Copyright
©The Author(s) 2015.
World J Diabetes. Jul 10, 2015; 6(7): 943-960
Published online Jul 10, 2015. doi: 10.4239/wjd.v6.i7.943
Published online Jul 10, 2015. doi: 10.4239/wjd.v6.i7.943
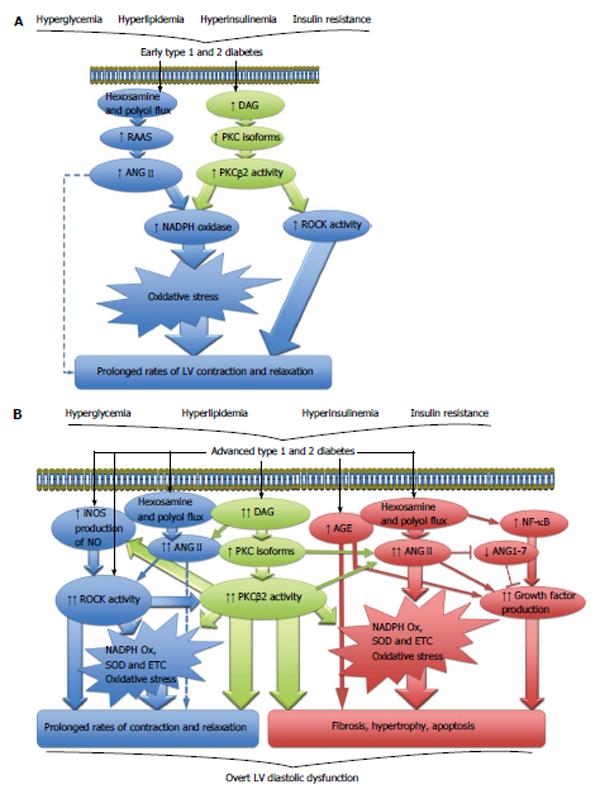
Figure 1 Cellular signaling pathways.
A: Cellular signaling pathways driving contractile dysfunction in early diabetic cardiomyopathy; B: Cellular signaling pathways involved in the development of overt LV diastolic dysfunction in diabetes. ANGII: Angiotensin II; DAG: Diacylglycerol; NADPH: Nicotinamide adenine dinucleotide phosphate; PKC: Protein kinase C; RAAS: Renin angiotensin aldosterone system; ROCK: Rho kinase; AGE: Advanced glycation end products; ANG1-7: Angiotensin 1-7; ETC: Electron transport chain; iNOS: Inducible nitric oxide synthase; NF-κB: Nuclear factor-κB; NO: Nitric oxide; SOD: Superoxide dismutase; LV: Left ventricle.
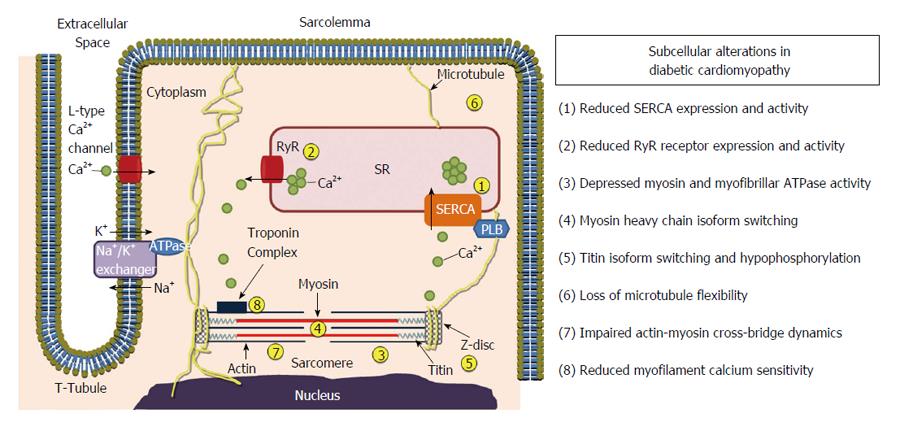
Figure 2 Subcellular alterations in various compartments of the cardiomyocyte in diabetic cardiomyopathy.
PLB: Phospholamban; SERCA: Sarcoplasmic reticulum Ca2+-ATPase; SR: Sarcoplasmic reticulum; RyR: Ryanodine receptor.
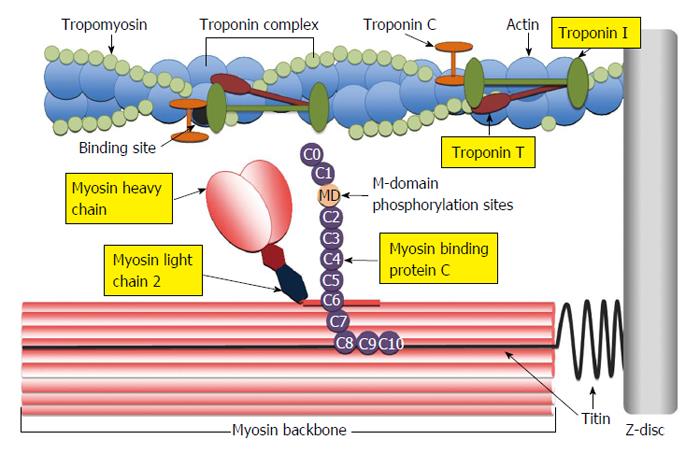
Figure 3 Illustration of the cardiac sarcomere indicating the location of actin thin-filament and myosin thick-filament accessory proteins involved in the regulation of actin-myosin cross-bridge dynamics and kinetics.
C0-C10: Immunoglobulin-like and fibronectin-like domains of myosin binding protein C; MD: M-domain of myosin binding protein C containing protein kinase phosphorylation sites.
- Citation: Waddingham MT, Edgley AJ, Tsuchimochi H, Kelly DJ, Shirai M, Pearson JT. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World J Diabetes 2015; 6(7): 943-960
- URL: https://www.wjgnet.com/1948-9358/full/v6/i7/943.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i7.943