Published online Apr 16, 2017. doi: 10.4253/wjge.v9.i4.196
Peer-review started: July 26, 2016
First decision: September 29, 2016
Revised: January 22, 2017
Accepted: February 28, 2017
Article in press: March 1, 2017
Published online: April 16, 2017
Processing time: 265 Days and 8.6 Hours
To review the role of multidisciplinary management in treating sporadic duodenal adenomas (SDA).
SDA managed at North Shore Hospital between 2009-2014 were entered into a prospective database. Pathology, endoscopic and surgical management as well as follow up were reviewed.
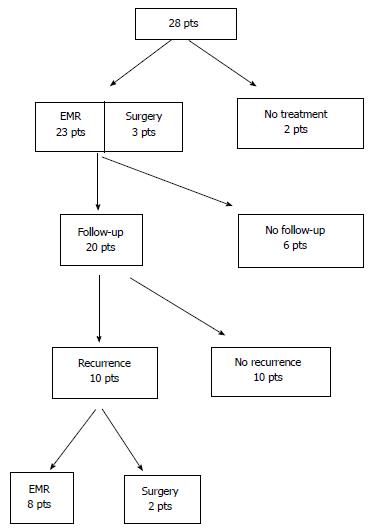
Twenty-eight patients (14 male: Median age 68 years) presented with SDA [18 were classified as non ampullary location (NA), 10 as ampullary location (A)]. All SDA were diagnosed on upper gastrointestinal endoscopy and were imaged with a contrast enhanced CT scan of the chest, abdomen and pelvis. Of the NA adenomas 14 were located in the second part, 2 in the first part and 2 in the third part of the duodenum. Two patients declined treatment, 3 patients underwent surgical resection (2 transduodenal resections and 1 pancreaticoduodenectomy), and 23 patients were treated with endoscopic mucosal resection (EMR). The only complication with endoscopic resection was mild pancreatitis post procedure. Patients were followed with gastroduodenoscopy for a median of 22 mo (range: 2-69 mo). There were 8 recurrences treated with EMR with one patient proceeding to pancreaticodeuodenectomy because of high grade dysplasia in the resected specimen and 2 NA recurrences were managed with surgical resection (distal gastrectomy for a lesion in the first part of the duodenum and a transduodenal resection of a lesion in the third part of the duodenum).
SDA can be treated endoscopically with minimal morbidity and piecemeal resection results in eradication in nearly three quarters of patients. Recurrent SDA can be treated with endoscopic reresection with surgical resection indicated when the lesions are large (> 4 cm in diameter) or demonstrate severe dysplasia or invasive cancer.
Core tip: Sporadic duodenal adenomas can be treated endoscopically with minimal morbidity and even piecemeal resection results in eradication in nearly three quarters of patients. Optimal surveillance strategies include re-endoscopy 6 mo after the initial resection is a satisfactory starting point. Recurrent sporadic adenomas can be treated with endoscopic re-resection with surgical resection indicated when the lesions are large (> 3 cm in diameter) or demonstrate severe dysplasia or invasive cancer.
- Citation: Rajkomar K, Kweon M, Khan I, Frankish P, Rodgers M, Koea JB. Endoscopic assessment and management of sporadic duodenal adenomas: The results of single centre multidisciplinary management. World J Gastrointest Endosc 2017; 9(4): 196-203
- URL: https://www.wjgnet.com/1948-5190/full/v9/i4/196.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i4.196
Sporadic duodenal adenomas (SDA) are rare lesions with a prevalence of 0.3%-1.5%[1]. Due to this rarity, the natural history of SDA is not well understood although it is known to follow an adenoma to carcinoma sequence similar to colorectal cancer[2]. The reported rate of malignant transformation of SDA ranges from 25% to 85% and this provides a rationale for preventative intervention and surveillance[2-4]. The majority of sporadic adenomas are sessile and occur in the second part of the duodenum[5,6] and can be divided into those with an ampullary location (A) or non ampullary location (NA)[1,2].
Currently there is no consensus on the optimal management of SDA and, in particular, the choice of surgical or endoscopic resection remains controversial since surgical resection involves either local resection by the transduodenal approach or by pancreaticoduodenectomy with the risk of significant morbidity and mortality. In contrast endoscopic mucosal resection (EMR) was first described in 1992 and has become increasingly favoured as the first line treatment modality[6-8].
This investigation describes the multidisciplinary management strategy for SDA as used at a single unit and involves contributions from surgery, endoscopy and gastroenterology. The specific aims of this study were to: (1) define the role of EMR of SDA; (2) define the role of whole vs piece meal endoscopic resection; (3) define an optimal surveillance strategy following endoscopic resection; and (4) define the optimal treatment for recurrence SDA following endoscopic resection.
Consecutive cases of duodenal adenoma diagnosed at North Shore Hospital (NSH) between 2009 and 2014 were reviewed. The pathology findings from all patients was entered into a prospective database. Demographic, diagnostic, biopsy, treatment and follow up information was then reviewed as well as details pertaining to local recurrence rate and salvage treatments.
This project was logged with the Awhina Research and Knowledge Centre at NSH and ethics approval was obtained from the Regional Ethics Committee.
Thirty-four patients were diagnosed with duodenal adenomas between 2009 and 2014 of which six patients were excluded because of an underlying diagnosis of familial adenomatous polyposis. Data from 28 patients was analysed for the investigation of whom 18 were classified as NA and 10 as A.
A summary of patient demographics, polyp morphology and investigations utilized in the management of the reported patients with SDA are presented in Table 1. All patients were New Zealand European with no Maori or Pacific Island patients presenting with SDA. Five patients (50%) with ampullary lesions presented with adenoma specific symptoms (iron deficiency anaemia 3, obstructive jaundice 2), while five (28%) of the NA patients presented with iron deficiency anaemia. The remaining patients with ampullary lesions underwent investigation for non-specific abdominal pain or following an incidental finding on ERCP for choledocolithiasis. In patients with NA upper gastrointestinal endoscopy was also undertaken for non-specific pain (4 patients), peptic ulcer disease or reflux (4 patients), and one patient each for globus, dysphagia, incidental finding during ERCP and investigation of Crohn’s disease and incidentally noted raised carcino-embryonic antigen. All SDA were diagnosed on upper gastrointestinal endoscopy and were biopsied (Table 1). All patients were imaged with a contrast enhanced CT scan of the abdomen to define signs of invasion or metastases. Of the non-ampullary adenomas 14 were located in the second part of the duodenum, two in the first part and two in the third part of the duodenum. Endoscopic ultrasound (EUS) was used selectively to locoregionally stage lesions that were large, ulcerated or had high grade dysplasia on biopsy (5 of 8 ampullary adenomas and 8 of 15 non-ampullary adenomas). EUS permitted detailed assessment of lesional size and depth and location of further biopsy specimens[4,5,7].
| Non-ampullary (n = 18) | Ampullary (n = 10) | |
| Demographics | ||
| Median age, yr (range) | 69 (47-88) | 67 (48-80) |
| Male: female | 9:9 | 5:5 |
| Morphology | ||
| Pedunculated | 3 (17) | 1 (10) |
| Sessile | 15 (83) | 9 (90) |
| Median size, mm (range) | 15 (9-24) | 20 (10-35) |
| Number ≥ 20 mm | 7 (39) | 6 (60) |
| Investigations | ||
| Biopsy | 7 (39) | 8 (80) |
| EUS | 3 (17) | 0 |
| ERCP | 0 | 10 (100) |
Patient management is summarised in Figure 1 and Table 2. All endoscopically treated patients had an EMR. All endoscopic procedures were undertaken in a specialist endoscopy suite with conscious sedation administered intravenously followed by recovery and same day discharge. Endoscopic resection was undertaken after submucosal injection of saline, epinephrine or methylene blue depending on the endoscopist’s preference. The median number of endoresections per patient was 1 and was higher for ampullary (median 2.5) than non-ampullary adenomas (median 1). Endoscopic en bloc resection was aimed for in all cases but, due to the size of the lesions, 11 NA and 6 A underwent piecemeal resection (Table 2). The only complication of endoscopic resection was one episode of mild pancreatitis post-procedure which was self-limiting.
| Non-ampullary (n = 18) | Ampullary (n = 10) | |
| Endoscopic treatment | 15 | 8 |
| Stenting | ||
| Biliary | 0 | 2 |
| Pancreatic | 0 | 5 |
| Specimen removal | ||
| Piecemeal | 11 | 6 |
| En bloc | 4 | 2 |
| Complications | 0 | 1 |
| Surgical resection | 2 | 1 |
| No treatment | 1 | 1 |
| Histology | ||
| 1 no dysplasia | 7 low grade dysplasia | |
| 13 low grade dysplasia | 1 high grade dysplasia | |
| 3 high grade dysplasia | 1 adenocarcinoma | |
| Concordance with biopsy | 4/7 | 7/8 |
| Recurrence | 5 | 5 |
Once removed specimens were orientated and sent for pathological examination. Overall biopsies were concordant with final pathology in 4 of 7 NA and 7 of 8 A (Table 2).
Two non-ampullary adenomas underwent surgical resection: Two patients underwent transduodenal resection of lesions in the second and third parts of the duodenum and one patient with a large ampullary adenoma, was treated with a pancreaticoduodenectomy. In addition, two elderly patients declined any treatment.
All patients had follow up gastroscopies although five patients declined follow up and one patient had undergone a pancreaticoduodenectomy (n = 1). The average time taken for the first endoscopic surveillance post resection was 7.9 mo for NA and 5.9 mo for A. The median follow up period was 22 mo (range 2-69 mo).
Details on recurrence rate in the 20 cases actively followed up are presented in Table 3 in addition to salvage therapy employed. EMR was used to treat 8 recurrences. Endoscopic ultrasound was used in two ampullary recurrences to rule out transmural invasion. One of eight patients treated with endoscopic resection was shown to be a high grade dysplastic lesion and was subsequently treated with a pancreaticoduodenectomy (final pathology T1N0 adenocarcinoma). Two non-ampullary recurrences were managed with surgical resection (distal gastrectomy for a lesion in the first part of the duodenum and a transduodenal resection of a lesion in the third part of the duodenum).
| Recurrence (n = 10) | No recurrence (n = 10) | |
| Non-ampullary/ ampullary | 5:5 | 7:3 |
| Median size (mm) | 20 mm | 10 mm |
| Treatment | ||
| Endoscopic resection | 10 | 8 |
| Surgical resection | 0 | 2 |
| Specimen retrieval | ||
| Piecemeal | 8 | 6 |
| En bloc | 2 | 4 |
| Margin positivity | 9 (90%) | 6 (60%) |
| Salvage therapy | ||
| Endoscopic resection | 8 | |
| Surgical resection | 2 |
This investigation was undertaken to review multidisciplinary management of SDA and confirms that the majority of SDA are not symptomatic and are found incidentally[6-9]. Endoscopically SDA tend to be large, sessile and located in the second part of the duodenum[6,10-13] and this series also confirms that most SDA harbour dysplasia[14-19]. Kim et al[13] found that all of their 17 non ampullary adenomas were dysplastic while a larger series from Japan[14] demonstrated that dysplasia was presented in all 233 non-ampullary adenomas assessed. The rate of low grade dysplasia in non-ampullary adenomas in our series was 73.3%, which was within range (52%-84%) of recently published series[13-15,19], while the rate of low grade dysplasia in our ampullary adenomas (78%) was higher than 53%-66% previously reported[16-18]. The processes responsible for the high rates of dysplasia in SDA are not clear however Rubio[19] suggested that the duodenum of those patients may exhibit gastric duodenal metaplasia and bile acids and pancreatic juices may provide a milieu that encourages the metaplasia to proceed onto the adenoma-carcinoma sequence. It is possible that SDA progress to dysplasia faster than other adenomas in the gastrointestinal tract[19].
The variable investigations performed during patient workup is a reflection of the lack of guidelines available in managing this rare entity.
Role of biopsy: There are no clear guidelines regarding the absolute need to biopsy all lesions and therefore the decision is often left to the discretion of the endoscopist. However a pre resection biopsy for SDAs may compromise a subsequent safe “lift off” technique of EMR and may increase the risk of perforation especially in the setting of a thin duodenal wall or a large duodenal tumour. Moreover morphological changes after biopsy may give the false impression of submucosal infiltration of a superficial lesion[20,21]. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggests that all suspicious lesions should be biopsied[22]. Although biopsy concordance with final pathology is commonly around 75%, as in this investigation[23-25], and the non-concordant biopsies usually fail to sample a small focus of malignancy within the SDA particularly ampullary adenomas[26]. Elek suggests taking large, multiple biopsies (up to 6) or doing papillectomies to improve the diagnostic yield[27].
Role of EUS: We pursued a selective policy of EUS prior to resection to define invasion or pancreatic ductal involvement in large SDA that were suspicious (large size, ulceration or the presence of high grade dysplasia on biopsy)[8,27-29]. However SDA size is a variable determinant of high grade dysplasia or malignant change with authors quoting a size > 10 mm[30], > 20 mm[8,27,28,31], and > 30 mm[29]. ASGE guidelines suggest the use of EUS in lesions > 2 cm in non-ampullary and > 1 cm in ampullary adenoma[22]. Currently the role of intraductal ultrasound is not well defined. Menzel et al[32] suggested it was more useful than EUS in tumour diagnosis but a recent prospective study suggested that it could overstage tumours[33].
Role of ERCP: This is the least controversial investigational tool for ampullary adenomas and was performed in all our patients since it provides an accurate means of assessing ductal involvement[34-36].
Most of the SDAs were resected endoscopically, which is in line with contemporary management[37].
Role for EMR: The factors affecting the suitability for a lesion to undergo endoscopic resection include size, presence of malignant signs, extension along the wall of the duodenum and extension into biliary/pancreatic ducts[37]. There is no consensus regarding the absolute size that would make a lesion suitable for endoscopic resection although a maximum size of 4-5 cm for an endoscopic ampullectomy has been suggested, due to the increased risk of malignancy. Large adenomas can be challenging to resect en bloc although Irani had a success rate of 84%, with a mean lesion size of 2.4 cm[38].
There has been a significant shift with respect to size criteria for non ampullary lesions. In 2003 Perez et al[8] suggested that lesions more than 2 cm ought to be resected surgically. In 2009 Alexander et al[7] showed that lesions with mean size of 27.6 mm could be resected endoscopically. Apart from size, the physical appearance of the lesion is important. If the depressed segment is < 10 mm and non-depressed segment < 50 mm then it will be suitable for endoscopic resection and the non-lift sign is a strong sign of malignancy[39].
Role for endoscopic submucosal dissection: In our institution we have favoured EMR as a method of endoscopic resection. In general it has a success rate of 79%-100% with ability to deal with any lesion in only one session in 80%. The complication rate been quoted as 0.6% for perforation and up to 9% for non-fatal bleeding. Endoscopic submucosal dissection has recently been trialled in duodenal adenomas and electrosurgical dissection with an endoscopic knife achieves a better en bloc resection of the lesion[11]. However the complication rate is higher with perforation rates of 31%, 15% for post-procedural bleeding and a longer procedural duration.
En bloc vs piecemeal resection: We have more commonly resorted to piecemeal resection for both types of adenoma. Ideally en bloc resection would allow an oncologically better resection of the tumour but this can be challenging for lesions > 2 cm[7,40]. Piecemeal resection allows tumours of larger size to be resected endoscopically with reduced risk of perforation, reduces resection time and uses less electrocautery. Unfortunately it does predispose to repeated subsequent resections[22] as there is increased risk of recurrence[7] especially when the lesion is > 20 mm[7,22].
Role of pancreatic stenting following ampullectomy: Pancreatic duct stenting has been shown to reduce the risk of post procedural pancreatitis in a prospective randomised trial[41], although the study only included 19 patients. A meta-analysis of five studies involving 481 patients showed that patients in the no stent group had a 3-fold increased risk of post-ERCP pancreatitis[42]. Our pancreatic stenting rate is only 62.5%, without however any trend towards significant pancreatitis post resection. There is no strong evidence regarding prophylactic biliary stenting, although it has a role should biliary drainage post procedure be a concern[33].
We reported a 4.3% complication rate. This was a single patient with self-limiting mild pancreatitis after a papillectomy. The rate of specific complications associated with endoscopic resections include pancreatitis (8%-15%), perforation (up to 4%), cholangitis (up to 2%), papillary stenosis (0%-8%)[22]. A recent prospective study showed a risk of minor bleeding of 18% and 6.5% for major bleeding[43]. The low rate of bleeding at our institution could be due to meticulous hemostasis being achieved once resection is completed.
In our series of cases, recurrences in ampullary adenomas occurred earlier and more often than in non ampullary SDA. The inherent risk of recurrence after endoscopic resection has been investigated separately in both subgroups of adenomas. Two series on ampullary adenomas showed a recurrence rate of 19% on followup[43,44] while a published case series of endoscopic resection of non-ampullary adenomas showed an average recurrence rate of 19.9%[31]. However subset analysis shows that the recurrence rate of 37% can go up to 63% if lesion of > 2 cm diameter are analysed separately[6]. Currently there is no accepted standardized follow up regime. Most commonly it is suggested that patients should have annual endoscopic follow up for first 2 years after complete resection[6], while Apel et al[10] suggests 3 monthly endoscopy for 1 year, increasing to 6 monthly for 2 years followed by annual endoscopy.
A treatment plan for recurrences should be devised by all units offering endoscopic therapy of duodenal adenomas as recurrences are common, especially if there has been more than one endoresection, the lesion was large or the resection was incomplete. Unfortunately there is no consensus on the optimal salvage therapy. As more experience is being gathered with endoresection it is increasingly becoming an attractive tool to treat recurrences, often coupled with ablative therapy such as argon beam coagulation (APC). Alexander et al[7] noted 5 recurrences after treating 23 patients with NA by EMR, with median size of 20 mm. Those were cleared with a further session of APC ± EMR with a mean follow up of 13 mo. Similarly a series of 54 patients with non-ampullary adenomas (mean size 15 mm)[45] had 16 recurrences of which 15 were eradicated with a further session of EMR ± APC. However, the median follow up period was only 10.8 mo.
Very few series have assessed ablation therapy in isolation. Lienert et al[46] assessed 16 cases of NA treated with APC ± polypectomy where 3 of the 4 recurrences were successfully treated with ablative therapy. Apel et al[10] had assessed 18 cases of non-ampullary adenoma, with a median size of 27.5 mm, treated with a combination of serial sessions of polypectomy and APC (33 sessions) carried out over 3 wk to achieve a 55% success rate although 6 cases could not be eradicated despite multimodal endoscopic therapy.
Recently Schneider et al[47] addressed the role of surgery to treat recurrences after failed endoscopic treatment of ampullary adenomas. Forty-four cases were referred for transduodenal surgical ampullectomy following a median of 3 endoscopic treatments before referral. The surgical cure rate was 84% with a post-operative morbidity of 24%, the majority being mild (Clavien-Dindo grade I/II). This was comparable to morbidity associated with endoresection (8%-27%).
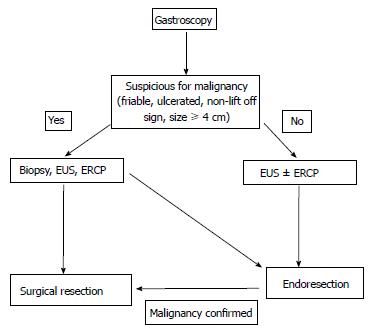
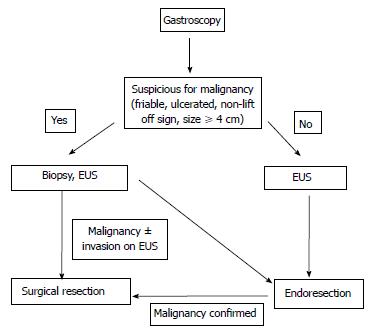
Based on this information a management algorithm for sporadic non-ampullary and ampullary adenomas is summarised in Figures 2 and 3 respectively. However, management does depend on the experience of the endoscopist (e.g., with respect to size of polyp), the availability of investigative tools (e.g., EUS) and the fitness of the patient to tolerate the treatment offered.
In conclusion, this investigation has confirmed that SDA can be treated endoscopically with minimal morbidity and that piecemeal resection results in eradication in nearly three quarters of patients. Optimal surveillance strategies following resection are not clearly established but re-endoscopy 6 mo after the initial resection is a satisfactory starting point. Recurrent SDA can be treated with endoscopic reresection with surgical resection indicated when the lesions are large (> 3 cm in diameter) or demonstrate severe dysplasia or invasive cancer.
The medical and nursing staff of wards 4, 6 and 8 and the Department of Gastroenterology at North Shore Hospital are gratefully acknowledged.
The optimal treatment strategy for sporadic duodenal adenomas (SDA) is not yet established although it is clear that this involves contributions from both advanced endoscopy and upper gastrointestinal surgery.
Developing algorithms to accurately predict the optimal treatment (endoscopic or surgical resection) based on morphology and pathology of both primary and recurrent SDA will assist in their multidisciplinary management.
Most SDA can be treated endoscopically with even piecemeal resection resulting in eradication in three quarters of patients. Surgical resection can be reserved for lesions > 4 cm in diameter or with malignant change.
With multidisciplinary review, endoscopic resection can be the primary treatment modality for SDA.
SDA is a management challenge due to their anatomical position and the often comorbid status of patients.
This manuscript is interesting due to the paucity of precise international guidelines regarding the topic.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: New Zealand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: de Angelis GL S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P, Kruse A, Thommesen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992;87:37-42. [PubMed] |
| 3. | Spigelman AD, Talbot IC, Penna C, Nugent KP, Phillips RK, Costello C, DeCosse JJ. Evidence for adenoma-carcinoma sequence in the duodenum of patients with familial adenomatous polyposis. The Leeds Castle Polyposis Group (Upper Gastrointestinal Committee). J Clin Pathol. 1994;47:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc. 2009;69:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Perez A, Saltzman JR, Carr-Locke DL, Brooks DC, Osteen RT, Zinner MJ, Ashley SW, Whang EE. Benign nonampullary duodenal neoplasms. J Gastrointest Surg. 2003;7:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Takahashi T, Ando T, Kabeshima Y, Kawakubo H, Shito M, Sugiura H, Omori T. Borderline cases between benignancy and malignancy of the duodenum diagnosed successfully by endoscopic submucosal dissection. Scand J Gastroenterol. 2009;44:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy. 2005;37:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Kim HK, Chung WC, Lee BI, Cho YS. Efficacy and long-term outcome of endoscopic treatment of sporadic nonampullary duodenal adenoma. Gut Liver. 2010;4:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Goda K, Kikuchi D, Yamamoto Y, Takimoto K, Kakushima N, Morita Y, Doyama H, Gotoda T, Maehata Y, Abe N. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: Multicenter case series. Dig Endosc. 2014;26 Suppl 2:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Kedia P, Brensinger C, Ginsberg G. Endoscopic predictors of successful endoluminal eradication in sporadic duodenal adenomas and its acute complications. Gastrointest Endosc. 2010;72:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Jeanniard-Malet O, Caillol F, Pesenti C, Bories E, Monges G, Giovannini M. Short-term results of 42 endoscopic ampullectomies: a single-center experience. Scand J Gastroenterol. 2011;46:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Laleman W, Verreth A, Topal B, Aerts R, Komuta M, Roskams T, Van der Merwe S, Cassiman D, Nevens F, Verslype C. Endoscopic resection of ampullary lesions: a single-center 8-year retrospective cohort study of 91 patients with long-term follow-up. Surg Endosc. 2013;27:3865-3876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Rubio CA. Gastric duodenal metaplasia in duodenal adenomas. J Clin Pathol. 2007;60:661-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Uno Y, Munakata A. The non-lifting sign of invasive colon cancer. Gastrointest Endosc. 1994;40:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 146] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Han KS, Sohn DK, Choi DH, Hong CW, Chang HJ, Lim SB, Choi HS, Jeong SY, Park JG. Prolongation of the period between biopsy and EMR can influence the nonlifting sign in endoscopically resectable colorectal cancers. Gastrointest Endosc. 2008;67:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Chathadi KV, Khashab MA, Acosta RD, Chandrasekhara V, Eloubeidi MA, Faulx AL, Fonkalsrud L, Lightdale JR, Salztman JR, Shaukat A. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773-781. [PubMed] |
| 23. | Elek G, Gyôri S, Tóth B, Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. 2003;9:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 24. | Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimens procured by endoscopic ampullectomy. Am J Clin Pathol. 2009;132:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Rodríguez C, Borda F, Elizalde I, Jiménez Pérez FJ, Carral D. How accurate is preoperative diagnosis by endoscopic biopsies in ampullary tumours? Rev Esp Enferm Dig. 2002;94:585-592. [PubMed] |
| 26. | Pandolfi M, Martino M, Gabbrielli A. Endoscopic treatment of ampullary adenomas. JOP. 2008;9:1-8. [PubMed] |
| 27. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Min YW, Min BH, Kim ER, Lee JH, Rhee PL, Rhee JC, Kim JJ. Efficacy and safety of endoscopic treatment for nonampullary sporadic duodenal adenomas. Dig Dis Sci. 2013;58:2926-2932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Lim GJ, Devereaux BM. EUS in the assessment of ampullary lesions prior to endoscopic resection. Tech Gastrointest Endosc. 2010;12:49-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Baillie J. Endoscopic ampullectomy. Am J Gastroenterol. 2005;100:2379-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Basford PJ, Bhandari P. Endoscopic management of nonampullary duodenal polyps. Therap Adv Gastroenterol. 2012;5:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Menzel J, Hoepffner N, Sulkowski U, Reimer P, Heinecke A, Poremba C, Domschke W. Polypoid tumors of the major duodenal papilla: preoperative staging with intraductal US, EUS, and CT--a prospective, histopathologically controlled study. Gastrointest Endosc. 1999;49:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Catalano MF, Linder JD, Chak A, Sivak MV, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Norton ID, Geller A, Petersen BT, Sorbi D, Gostout CJ. Endoscopic surveillance and ablative therapy for periampullary adenomas. Am J Gastroenterol. 2001;96:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Moon JH, Choi HJ, Lee YN. Current status of endoscopic papillectomy for ampullary tumors. Gut Liver. 2014;8:598-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Kahaleh M, Shami VM, Brock A, Conaway MR, Yoshida C, Moskaluk CA, Adams RB, Tokar J, Yeaton P. Factors predictive of malignancy and endoscopic resectability in ampullary neoplasia. Am J Gastroenterol. 2004;99:2335-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, Howell DA. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Singh P, Das A, Isenberg G, Wong RC, Sivak MV, Agrawal D, Chak A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 43. | Aschmoneit-Messer I, Richl J, Pohl J, Ell C, May A. Prospective study of acute complication rates and associated risk factors in endoscopic therapy for duodenal adenomas. Surg Endosc. 2015;29:1823-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Zádorová Z, Dvofák M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Navaneethan U, Lourdusamy D, Mehta D, Lourdusamy V, Venkatesh PG, Sanaka MR. Endoscopic resection of large sporadic non-ampullary duodenal polyps: efficacy and long-term recurrence. Surg Endosc. 2014;28:2616-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Lienert A, Bagshaw PF. Treatment of duodenal adenomas with endoscopic argon plasma coagulation. ANZ J Surg. 2007;77:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Schneider L, Contin P, Fritz S, Strobel O, Büchler MW, Hackert T. Surgical ampullectomy: an underestimated operation in the era of endoscopy. HPB (Oxford). 2016;18:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |