Published online Nov 15, 2021. doi: 10.4239/wjd.v12.i11.1818
Peer-review started: February 25, 2021
First decision: May 3, 2021
Revised: May 27, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 15, 2021
Organophosphate is a commonly used pesticide in the agricultural sector. The main action of organophosphate focuses on acetylcholinesterase inhibition, and it therefore contributes to acute cholinergic crisis, intermediate syndrome and delayed neurotoxicity. From sporadic case series to epidemiologic studies, organophosphate has been linked to hyperglycemia and the occurrence of new-onset diabetes mellitus. Organophosphate-mediated direct damage to pancreatic beta cells, insulin resistance related to systemic inflammation and excessive hepatic gluconeogenesis and polymorphisms of the enzyme governing organophosphate elimination are all possible contributors to the development of new-onset diabetes mellitus. To date, a preventive strategy for organophosphate-mediated new-onset diabetes mellitus is still lacking. However, lowering reactive oxygen species levels may be a practical method to reduce the risk of developing hyperglycemia.
Core Tip: Organophosphate may induce acute hyperglycemia by damaging pancreatic cells and result in new-onset diabetes mellitus after chronic exposure to organophosphate compounds. Organophosphate-mediated new-onset diabetes mellitus might be mediated by a polymorphism of paraoxonase-1, which is associated with organophosphate elimination in hepatocytes. Pancreatic beta cell damage, excessive gluconeogenesis, hepatic steatosis, systemic inflammation and possibly sarcopenia all contribute to insulin resistance and therefore hyperglycemia.
- Citation: Chung YL, Hou YC, Wang IK, Lu KC, Yen TH. Organophosphate pesticides and new-onset diabetes mellitus: From molecular mechanisms to a possible therapeutic perspective. World J Diabetes 2021; 12(11): 1818-1831
- URL: https://www.wjgnet.com/1948-9358/full/v12/i11/1818.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i11.1818
Organophosphate is a commonly used pesticide in the agricultural sector because of its bioavailability. The main action of the organophosphate focuses on acetylcholinesterase inhibition. Because of its wide use, intoxication of organophosphate has been commonly encountered by physicians. Intoxication can be divided into acute cholinergic crisis, intermediate syndrome and delayed neuropathy[1]. Among the complications induced by organophosphates, diabetes mellitus is a common yet often overlooked metabolic complication. The aim of this review is to analyze the molecular pathogenesis mechanisms of new-onset diabetes mellitus after organophosphate exposure.
The main action of organophosphate is to inhibit acetylcholinesterase within the nervous system, and therefore, acetylcholine overactivity exists within the synapse and neuromuscular junction[2]. Neurological manifestations are the cardinal symptoms of organophosphate intoxication through the activation of muscarinic receptors and include myosis, excessive secretions, seizures, severe muscle paralysis, cardiorespiratory depression and even death in organophosphate overdose patients[3]. The hydrophobic character of organophosphate leads to its accumulation in adipose tissue, and therefore, intermediate syndrome with delayed neurologic injury might occur through the generation of oxidative stress. Gultekin et al[4] demonstrated that organophosphate treatment could activate lipid peroxidase and therefore generate reactive oxygen species (ROS) by exhausting glutathione and superoxide dismutase in a dose-dependent manner. Similar oxidative stress with excessive acetylcholinesterase activity has been reported in workers with chronic exposure to organophosphate[5]. Apart from neurotoxicity, accumulation within different tissues could cause different end-organ damage in the chronic phase. The mitogen-activated protein kinase (MAPK) signaling pathway could activate associated kinases, such as extracellular responsive kinases, c-Jun N-terminal kinase (JNK) and p38 MAPK, which could worsen downstream apoptosis[6]. The main contributor to MAPK signaling from organophosphates is mediated by oxidative stress. From in vitro studies, the administration of organophosphate could activate the expression of quinone oxidoreductase-1, heme oxygenase 1, paraoxonase-1, catalase or superoxide dismutase in blood mesenchymal stem cells[7] or human umbilical vein endothelial cells[8]. Therefore, distant organ damage should arouse concern in chronic organophosphate-intoxicated subjects.
Previous studies revealed that organophosphate exposure could increase the risk of new-onset diabetes mellitus (Tables 1 and 2). Moore and James[9] first noticed that acute organophosphate ingestion was associated with hyperglycemia, and hyperglycemia required insulin intervention for blood sugar control (Table 1). Serial studies also demonstrated that organophosphate-mediated acute pancreatic injury might induce hyperglycemia[10,11]. In 2008, Montgomery et al[12] provided epidemiologic data to link chronic exposure to organophosphate with diabetes mellitus (Table 2). Within the 5-year follow-up, the incidence of diabetes mellitus increased in organophosphate users. The study conducted by Liu et al[13] demonstrated that acute exposure to organophosphate led to hyperglycemia, but the effect on the development of diabetes mellitus was only marginal. In a meta-analysis study conducted by Lakshmi et al[14], hyperglycemia was common. A recent study by Panda et al[15] demonstrated that organophosphate exposure was associated with higher insulin resistance and higher plasma glycated hemoglobin levels. From the clinical study, acute organophosphate exposure was associated with hyperglycemia and then regressed after atropine treatment. From the study published by Leonel Javeres et al[16], red blood cell acetylcholinesterase activity decreased within the organophosphate exposure group, and the plasma concentrations of lipase/amylase and insulin increased in the organophosphate-exposed group. Such evidence demonstrated the effect of organophosphate on insulin resistance and direct damage to pancreatic cells in clinical investigations.
| Ref. | Area | Pesticide | Exposure | Sample size | Association |
| Moore and James[9], 1980 | Australia | Coumaphos | Acute | 1 | Hyperglycemia |
| Hui[10], 1983 | Hong Kong | Organophosphate | Acute | 2 | Hyperglycemia |
| Weizman and Sofer[11], 1992 | Israel | Organophosphate and carbamate | Acute | 17 | Hyperglycemia in 29.4% of patients |
| Yurumez et al[98], 2007 | Turkey | Organophosphate | Acute | 220 | Hyperglycemia in 67.7% of patients |
| Liu et al[13], 2014 | Taiwan | Organophosphate | Acute | 118 | Hyperglycemia after poisoning was not associated with higher mortality |
| Moon et al[99], 2016 | South Korea | Organophosphate | Acute | 184 | Hyperglycemia after poisoning was associated with higher mortality |
| Ref. | Area | Pesticide | Exposure | Sample size | Association |
| Montgomery et al[12], 2008 | United States | Organophosphate and organochlorine | Chronic | 33457 | Positive association with diabetes |
| Raafat et al[100], 2012 | Egypt | Malathion | Chronic | 98 | Positive associations among blood malathion concentration, waist circumference and insulin resistance |
| Velmurugan et al[30], 2017 | India | Organophosphate | Chronic | 3080 | Positive association between blood organophosphate residues and glycated hemoglobin levels |
| Velmurugan et al[90], 2020 | India | Organophosphate and arsenic | Chronic | 865 | Positive associations of organophosphate and arsenic with diabetes, prediabetes and atherosclerosis |
Clinical studies have shown that acute hyperglycemia develops in acute organophosphate-intoxicated subjects and that such hyperglycemia is associated with poor clinical patient outcomes. However, hyperglycemic status was mostly observed in animals with chronic or subchronic exposure[17,18]. Several in vivo studies demonstrated the acute effect of organophosphate on the variation of blood sugar. Rodrigues et al[19] reported variations in blood sugar after acute organophosphate exposure. For rats receiving a single intraperitoneal injection of malathion, blood glucose increased within 2 h, followed by hypoglycemia 8 h after injection[19]. In brain tissue, organophosphates can decrease the storage of glycogen within the brain by activating glycogenolytic enzymes such as glycogen phosphorylase and phosphoglucomutase[20]. Glycolytic enzymes, such as phosphofructokinase and hexokinase, might decrease in the acute exposure of organophosphate[21]. Collectively, these mechanisms could explain the occurrence of acute hyperglycemia following organophosphate exposure.
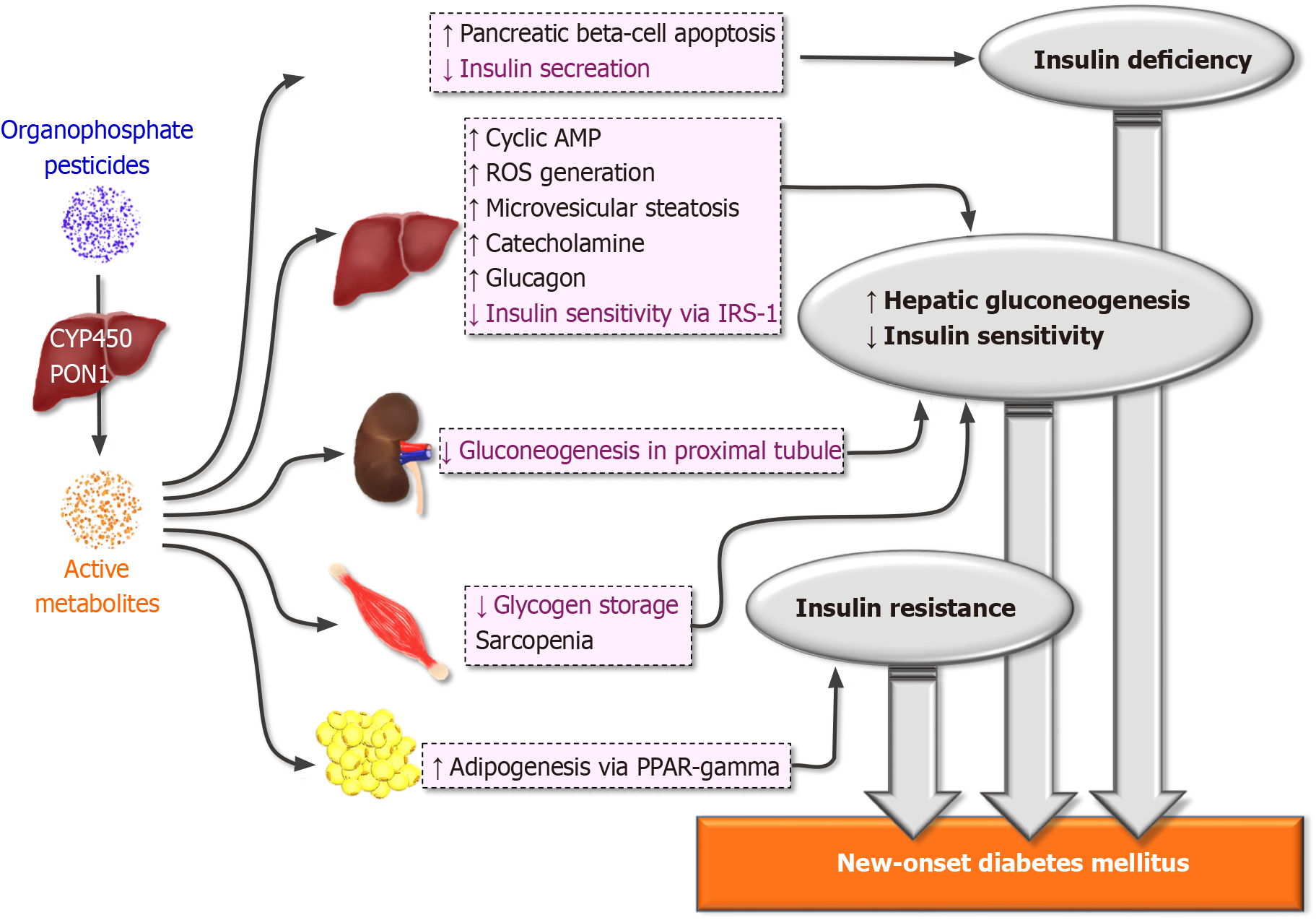
Nagaraju and Rajini[22] reported that rats receiving chronic organophosphate had higher insulin secretion from pancreatic islet cells and associated pancreatic hypertrophy. Insulin plays an important role in activating glucose transporter 9-mediated glucose transport into cells. Therefore, the regulation of insulin secretion is important in mediating the plasma concentration of glucose. Acetylcholinesterase lies within the pancreas either within acinar cells or insulin-secreting beta cells[23,24]. In insulin-secreting beta cells, acetylcholine binds to the muscarinic receptors of beta cells and then increases the cytosolic calcium concentration and enhances the efficiency of calcium-mediated exocytosis, which activates insulin-secreting activity[24]. Acetylcholinesterase also occurred within the alpha cells of the pancreas. Alpha cells stimulate insulin secretion in a paracrine manner within the pancreas[25]. Bendayan and Gisiger[23] also reported that acetylcholinesterase existed within acinar cells. Acinar cells are commonly regarded as governing lipase, but insulin secretion ability has been noted in several human studies beyond alpha and beta islet cells[26]. Case series studies showed that organophosphate overdose could induce pancreatitis and elevation of serum amylase[27]. Such clinical studies have provided evidence of organophosphate-mediated pancreatic damage. In addition, the acetylcholinergic receptor also governs the viability of pancreatic cells. The study conducted by Pfitzinger et al[28] demonstrated that cholinergic activation slowed the progression of pancreatic cancer. On the other hand, Zhang et al[29] presented evidence in a type I diabetes mellitus animal model mediated by streptozotocin that an acetylcholinesterase inhibitor protected pancreatic beta cells against apoptosis. Therefore, organophosphates might disrupt insulin secretion directly by dysregulating acetylcholinesterase activity (Figure 1).
The pathogenesis of diabetes mellitus involves impaired regulation of hepatic gluconeogenesis. Hypersensitive glucose production in response to gluconeogenic stimuli poses organophosphate exposure as a risk factor for prediabetes[30,31]. As organophosphates are ingested via the intestine, the conversion of organophosphates by cytochrome 450 enhances cholinergic inhibition up to 70%[32]. As organophosphate accumulates within hepatocytes, the activation of adenylyl cyclase produces excessive cyclic adenosine monophosphate[31], which increases hepatic glucose production and therefore increases body weight along with adipose tissue[33]. In a study conducted by Velmurugan et al[30], acetic acid increased hepatic glucose-6 phosphate and citric acid production after inducing inflammation. Apart from activation of cyclic adenosine monophosphate, the organophosphate itself also increases oxidative stress within hepatocytes by exhausting enzymes that reverse oxidative stress[34], and such oxidative stress may disrupt membranous lipids by activating lipid peroxidation[35]. Hepatic injury also occurs in organophosphate intoxication, and therefore, sequential sinusoidal dilatation and microvesicular steatosis impair glycogen synthesis[36]. Insulin mediates the suppression of adipose lipolysis physiologically and therefore downregulates gluconeogenesis[37]. Ince et al[38] demonstrated that lipid metabolism was disturbed in organophosphate-treated mice, with excessive end-products of lipid peroxidation. As excessive acetylcholinesterase leads to subjects having chronic hypercholinergic status, dietary habits are altered. From the study by Slotkin et al[39], neonatal rats exposed to organophosphate had hyperactive acetylcholine function within the neuron body, and such activity could be ameliorated only by a high-fat diet.
Under hyperglycemic conditions, glycogen storage could lower circulating glucose and enhance the anabolism process rather than catabolism. Glycogen phosphorylase, which counteracts glycogen storage, is activated by organophosphates[40]. Dichlorvos, as an example, increased the messenger ribonucleic acid expression of glycogen phosphorylase and decreased glycogen storage[41]. However, the different organophosphates had diverse actions on glycogen-storing proteins. Malathion, while activating the gluconeogenesis process, decreased glycogen phosphorylase but resulted in compensatory hepatomegaly[42,43]. The modulation of organophosphate on glycogen storage could contribute to the gluconeogenesis process.
Glucagon and catecholamine are the major hormones regulating gluconeogenesis[37]. Glucagon and catecholamine could directly enhance hepatic gluconeogenesis by activating cyclic adenosine monophosphate via phosphorylation of protein kinase A activity[44] and bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 (PFK2/FBPase-2)[45]. Glucagon activity also decreased glycogen storage by coupling with the inhibitory G protein[46]. Catecholamine, on the other hand, also activates gluconeogenesis via cyclic adenosine monophosphate activity[47]. Stress-associated catecholamine release can increase the gluconeogenesis process and therefore insulin resistance, and organophosphate itself activates catecholamine release after inhibiting acetylcholinesterase activity[42]. Organophosphate could activate catecholamine release within neurons, and unbalanced catecholamine release might prolong the gluconeogenesis process.
Insulin resistance, excessive gluconeogenesis and insufficient glucose uptake in the presence of insulin place subjects as hyperglycemia status and therefore invoke sequential adipose tissue formation. Physiologically, insulin activates the insulin receptor by tyrosine phosphorylation of insulin receptor substrate-1[48], and serine phosphorylation inhibits the insulin receptor and offsets insulin activity[49]. Insulin resistance is common in chronic organophosphate exposure subjects. From the in vivo study conducted by Nagaraju and Rajini[22], insulin hypertrophy and the increased secretion of insulin were accompanied by circulating insulin-like growth factor 1, free fatty acids, corticosterone, and paraoxonase activity. As organophosphate increases excessive cholinergic activity, insulin resistance is associated with systemic inflammation. From the study reported by Liang et al[50], organophosphates could induce an increase in body weight in experimental mice treated with a high-fat diet. In organophosphate mice treated with a high-fat diet, systemic inflammation mediated by lipopolysaccharide might occur. Systemic inflammation mediated by the intestinal barrier might activate systemic inflammation. In addition, the lipid peroxidation end product malondialdehyde (MDA) increased in organophosphate-treated rats, and the oxidative end product was associated with a higher level of inflammation[38].
Chronic exposure to organophosphate could directly enhance systemic inflammation. In a study by Ince et al[38], organophosphate-treated rats had higher proinflammatory cytokines, such as interferon gamma, interleukin 1 beta, tumor necrosis factor alpha, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), than control rats. Hepatocytes are the major cells confronting proinflammatory cytokines. As NF-κB is activated by inflammatory cytokines, IκB kinase-β might be activated and therefore hamper the downstream action of insulin[51]. In addition to IκB kinase-β, JNK signaling was also activated in organophosphate intoxication. When endoplasmic reticulum stress is enhanced after organophosphate-mediated excessive oxidative stress, JNK is activated under conditions of proinflammatory cytokine release via NF-κB[52]. Based on the evidence above, organophosphates might induce inflammation and produce proinflammatory cytokines mediating insulin resistance.
Excessive oxidative stress is associated with insulin resistance. Polyunsaturated fatty acids are the main source of oxidative stress. Low concentrations of ROS mediate the proliferative signals of insulin by phosphatidylinositol 3-kinase and protein kinase B[53]. In acute or chronic organophosphate intoxication, ROS regeneration is common as the exhaustion of endogenous antioxidant species occurs. The MDA level and superoxide dismutase increased in organophosphate intoxication, and reduced glutathione was depleted[5]. Possamai et al[54] showed that both acute and chronic exposure to malathion could generate ROS within the kidney and brain acutely and liver and skeletal muscle chronically. The study performed by Aly et al[55] also demonstrated that the liver serves as the reservoir in chronic organophosphate exposure with the generation of ROS. To enhance the elimination of ROS, adenosine triphosphate is generated from the activated gluconeogenesis process within the liver[56]. In addition, ROS also directly disturb insulin receptor signaling. Morino et al[57] demonstrated that ROS activated serine residues on insulin receptor substrate 1 and therefore inhibited glucose transporter type 4. From the aspect above, the ROS generated by organophosphate could disturb insulin signaling and therefore worsen insulin resistance.
Peroxisome proliferator-activated receptor (PPAR) is a transcriptional receptor within the nucleus, and its main action governs the proliferation of peroxisomes within the nucleus. PPARs regulate the metabolism of carbohydrates, lipids and proteins along with insulin sensitivity. The role of organophosphates in lipid metabolism has been demonstrated. Since organophosphate is a highly fat-soluble component, accumulation within adipose tissue could prolong its toxicity and generate oxidative stress within adipose tissue[58]. Smith et al[59] demonstrated that diazinon induces adipogenesis within preadipocytes by activating PPAR gamma receptors along with the transcription factor CCAAT-enhancer-binding protein α (C/EBPα).
Incretin secreted from the intestine is important for insulin secretion. As carbohydrates enter the duodenum, the K cells within the duodenum secrete glucose-dependent insulinotropic polypeptides into the brain via the vagus nerve. Activated vagal tone enhances acetylcholine release to M cells within the distal ileum and therefore increases glucagon-like peptide 1 (GLP-1). GLP-1 could therefore increase insulin release and lower blood glucose. Organophosphates might increase the acetylcholine concentration within the neural cleft and therefore downregulate muscarinic receptors. Downregulated muscarinic receptors attenuate GLP-1 release and therefore further insulin release[60]. From the study by Rathis et al[61], the GLP-1 response was attenuated in subjects with acute exposure to organophosphate with atropine treatment. Chronic exposure to organophosphate might downregulate the incretin-mediated glucose-lowering effect.
From clinical observations, victims of organophosphate exposure had transient glycosuria, which was relevant to euglycemia[62]. Based on the evidence, acute tubular necrosis might be noticed in organophosphate intoxication subjects. From the study reported by Kaya et al[63], acute organophosphate intoxication could mediate the vacuolization of tubular epithelial cells and tubular structure approaching atrophy within the proximal tubules. The oxidative stress mediated by organophosphates worsened proximal tubular damage in an in vitro study performed by Poovala et al[64]. The activation of the MAPK signaling pathway within nephron precursor cells demonstrated direct nephrotoxicity after activating JNK and caspase-3[65]. Proximal tubular cells primarily serve as gluconeogenic cells through the utilization of adenosine triphosphate, and therefore, damaged proximal tubules might impair endogenous gluconeogenesis[66] and are associated with higher mortality and the need for dialysis in critically ill patients[67,68], including organophosphate in
As described in the previous sections, the major clinical manifestation of cholinergic crisis was the overactivation of the parasympathetic tone with tachycardia, myosis or neurologic complications such as seizures. From a previous study by Liu et al[13], acute organophosphate intoxication was minimally predictive of new-onset diabetes mellitus. Intermediate syndrome by organophosphate could induce myopathy, especially in the proximal skeletal muscle and respiratory muscle[71]. Although the direct mechanism is still unknown, necrosis of skeletal muscle and associated myopathy might be an entity in chronic organophosphate intoxication subjects[72]. In addition, organophosphate-mediated peripheral motor neuropathy is accompanied by weakness after acute intoxication[73]. From a review of the literature, a correlation between organophosphate intoxication and sarcopenia was rare. However, in patients with diabetic neuropathy, sarcopenia was more obvious than in those without neuropathy[74]. Persistent muscle weakness in intermediate syndrome might lead to sarcopenic status in organophosphate patients, and sarcopenia alone might enhance the risk of developing diabetes mellitus. In the study by Hong et al[75], skeletal muscle mass was negatively associated with the development of type 2 diabetes mellitus. Since skeletal muscle serves as the pool of glucose mediated by insulin, the decreased skeletal mass would reduce glucose disposal and therefore worsen the inflammation of skeletal muscle and insulin resistance[76].
In acute organophosphate intoxication, the application of atropine is the mandatory therapeutic strategy in treating cholinergic crises. The association between atropine and insulin secretion has been discussed. In 1978, cholinergic blockade by atropine was known to decrease insulin secretion mediated by gastric inhibitory polypeptides and gastrin release[77,78]. The action of atropine on gastric inhibitory polypeptides lowered postprandial insulin secretion. From the study published by Schafer et al[79] and Afonso et al[80], atropine inhibited the release of hepatic insulin-sensitizing substances, which therefore lessened insulin sensitivity during feeding. The parasympathetic nerves directly stimulate postprandial insulin secretion; therefore, atropine might play an inhibitory role in blood sugar control. However, a study by Svensson et al[81] showed that atropine improved insulin sensitivity in both lean and obese subjects. In the atropine-treated group, glucose uptake was higher than that in the subjects treated with saline alone. In summary, parasympathetic blockade might directly decrease insulin secretion mediated by gastric inhibitory polypeptides and delay intestinal emptying under cellular dehydration conditions[82]. However, atropine might improve insulin sensitivity based on the clinical trial mediated by Svensson et al[81]. Since atropine might only be given in the acute intoxication of organophosphate conditions, the acute adverse effect might not be potentiated.
From the evidence mentioned above, the oxidative stress generated by organophosphate increased gluconeogenesis and decreased insulin sensitivity. Therefore, interventions to lessen ROS generation have been proposed to prevent the development of organophosphate-mediated diabetes mellitus. N-Acetylcysteine is a widely used scavenger for ROS due to its regeneration of glutathione. From clinical trials, N-acetylcysteine has been applied to treat acute organophosphate intoxication. From the clinical trial reported by El-Ebiary et al[83], n-acetylcysteine could achieve less atropine use and shorter hospitalization stays in acute organophosphate intoxication subjects. Falach-Malik et al[84] demonstrated that in diabetes-prone mice treated with a high-fat diet, n-acetylcysteine alleviated glucose intolerance by lessening hepatic steatosis. Charron et al[85] also demonstrated that in high-fat diet-fed maternal mice, n-acetylcysteine supplementation in the maternal stage decreased diabetes mellitus development in offspring. A similar effect was also demonstrated in a type 1 diabetes mellitus animal model under insulin deficiency[86]. N-Acetylcysteine also lessened organophosphate-mediated toxicity in vivo. A report from Yurumez et al[87] demonstrated that N-acetylcysteine could rescue antioxidative glutathione, nitrite and nitrate and decrease MDA generation in organophosphate-treated mice. The study conducted by Bayir et al[88] demonstrated that in organophosphate-poisoned mice, n-acetylcysteine alone could restore the cholinesterase concentration within erythrocytes, and the liver MDA level was lessened in n-acetylcysteine-treated mice rather than pralidoxime-atropine-treated mice or sham mice. From the aspect of decreasing organophosphate-mediated oxidative stress and the sequential development of diabetes mellitus, a therapeutic strategy for lowering ROS should be considered.
Since the development of diabetes mellitus is common in organophosphate-exposed subjects, risk stratification should be emphasized. The specific brand of organophosphate pesticide could influence the development of diabetes mellitus. Juntarawijit and Juntarawijit[89] noticed that endosulfan, mevinphos, carbamate and one fungicide (benlates) contributed to the development of diabetes mellitus in the Thai population. Apart from the specific insecticides, the environmental heavy metal content might play a synergistic role in the development of diabetes mellitus. From the study by Velmurugan et al[90], arsenics could synergize with organophosphate-mediated diabetes mellitus. At the same time, genetic polymorphisms should play a role in the development of organophosphate-induced diabetes mellitus. As the previous section mentioned, organophosphates could be metabolized by hepatic cytochrome p450, and metabolites might generate genotoxicity if the polymorphism existed within the subjects[91]. The first pass effect of cytochrome p450 generates toxic oxon organophosphate, which would be further oxidatively cleaved by cytochrome or hydroxylated by paraoxonase-1[92,93]. From the study by Al-Hakeem et al[94], the polymorphism in paraoxonase-1 with glutamine 192 to arginine made the subject vulnerable to gestational diabetes mellitus. The evidence shows a link between the polymorphism and organophosphate-mediated diabetes mellitus. In addition to diabetes mellitus development, lipid metabolism might be altered by paraoxonase-1 polymorphisms. The study conducted by Onat and et al[95] Leonel Javeres et al[96] demonstrated that the paraoxonase-1 polymorphism with the rs662 genotype was associated with ApoA1 and ApoB, which also reflected dyslipidemia in metabolic syndrome. Finally, personal protective equipment plays an important role in moderating the organophosphate metabolites associated with insulin resistance. Seesen conducted a study analyzing urinary organophosphate metabolites in pesticide sprayers and nonfarm workers[97]. In this study, the pesticide sprayer had a higher incidence of insulin resistance, and the only different organophosphate metabolite was diethylthiophosphate. No correlation was identified between diethylthiophosphate and the severity of insulin resistance. However, personal protection equipment lowered organophosphate metabolite generation. Personal protective equipment might play a preventive role in alleviating insulin resistance in organophosphate intoxication subjects.
Organophosphate pesticides have been linked to both acute and chronic intoxication. In acute intoxication, organophosphate-mediated cholinergic crisis might sequentially be followed by intermediate syndrome. Intermediate syndrome might hamper chronic muscle wasting and sarcopenia, therefore increasing the risk of diabetes mellitus. With chronic exposure to organophosphates, diabetes mellitus might develop by direct damage to the pancreas and insulin resistance mediated by lipolysis, oxidative stress and chronic inflammation. Distal organ damage, such as acute kidney injury, might worsen possible organophosphate-mediated diabetes mellitus. The standard therapeutic strategy for cholinergic crisis may play a controversial role in managing organophosphate-mediated diabetes mellitus. However, reducing ROS might be a possible therapeutic strategy. In addition, elucidating the possible genetic polymorphisms to predict the development of diabetes mellitus with organophosphate intoxication might be essential.
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mei XF S-Editor: Gao CC L-Editor: A P-Editor: Wang LYT
| 1. | Liu HF, Ku CH, Chang SS, Chang CM, Wang IK, Yang HY, Weng CH, Huang WH, Hsu CW, Yen TH. Outcome of patients with chlorpyrifos intoxication. Hum Exp Toxicol. 2020;39:1291-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Pearson JN, Patel M. The role of oxidative stress in organophosphate and nerve agent toxicity. Ann N Y Acad Sci. 2016;1378:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Brown MA, Brix KA. Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J Appl Toxicol. 1998;18:393-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 4. | Gultekin F, Ozturk M, Akdogan M. The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch Toxicol. 2000;74:533-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Ranjbar A, Pasalar P, Abdollahi M. Induction of oxidative stress and acetylcholinesterase inhibition in organophosphorous pesticide manufacturing workers. Hum Exp Toxicol. 2002;21:179-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Aggeli IK, Gaitanaki C, Lazou A, Beis I. Activation of multiple MAPK pathways (ERKs, JNKs, p38-MAPK) by diverse stimuli in the amphibian heart. Mol Cell Biochem. 2001;221:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Jahan S, Kumar D, Singh S, Kumar V, Srivastava A, Pandey A, Rajpurohit CS, Khanna VK, Pant AB. Resveratrol Prevents the Cellular Damages Induced by Monocrotophos via PI3K Signaling Pathway in Human Cord Blood Mesenchymal Stem Cells. Mol Neurobiol. 2018;55:8278-8292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ellis RJ, Small DM, Ng KL, Vesey DA, Vitetta L, Francis RS, Gobe GC, Morais C. Indoxyl Sulfate Induces Apoptosis and Hypertrophy in Human Kidney Proximal Tubular Cells. Toxicol Pathol. 2018;46:449-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Moore PG, James OF. Acute pancreatitis induced by acute organophosphate poisoning. Postgrad Med J. 1981;57:660-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Hui KS. Metabolic disturbances in organophosphate insecticide poisoning. Arch Pathol Lab Med. 1983;107:154. [PubMed] [Cited in This Article: ] |
| 11. | Weizman Z, Sofer S. Acute pancreatitis in children with anticholinesterase insecticide intoxication. Pediatrics. 1992;90:204-206. [PubMed] [Cited in This Article: ] |
| 12. | Montgomery MP, Kamel F, Saldana TM, Alavanja MC, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993-2003. Am J Epidemiol. 2008;167:1235-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Liu SH, Lin JL, Shen HL, Chang CC, Huang WH, Weng CH, Hsu CW, Wang IK, Liang CC, Yen TH. Acute large-dose exposure to organophosphates in patients with and without diabetes mellitus: analysis of mortality rate and new-onset diabetes mellitus. Environ Health. 2014;13:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Lakshmi J, Mukhopadhyay K, Ramaswamy P, Mahadevan S. A Systematic Review on Organophosphate Pesticide and Type II Diabetes Mellitus. Curr Diabetes Rev. 2020;16:586-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Panda S, Nanda R, Mangaraj M, Rathod PK, Mishra PK. Glycemic Status in Organophosphorus Poisoning. J Nepal Health Res Counc. 2015;13:214-219. [PubMed] [Cited in This Article: ] |
| 16. | Leonel Javeres MN, Raza S, Judith N, Anwar F, Habib R, Batool S, Nurulain SM. Mixture of Organophosphates Chronic Exposure and Pancreatic Dysregulations in Two Different Population Samples. Front Public Health. 2020;8:534902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Deotare ST, Chakrabarti CH. Effect of acephate (orthene) on tissue levels of thiamine, pyruvic acid, lactic acid, glycogen and blood sugar. Indian J Physiol Pharmacol. 1981;25:259-264. [PubMed] [Cited in This Article: ] |
| 18. | Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp Biochem Physiol C Toxicol Pharmacol. 2004;137:343-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Rodrigues MA, Puga FR, Chenker E, Mazanti MT. Short-term effect of malathion on rats' blood glucose and on glucose utilization by mammalian cells in vitro. Ecotoxicol Environ Saf. 1986;12:110-113. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Husain K, Ansari RA. Effectiveness of certain drugs in acute malathion intoxication in rats. Ecotoxicol Environ Saf. 1990;19:271-275. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Sarin S, Gill KD. Dichlorvos induced alterations in glucose homeostasis: possible implications on the state of neuronal function in rats. Mol Cell Biochem. 1999;199:87-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Nagaraju R, Rajini PS. Adaptive response of rat pancreatic β-cells to insulin resistance induced by monocrotophos: Biochemical evidence. Pestic Biochem Physiol. 2016;134:39-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Bendayan M, Gisiger V. Demonstration of acetylcholinesterase molecular forms in a continuous tubular lysosomal system of rat pancreatic acinar cells. J Histochem Cytochem. 2001;49:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22:565-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011;17:888-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 26. | Yu L, Luo JX, Wei JL, Mu XJ, Ren XX, Yang Z, Peng YT, Zhao HL. Insulin-producing acinar cells in adult human pancreas. Pancreas. 2014;43:592-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Yoshida S, Okada H, Nakano S, Shirai K, Yuhara T, Kojima H, Doi T, Kato H, Suzuki K, Morishita K, Murakami E, Ushikoshi H, Toyoda I, Ogura S. Much caution does no harm! J Intensive Care. 2015;3:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Pfitzinger PL, Fangmann L, Wang K, Demir E, Gürlevik E, Fleischmann-Mundt B, Brooks J, D'Haese JG, Teller S, Hecker A, Jesinghaus M, Jäger C, Ren L, Istvanffy R, Kühnel F, Friess H, Ceyhan GO, Demir IE. Indirect cholinergic activation slows down pancreatic cancer growth and tumor-associated inflammation. J Exp Clin Cancer Res. 2020;39:289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Zhang B, Yang L, Yu L, Lin B, Hou Y, Wu J, Huang Q, Han Y, Guo L, Ouyang Q, Zhang B, Lu L, Zhang X. Acetylcholinesterase is associated with apoptosis in β cells and contributes to insulin-dependent diabetes mellitus pathogenesis. Acta Biochim Biophys Sin (Shanghai). 2012;44:207-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Velmurugan G, Ramprasath T, Swaminathan K, Mithieux G, Rajendhran J, Dhivakar M, Parthasarathy A, Babu DD, Thumburaj LJ, Freddy AJ, Dinakaran V, Puhari SS, Rekha B, Christy YJ, Anusha S, Divya G, Suganya K, Meganathan B, Kalyanaraman N, Vasudevan V, Kamaraj R, Karthik M, Jeyakumar B, Abhishek A, Paul E, Pushpanathan M, Rajmohan RK, Velayutham K, Lyon AR, Ramasamy S. Gut microbial degradation of organophosphate insecticides-induces glucose intolerance via gluconeogenesis. Genome Biol. 2017;18:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod Toxicol. 2011;31:297-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 345] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 33. | Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J Med Toxicol. 2007;3:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Sharma Y, Bashir S, Irshad M, Gupta SD, Dogra TD. Effects of acute dimethoate administration on antioxidant status of liver and brain of experimental rats. Toxicology. 2005;206:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Altuntas I, Delibas N, Demirci M, Kilinc I, Tamer N. The effects of methidathion on lipid peroxidation and some liver enzymes: role of vitamins E and C. Arch Toxicol. 2002;76:470-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Sutcu R, Altuntas I, Yildirim B, Karahan N, Demirin H, Delibas N. The effects of subchronic methidathion toxicity on rat liver: role of antioxidant vitamins C and E. Cell Biol Toxicol. 2006;22:221-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13:572-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 608] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 38. | Ince S, Arslan-Acaroz D, Demirel HH, Varol N, Ozyurek HA, Zemheri F, Kucukkurt I. Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats. Biomed Pharmacother. 2017;96:263-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Slotkin TA, Lassiter TL, Ryde IT, Wrench N, Levin ED, Seidler FJ. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environ Health Perspect. 2009;117:916-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Koundinya PR, Ramamurthi R. Effect of organophosphate pesticide Sumithion (Fenitrothion) on some aspects of carbohydrate metabolism in a freshwater fish, Sarotherodon (Tilapia) mossambicus (Peters). Experientia. 1979;35:1632-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Romero-Navarro G, Lopez-Aceves T, Rojas-Ochoa A, Fernandez Mejia C. Effect of dichlorvos on hepatic and pancreatic glucokinase activity and gene expression, and on insulin mRNA levels. Life Sci. 2006;78:1015-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Rezg R, Mornagui B, El-Fazaa S, Gharbi N. Caffeic acid attenuates malathion induced metabolic disruption in rat liver, involvement of acetylcholinesterase activity. Toxicology. 2008;250:27-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Rezg R, Mornagui B, El-Arbi M, Kamoun A, El-Fazaa S, Gharbi N. Effect of subchronic exposure to malathion on glycogen phosphorylase and hexokinase activities in rat liver using native PAGE. Toxicology. 2006;223:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Lee Y, Berglund ED, Yu X, Wang MY, Evans MR, Scherer PE, Holland WL, Charron MJ, Roth MG, Unger RH. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc Natl Acad Sci U S A. 2014;111:13217-13222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 46. | Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell-signaling cascades controlling metabolism: differential effects of diazinon and parathion. Environ Health Perspect. 2010;118:210-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Siddle K, Kane-Maguire B, Campbell AK. The effects of glucagon and insulin on adenosine 3':5'-cyclic monophosphate concentrations in an organ culture of mature rat liver. Biochem J. 1973;132:765-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 37] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413-E422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 607] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 49. | Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650-1656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 613] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 50. | Liang Y, Zhan J, Liu D, Luo M, Han J, Liu X, Liu C, Cheng Z, Zhou Z, Wang P. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome. 2019;7:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 51. | Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1323] [Cited by in F6Publishing: 1336] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 52. | Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047-9054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1069] [Cited by in F6Publishing: 1064] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 53. | Dröge W. Oxidative enhancement of insulin receptor signaling: experimental findings and clinical implications. Antioxid Redox Signal. 2005;7:1071-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Possamai FP, Fortunato JJ, Feier G, Agostinho FR, Quevedo J, Wilhelm Filho D, Dal-Pizzol F. Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats. Environ Toxicol Pharmacol. 2007;23:198-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Aly N, El-Gendy K, Mahmoud F, El-Sebae AK. Protective effect of vitamin c against chlorpyrifos oxidative stress in male mice. Pestic Biochem Physiol. 2010;97:7-12. [DOI] [Cited in This Article: ] |
| 56. | Rahimi R, Abdollahi M. A review on the mechanisms involved in hyperglycemia induced by organophosphorus pesticides. Pestic Biochem Physiol. 2007;88:115-121. [DOI] [Cited in This Article: ] |
| 57. | Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 Suppl 2:S9-S15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 587] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 58. | De Bleecker JL. The intermediate syndrome in organophosphate poisoning: an overview of experimental and clinical observations. J Toxicol Clin Toxicol. 1995;33:683-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Smith A, Yu X, Yin L. Diazinon exposure activated transcriptional factors CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and induced adipogenesis in 3T3-L1 preadipocytes. Pestic Biochem Physiol. 2018;150:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Rathish D, Agampodi SB, Jayasumana MACS, Siribaddana SH. From organophosphate poisoning to diabetes mellitus: The incretin effect. Med Hypotheses. 2016;91:53-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Rathish D, Senavirathna I, Jayasumana C, Agampodi S, Siribaddana S. A low GLP-1 response among patients treated for acute organophosphate and carbamate poisoning: a comparative cross-sectional study from an agrarian region of Sri Lanka. Environ Sci Pollut Res Int. 2019;26:2864-2872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Shobha TR, Prakash O. Glycosuria in organophosphate and carbamate poisoning. J Assoc Physicians India. 2000;48:1197-1199. [PubMed] [Cited in This Article: ] |
| 63. | Kaya Y, Bas O, Hanci H, Cankaya S, Nalbant I, Odaci E, Avni Uydu H, Aslan A. Acute renal involvement in organophosphate poisoning: histological and immunochemical investigations. Ren Fail. 2018;40:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Poovala VS, Huang H, Salahudeen AK. Role of reactive oxygen metabolites in organophosphate-bidrin-induced renal tubular cytotoxicity. J Am Soc Nephrol. 1999;10:1746-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Ren X, Han HJ, Lee YJ, Lee SH, Ng HY, Chae KJ, Kim IS. Proapoptotic effect of a micropollutant (tris-(2-chloroethyl)-phosphate) at environmental level in primary cultured renal proximal tubule cells. J Water Health. 2012;10:522-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Lewy PR, Quintanilla A, Levin NW, Kessler RH. Renal energy metabolism and sodium reabsorption. Annu Rev Med. 1973;24:365-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Lin YF, Lin SL, Huang TM, Yang SY, Lai TS, Chen L, Wu VC, Chu TS, Wu KD; National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF). New-Onset Diabetes After Acute Kidney Injury Requiring Dialysis. Diabetes Care. 2018;41:2105-2110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Legouis D, Ricksten SE, Faivre A, Verissimo T, Gariani K, Verney C, Galichon P, Berchtold L, Feraille E, Fernandez M, Placier S, Koppitch K, Hertig A, Martin PY, Naesens M, Pugin J, McMahon AP, Cippà PE, de Seigneux S. Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat Metab. 2020;2:732-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 69. | Lee FY, Chen WK, Lin CL, Lai CY, Wu YS, Lin IC, Kao CH. Organophosphate Poisoning and Subsequent Acute Kidney Injury Risk: A Nationwide Population-Based Cohort Study. Medicine (Baltimore). 2015;94:e2107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Jiang YD, Chang CH, Tai TY, Chen JF, Chuang LM. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000-2009 Nationwide Health Insurance database. J Formos Med Assoc. 2012;111:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 71. | Karalliedde L, Baker D, Marrs TC. Organophosphate-induced intermediate syndrome: aetiology and relationships with myopathy. Toxicol Rev. 2006;25:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Ahlgren JD, Manz HJ, Harvey JC. Myopathy of chronic organophosphate poisoning: a clinical entity? South Med J. 1979;72:555-558, 563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Miranda J, McConnell R, Wesseling C, Cuadra R, Delgado E, Torres E, Keifer M, Lundberg I. Muscular strength and vibration thresholds during two years after acute poisoning with organophosphate insecticides. Occup Environ Med. 2004;61:e4. [PubMed] [Cited in This Article: ] |
| 74. | Yang Q, Zhang Y, Zeng Q, Yang C, Shi J, Zhang C, Ni X, Du Z, Tang Z, Hu J, Li X, Cai J, Li Q, Cheng Q. Correlation Between Diabetic Peripheral Neuropathy and Sarcopenia in Patients with Type 2 Diabetes Mellitus and Diabetic Foot Disease: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2020;13:377-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Hong S, Chang Y, Jung HS, Yun KE, Shin H, Ryu S. Relative muscle mass and the risk of incident type 2 diabetes: A cohort study. PLoS One. 2017;12:e0188650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Scott D, de Courten B, Ebeling PR. Sarcopenia: a potential cause and consequence of type 2 diabetes in Australia's ageing population? Med J Aust. 2016;205:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 77. | Larrimer JN, Mazzaferri EL, Cataland S, Mekhjian HS. Effect of atropine on glucose-stimulated gastric inhibitory polypeptide. Diabetes. 1978;27:638-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Baumert JE Jr, Cataland S, Tetirick CE Jr, Pace WG, Mazzaferri EL. Effect of atropine on meal-stimulated gastrin and gastric inhibitory polypeptide (GIP) release. J Clin Endocrinol Metab. 1978;46:473-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Schafer J, Legare DJ, Lautt WW. Acetylcholinesterase antagonist potentiated insulin action in fed but not fasted state. J Pharmacol Exp Ther. 2010;333:621-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Afonso RA, Lautt WW, Ribeiro RT, Legare DJ, Macedo MP. Insulin resistance in two animal models of obesity: A comparison of HISS-dependent and HISS-independent insulin action in high-fat diet-fed and Zucker rats. Proc West Pharmacol Soc. 2007;50:110-114. [PubMed] [Cited in This Article: ] |
| 81. | Svensson MK, Jansson PA, Persson AL, Sjöstrand M, Eriksson JW. Atropine improves insulin sensitivity in both lean and abdominally obese subjects. J Clin Endocrinol Metab. 2011;96:E1843-E1847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Gundamaraju R, Vemuri R. Pathophysiology of Greedy Colon and Diabetes: Role of Atropine in worsening of Diabetes. Euroasian J Hepatogastroenterol. 2014;4:51-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | El-Ebiary AA, Elsharkawy RE, Soliman NA, Soliman MA, Hashem AA. N-acetylcysteine in Acute Organophosphorus Pesticide Poisoning: A Randomized, Clinical Trial. Basic Clin Pharmacol Toxicol. 2016;119:222-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Falach-Malik A, Rozenfeld H, Chetboun M, Rozenberg K, Elyasiyan U, Sampson SR, Rosenzweig T. N-Acetyl-L-Cysteine inhibits the development of glucose intolerance and hepatic steatosis in diabetes-prone mice. Am J Transl Res. 2016;8:3744-3756. [PubMed] [Cited in This Article: ] |
| 85. | Charron MJ, Williams L, Seki Y, Du XQ, Chaurasia B, Saghatelian A, Summers SA, Katz EB, Vuguin PM, Reznik SE. Antioxidant Effects of N-Acetylcysteine Prevent Programmed Metabolic Disease in Mice. Diabetes. 2020;69:1650-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Argaev Frenkel L, Rozenfeld H, Rozenberg K, Sampson SR, Rosenzweig T. N-Acetyl-l-Cysteine Supplement in Early Life or Adulthood Reduces Progression of Diabetes in Nonobese Diabetic Mice. Curr Dev Nutr. 2019;3:nzy097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Yurumez Y, Cemek M, Yavuz Y, Birdane YO, Buyukokuroglu ME. Beneficial effect of N-acetylcysteine against organophosphate toxicity in mice. Biol Pharm Bull. 2007;30:490-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 88. | Bayır A, Kara H, Köylü Ö, Kocabaş R, Ak A. Effects of n-acetylcysteine on the erythrocyte and liver cholinesterase, nitric oxide and malondialdehyde levels in acute organophosphate toxicity. Crit Care. 2011;15:P366. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Juntarawijit C, Juntarawijit Y. Association between diabetes and pesticides: a case-control study among Thai farmers. Environ Health Prev Med. 2018;23:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 90. | Velmurugan G, Swaminathan K, Mohanraj S, Dhivakar M, Veerasekar G, Alexander T, Cherian M, Palaniswami NG, Pradeep T. Association of co-accumulation of arsenic and organophosphate insecticides with diabetes and atherosclerosis in a rural agricultural community: KMCH-NNCD-I study. Acta Diabetol. 2020;57:1159-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Abass K, Pelkonen O. The inhibition of major human hepatic cytochrome P450 enzymes by 18 pesticides: comparison of the N-in-one and single substrate approaches. Toxicol In Vitro. 2013;27:1584-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Kaur G, Jain AK, Singh S. CYP/PON genetic variations as determinant of organophosphate pesticides toxicity. J Genet. 2017;96:187-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 94. | Al-Hakeem MM, Abotalib Z, Alharbi KK, Khan IA. Relationship between the paraoxonase 1 gene glutamine 192 to arginine polymorphism and gestational diabetes mellitus in Saudi women. Clin Biochem. 2014;47:122-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Onat A, Can G, Hergenç G, Yazici M, Karabulut A, Albayrak S. Serum apolipoprotein B predicts dyslipidemia, metabolic syndrome and, in women, hypertension and diabetes, independent of markers of central obesity and inflammation. Int J Obes (Lond). 2007;31:1119-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 96. | Leonel Javeres MN, Habib R, Judith N, Iqbal M, Nepovimova E, Kuca K, Batool S, Nurulain SM. Analysis of PON1 gene polymorphisms (rs662 and rs854560) and inflammatory markers in organophosphate pesticides exposed cohorts from two distinct populations. Environ Res. 2020;191:110210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Seesen M, Lucchini RG, Siriruttanapruk S, Sapbamrer R, Hongsibsong S, Woskie S, Kongtip P. Association between Organophosphate Pesticide Exposure and Insulin Resistance in Pesticide Sprayers and Nonfarmworkers. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Yurumez Y, Durukan P, Yavuz Y, Ikizceli I, Avsarogullari L, Ozkan S, Akdur O, Ozdemir C. Acute organophosphate poisoning in university hospital emergency room patients. Intern Med. 2007;46:965-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 99. | Moon JM, Chun BJ, Cho YS. Hyperglycemia at presentation is associated with in hospital mortality in non-diabetic patient with organophosphate poisoning. Clin Toxicol (Phila). 2016;54:252-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Raafat N, Abass MA, Salem HM. Malathion exposure and insulin resistance among a group of farmers in Al-Sharkia governorate. Clin Biochem. 2012;45:1591-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |