Copyright
©The Author(s) 2021.
World J Diabetes. Dec 15, 2021; 12(12): 1979-1999
Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.1979
Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.1979
Figure 1 A diagram showing sterile inflammation and microbial inflammation.
Inflammation results from activation of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). Examples of PAMPs include microorganisms, its byproducts or subcellular components. Examples of DAMPs include glucose, saturated fatty acids, uric acid, or amyloid beta plaques. DAMP: Damage-associated molecular pattern; PAMP: Pathogen-associated molecular pattern; AD: Alzheimer’s disease.
Figure 2 A diagram that depicts the five major classes of protein recognition receptors that are identified for sensing pathogen-associated molecular patterns and damage-associated molecular patterns and subsequent stimulation of proinflammatory responses.
The cell surface pattern recognition receptors (PRRs) include Toll-like receptors and c-type lectin receptors. The cytoplasmic PRRs include NOD-like receptors (NLRs), retinoic acid-inducible gene-1-like receptors (RLRs), and the non-NLRs. RLRs recognize double-stranded RNA viruses and activate NFκB to increase the transcription of cytokines. The signaling of NLRs requires the initial expression of inflammasome and cytokine precursors such as pro-IL-1β or pro-IL-18. Assembly of the NLR-inflammasome results in caspase-1 activation and subsequently processing and secretion of cytokines IL-1β and IL-18. Non-NLRs, known also as AIM-2 can recognize double-stranded DNA viruses. Similarly, AIM-2 signals via activation of cleavage and release of active caspase-1 to process and mature IL-1β and IL-18. TLRs: Toll-like receptors; NLRs: NOD-like receptors; CLRs: c-type lectin receptors; PRR: Pattern recognition receptors; RLRs: Retinoic acid-inducible gene-1-like receptors.
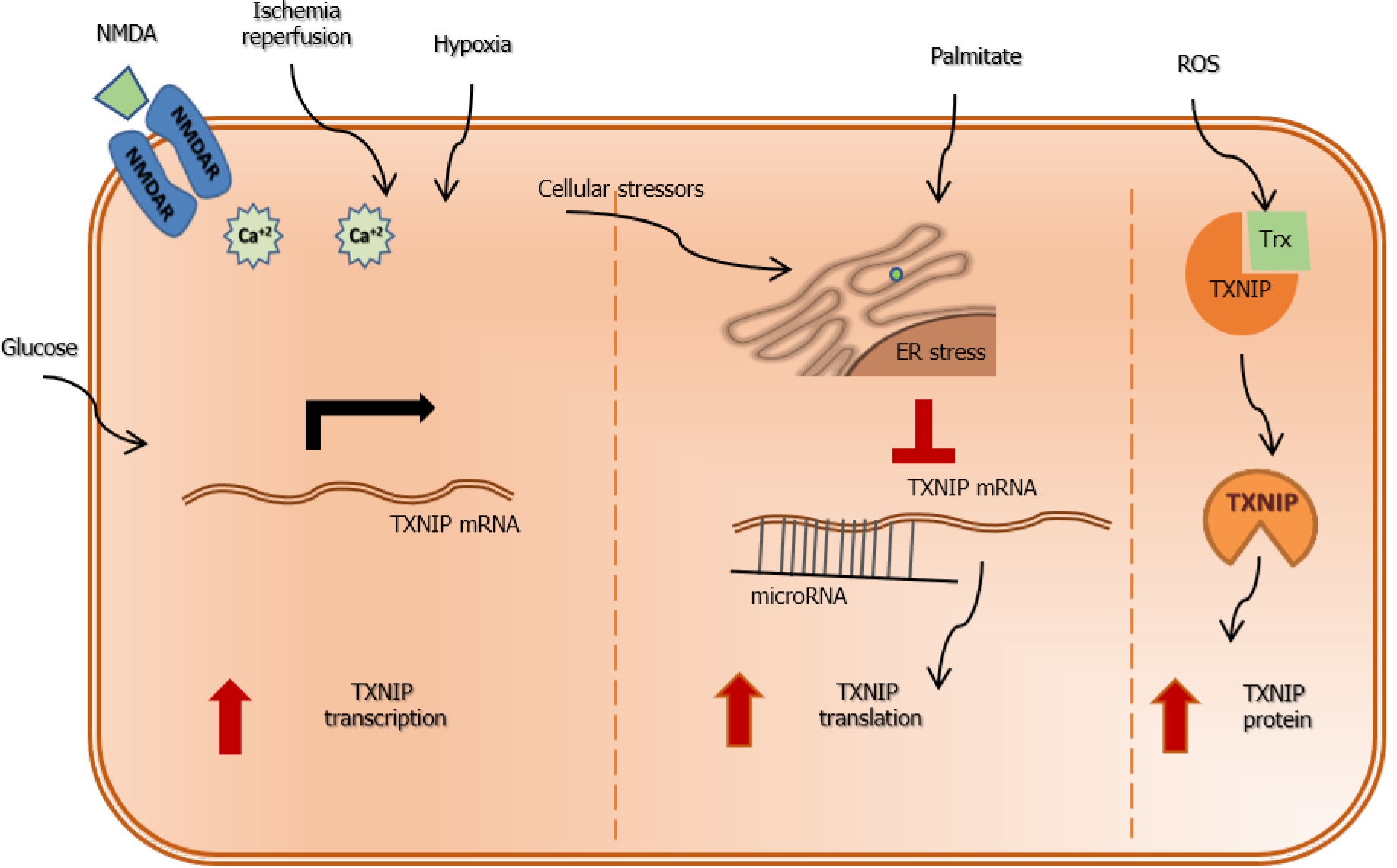
Figure 3 A diagram that depicts various ways of regulation of thioredoxin interacting protein expression.
At the transcriptional level, thioredoxin interacting protein (TXNIP) expression can be triggered by hyperglycemia as it contains carbohydrate response element. Ischemia-reperfusion injury, hypoxia and activation of the n-methyl D-aspartate receptor result in significant increase in calcium influx that trigger TXNIP expression via activation of the Ca-response element. Further, TXNIP can be post-transcriptionally regulated by endoplasmic reticulum (ER) stress and microRNA (miRNA) that traditionally bind to the 3 UTR region of TXNIP mRNA and repress its translation. Under conditions of cellular stressors, saturated fatty acids such as palmitate result in increases in ER stress and degradation of miRNA resulting in increases in TXNIP expression. Finally, oxidative stress and formation of reactive oxygen species dissociates TXNIP from thioredoxin and increase its level that facilitate activation of NLRP3-inflammsome and release of inflammatory mediators. TXNIP: Thioredoxin interacting protein; NMDA: N-methyl D-aspartate; ER: Endoplasmic reticulum; ROS: Reactive oxygen species.
- Citation: Mohamed IN, Li L, Ismael S, Ishrat T, El-Remessy AB. Thioredoxin interacting protein, a key molecular switch between oxidative stress and sterile inflammation in cellular response. World J Diabetes 2021; 12(12): 1979-1999
- URL: https://www.wjgnet.com/1948-9358/full/v12/i12/1979.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i12.1979