Am Heart J. 2018;196:82-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Dev K, Sharma SB, Garg S, Aggarwal A, Madhu SV. Glycated apolipoprotein B-A surrogate marker of subclinical atherosclerosis. Diabetes Metab Syndr. 2016;10:78-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Zhang Y, Jin JL, Cao YX, Zhang HW, Guo YL, Wu NQ, Zhu CG, Gao Y, Hua Q, Li YF, Xu RX, Li JJ. Lipoprotein (a) predicts recurrent worse outcomes in type 2 diabetes mellitus patients with prior cardiovascular events: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19:111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 54. | Won KB, Lee BK, Park HB, Heo R, Lee SE, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, Sung JM, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Chun EJ, Cademartiri F, Maffei E, Marques H, de Araújo Gonçalves P, Leipsic JA, Shin S, Choi JH, Virmani R, Samady H, Chinnaiyan K, Raff GL, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK, Chang HJ. Quantitative assessment of coronary plaque volume change related to triglyceride glucose index: The Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry. Cardiovasc Diabetol. 2020;19:113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, Tunnicliffe D, Ruospo M, Natale P, Saglimbene V, Nicolucci A, Johnson DW, Tonelli M, Rossi MC, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque LI, Lloyd A, Ahmad N, Liu Y, Tiv S, Millard T, Gagliardi L, Kolanu N, Barmanray RD, McMorrow R, Raygoza Cortez AK, White H, Chen X, Zhou X, Liu J, Rodríguez AF, González-Colmenero AD, Wang Y, Li L, Sutanto S, Solis RC, Díaz González-Colmenero F, Rodriguez-Gutierrez R, Walsh M, Guyatt G, Strippoli GFM. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 275] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 56. | Cholesterol Treatment Trialists' (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1303] [Cited by in F6Publishing: 1338] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 57. | Sharma JK, Rohatgi A, Sharma D. Diabetic autonomic neuropathy: a clinical update. J R Coll Physicians Edinb. 2020;50:269-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Bakkar NZ, Dwaib HS, Fares S, Eid AH, Al-Dhaheri Y, El-Yazbi AF. Cardiac Autonomic Neuropathy: A Progressive Consequence of Chronic Low-Grade Inflammation in Type 2 Diabetes and Related Metabolic Disorders. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Spallone V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab J. 2019;43:3-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 60. | Williams SM, Eleftheriadou A, Alam U, Cuthbertson DJ, Wilding JPH. Cardiac Autonomic Neuropathy in Obesity, the Metabolic Syndrome and Prediabetes: A Narrative Review. Diabetes Ther. 2019;10:1995-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes. 2014;5:17-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 211] [Cited by in F6Publishing: 173] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 62. | Fisher VL, Tahrani AA. Cardiac autonomic neuropathy in patients with diabetes mellitus: current perspectives. Diabetes Metab Syndr Obes. 2017;10:419-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 63. | Agashe S, Petak S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc J. 2018;14:251-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 64. | Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 769] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 65. | Safavi-Naeini P, Razavi M. Postural Orthostatic Tachycardia Syndrome. Tex Heart Inst J. 2020;47:57-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Serhiyenko VA, Serhiyenko AA. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J Diabetes. 2018;9:1-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 104] [Cited by in F6Publishing: 103] [Article Influence: 17.2] [Reference Citation Analysis (3)] |
| 67. | Pappachan JM, Sebastian J, Bino BC, Jayaprakash K, Vijayakumar K, Sujathan P, Adinegara LA. Cardiac autonomic neuropathy in diabetes mellitus: prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgrad Med J. 2008;84:205-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Stino AM, Rumora AE, Kim B, Feldman EL. Evolving concepts on the role of dyslipidemia, bioenergetics, and inflammation in the pathogenesis and treatment of diabetic peripheral neuropathy. J Peripher Nerv Syst. 2020;25:76-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 69. | Feldman EL, Nave KA, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron. 2017;93:1296-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 508] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 70. | Maamoun H, Benameur T, Pintus G, Munusamy S, Agouni A. Crosstalk Between Oxidative Stress and Endoplasmic Reticulum (ER) Stress in Endothelial Dysfunction and Aberrant Angiogenesis Associated With Diabetes: A Focus on the Protective Roles of Heme Oxygenase (HO)-1. Front Physiol. 2019;10:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 71. | Farias VX, Uchoa PN, Aquino CP, Britto LRG, Fonteles MC, Leal-Cardoso JH, Silva-Alves KS, Havt A, Prata MMG, Heimark DB, Nascimento NRF, Santos CF. Expression of myo-inositol cotransporters in the sciatic nerve and dorsal root ganglia in experimental diabetes. Braz J Med Biol Res. 2019;52:e8589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Mizukami H, Osonoi S, Takaku S, Yamagishi SI, Ogasawara S, Sango K, Chung S, Yagihashi S. Role of glucosamine in development of diabetic neuropathy independent of the aldose reductase pathway. Brain Commun. 2020;2:fcaa168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 73. | Mizukami H, Osonoi S. Pathogenesis and Molecular Treatment Strategies of Diabetic Neuropathy Collateral Glucose-Utilizing Pathways in Diabetic Polyneuropathy. Int J Mol Sci. 2020;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 642] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 75. | Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho CY, Russell JW. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 2019;145:177-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 76. | Yang H, Sloan G, Ye Y, Wang S, Duan B, Tesfaye S, Gao L. New Perspective in Diabetic Neuropathy: From the Periphery to the Brain, a Call for Early Detection, and Precision Medicine. Front Endocrinol (Lausanne). 2019;10:929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 77. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 557] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 78. | Jang ER, Lee CS. 7-ketocholesterol induces apoptosis in differentiated PC12 cells via reactive oxygen species-dependent activation of NF-κB and Akt pathways. Neurochem Int. 2011;58:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 79. | Othman A, Bianchi R, Alecu I, Wei Y, Porretta-Serapiglia C, Lombardi R, Chiorazzi A, Meregalli C, Oggioni N, Cavaletti G, Lauria G, von Eckardstein A, Hornemann T. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes. 2015;64:1035-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 80. | Callaghan BC, Gallagher G, Fridman V, Feldman EL. Diabetic neuropathy: what does the future hold? Diabetologia. 2020;63:891-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 81. | Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P; Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 536] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 82. | Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:136-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1095] [Cited by in F6Publishing: 1142] [Article Influence: 163.1] [Reference Citation Analysis (1)] |
| 83. | Balcıoğlu AS, Müderrisoğlu H. Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes. 2015;6:80-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 109] [Cited by in F6Publishing: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 84. | Yun JS, Park YM, Cha SA, Ahn YB, Ko SH. Progression of cardiovascular autonomic neuropathy and cardiovascular disease in type 2 diabetes. Cardiovasc Diabetol. 2018;17:109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Bissinger A. Cardiac Autonomic Neuropathy: Why Should Cardiologists Care about That? J Diabetes Res. 2017;2017:5374176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Mala S, Hoskovcova L, Riedlbauchova L, Nedelka T ,Broz J. Relationship between cardiac autonomic neuropathy and atherosclerosis in patients with diabetes mellitus. Curr Res Diabetes Obes J. 2018;9:555753. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Howorka K, Pumprla J, Haber P, Koller-Strametz J, Mondrzyk J, Schabmann A. Effects of physical training on heart rate variability in diabetic patients with various degrees of cardiovascular autonomic neuropathy. Cardiovasc Res. 1997;34:206-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Eschlböck S, Wenning G, Fanciulli A. Evidence-based treatment of neurogenic orthostatic hypotension and related symptoms. J Neural Transm (Vienna). 2017;124:1567-1605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 89. | Aronow WS, Ahn C. Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest. 1999;115:867-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschöpe C, Hoes AW, Seferović JP, Logue J, McDonagh T, Riley JP, Milinković I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 370] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 91. | van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18:242-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 445] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 92. | Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019;62:1550-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 93. | Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet. 2011;378:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 94. | Lind M, Olsson M, Rosengren A, Svensson AM, Bounias I, Gudbjörnsdottir S. The relationship between glycaemic control and heart failure in 83,021 patients with type 2 diabetes. Diabetologia. 2012;55:2946-2953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Cavender MA, Steg PG, Smith SC Jr, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL; REACH Registry Investigators. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years From the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 96. | MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ; CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377-1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 464] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 97. | Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 964] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 98. | Athithan L, Gulsin GS, McCann GP, Levelt E. Diabetic cardiomyopathy: Pathophysiology, theories and evidence to date. World J Diabetes. 2019;10:490-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 99. | Borghetti G, von Lewinski D, Eaton DM, Sourij H, Houser SR, Wallner M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front Physiol. 2018;9:1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 100. | Bouthoorn S, Valstar GB, Gohar A, den Ruijter HM, Reitsma HB, Hoes AW, Rutten FH. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diab Vasc Dis Res. 2018;15:477-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 101. | Bouthoorn S, Gohar A, Valstar G, den Ruijter HM, Reitsma JB, Hoes AW, Rutten FH; Queen of Hearts Consortium. Prevalence of left ventricular systolic dysfunction and heart failure with reduced ejection fraction in men and women with type 2 diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2018;17:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic Origins of Heart Failure. JACC Basic Transl Sci. 2017;2:297-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 103. | Palomer X, Pizarro-Delgado J, Vázquez-Carrera M. Emerging Actors in Diabetic Cardiomyopathy: Heartbreaker Biomarkers or Therapeutic Targets? Trends Pharmacol Sci. 2018;39:452-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 104. | Pappachan JM, Varughese GI, Sriraman R, Arunagirinathan G. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J Diabetes. 2013;4:177-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 111] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 105. | Egaña-Gorroño L, López-Díez R, Yepuri G, Ramirez LS, Reverdatto S, Gugger PF, Shekhtman A, Ramasamy R, Schmidt AM. Receptor for Advanced Glycation End Products (RAGE) and Mechanisms and Therapeutic Opportunities in Diabetes and Cardiovascular Disease: Insights From Human Subjects and Animal Models. Front Cardiovasc Med. 2020;7:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 106. | Ducheix S, Magré J, Cariou B, Prieur X. Chronic O-GlcNAcylation and Diabetic Cardiomyopathy: The Bitterness of Glucose. Front Endocrinol (Lausanne). 2018;9:642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 107. | Gupta MP. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J Mol Cell Cardiol. 2007;43:388-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 108. | Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17:585-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 322] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 109. | Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 446] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 110. | Fu S, Zhang Y, Li Y, Luo L, Zhao Y, Yao Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020;6:68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 111. | Dong X, Yu S, Wang Y, Yang M, Xiong J, Hei N, Dong B, Su Q, Chen J. (Pro)renin receptor-mediated myocardial injury, apoptosis, and inflammatory response in rats with diabetic cardiomyopathy. J Biol Chem. 2019;294:8218-8226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 112. | Zaveri MP, Perry JC, Schuetz TM, Memon MD, Faiz S, Cancarevic I. Diabetic Cardiomyopathy as a Clinical Entity: Is It a Myth? Cureus. 2020;12:e11100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Ernande L, Bergerot C, Girerd N, Thibault H, Davidsen ES, Gautier Pignon-Blanc P, Amaz C, Croisille P, De Buyzere ML, Rietzschel ER, Gillebert TC, Moulin P, Altman M, Derumeaux G. Longitudinal myocardial strain alteration is associated with left ventricular remodeling in asymptomatic patients with type 2 diabetes mellitus. J Am Soc Echocardiogr. 2014;27:479-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 114. | Lee WS, Kim J. Diabetic cardiomyopathy: where we are and where we are going. Korean J Intern Med. 2017;32:404-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 115. | Ernande L, Audureau E, Jellis CL, Bergerot C, Henegar C, Sawaki D, Czibik G, Volpi C, Canoui-Poitrine F, Thibault H, Ternacle J, Moulin P, Marwick TH, Derumeaux G. Clinical Implications of Echocardiographic Phenotypes of Patients With Diabetes Mellitus. J Am Coll Cardiol. 2017;70:1704-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 116. | Levelt E, Gulsin G, Neubauer S, McCann GP. MECHANISMS IN ENDOCRINOLOGY: Diabetic cardiomyopathy: pathophysiology and potential metabolic interventions state of the art review. Eur J Endocrinol. 2018;178:R127-R139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 117. | From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 118. | Faller KM, Lygate CA, Neubauer S, Schneider JE. (1)H-MR spectroscopy for analysis of cardiac lipid and creatine metabolism. Heart Fail Rev. 2013;18:657-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539-2550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1826] [Cited by in F6Publishing: 1800] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 120. | Tate M, Grieve DJ, Ritchie RH. Are targeted therapies for diabetic cardiomyopathy on the horizon? Clin Sci (Lond). 2017;131:897-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 121. | Al Hroob AM, Abukhalil MH, Hussein OE, Mahmoud AM. Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate. Biomed Pharmacother. 2019;109:2155-2172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 122. | Tang SG, Liu XY, Wang SP, Wang HH, Jovanović A, Tan W. Trimetazidine prevents diabetic cardiomyopathy by inhibiting Nox2/TRPC3-induced oxidative stress. J Pharmacol Sci. 2019;139:311-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 123. | Jiang Z, Fu L, Xu Y, Hu X, Yang H, Zhang Y, Luo H, Gan S, Tao L, Liang G, Shen X. Cyclovirobuxine D protects against diabetic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Sci Rep. 2020;10:6427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 124. | Laina A, Gatsiou A, Georgiopoulos G, Stamatelopoulos K, Stellos K. RNA Therapeutics in Cardiovascular Precision Medicine. Front Physiol. 2018;9:953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 125. | Fernandes JCR, Acuña SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA. 2019;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 126. | Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol. 2019;16:661-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 127. | Cannatà A, Ali H, Sinagra G, Giacca M. Gene Therapy for the Heart Lessons Learned and Future Perspectives. Circ Res. 2020;126:1394-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 128. | Yue Z, Zhang L, Li C. Advances and potential of gene therapy for type 2 diabetes mellitus. Biotechnol Equip. 2019;33:1150-1157. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 129. | Tang N, Jiang S, Yang Y, Liu S, Ponnusamy M, Xin H, Yu T. Noncoding RNAs as therapeutic targets in atherosclerosis with diabetes mellitus. Cardiovasc Ther. 2018;36:e12436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 130. | Nutter CA, Kuyumcu-Martinez MN. Emerging roles of RNA-binding proteins in diabetes and their therapeutic potential in diabetic complications. Wiley Interdiscip Rev RNA. 2018;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 131. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5314] [Cited by in F6Publishing: 5000] [Article Influence: 312.5] [Reference Citation Analysis (0)] |
| 132. | ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4759] [Cited by in F6Publishing: 4705] [Article Influence: 294.1] [Reference Citation Analysis (0)] |
| 133. | Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7:e013927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 134. | Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125-2135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1025] [Cited by in F6Publishing: 988] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 135. | Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, Gerstein HC, Gray R, Huo Y, Lang Z, McMurray JJ, Rydén L, Schröder S, Sun Y, Theodorakis MJ, Tendera M, Tucker L, Tuomilehto J, Wei Y, Yang W, Wang D, Hu D, Pan C; ACE Study Group. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:877-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 136. | NAVIGATOR Study Group. Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 137. | Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, Davidson JA, Nicolau JC, Corbalan R, Hirshberg B, Frederich R, Im K, Umez-Eronini AA, He P, McGuire DK, Leiter LA, Raz I, Scirica BM; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 Trial. Diabetes Care. 2015;38:696-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 138. | Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin vs placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 561] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 139. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1831] [Cited by in F6Publishing: 1772] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 140. | Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, Keller A, Mattheus M, Baanstra D, Meinicke T, George JT, von Eynatten M, McGuire DK, Marx N; CAROLINA Investigators. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 141. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7124] [Cited by in F6Publishing: 7330] [Article Influence: 814.4] [Reference Citation Analysis (0)] |
| 142. | Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 143. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3164] [Cited by in F6Publishing: 3546] [Article Influence: 709.2] [Reference Citation Analysis (0)] |
| 144. | Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK; VERTIS CV Investigators. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med. 2020;383:1425-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 673] [Cited by in F6Publishing: 800] [Article Influence: 200.0] [Reference Citation Analysis (0)] |
| 145. | Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247-2257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1573] [Cited by in F6Publishing: 1544] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 146. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4164] [Cited by in F6Publishing: 4200] [Article Influence: 525.0] [Reference Citation Analysis (0)] |
| 147. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3025] [Cited by in F6Publishing: 2879] [Article Influence: 359.9] [Reference Citation Analysis (0)] |
| 148. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1210] [Cited by in F6Publishing: 1249] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 149. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1213] [Cited by in F6Publishing: 1356] [Article Influence: 271.2] [Reference Citation Analysis (0)] |
| 150. | Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 957] [Cited by in F6Publishing: 1005] [Article Influence: 167.5] [Reference Citation Analysis (0)] |