Published online Apr 25, 2016. doi: 10.4253/wjge.v8.i8.368
Peer-review started: October 27, 2015
First decision: December 22, 2015
Revised: December 29, 2015
Accepted: February 23, 2016
Article in press: February 24, 2016
Published online: April 25, 2016
Perforation is an important procedural complication of endoscopic submucosal dissection (ESD) for early gastric cancer. Although the incidence of delayed perforation after ESD is low, extreme caution is necessary because many cases require surgical intervention. Among 1984 lesions of early gastric cancer treated in our hospital by ESD in 1588 patients from September 2002 through March 2015, delayed perforation developed in 4 patients (4 lesions, 0.25%). A diagnosis of delayed perforation requires prompt action, including surgical intervention when required.
Core tip: Delayed perforation is a serious complication of endoscopic submucosal dissection for early gastric cancer. A diagnosis of delayed perforation requires prompt action, including surgical intervention when required.
- Citation: Yano T, Tanabe S, Ishido K, Azuma M, Wada T, Suzuki M, Kawanishi N, Yamane S, Sasaki T, Katada C, Mikami T, Katada N, Koizumi W. Delayed perforation after endoscopic submucosal dissection for early gastric cancer: Clinical features and treatment. World J Gastrointest Endosc 2016; 8(8): 368-373
- URL: https://www.wjgnet.com/1948-5190/full/v8/i8/368.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i8.368
The development of endoscopic submucosal dissection (ESD) has facilitated the en bloc endoscopic resection of larger lesions, as well as lesions with an ulcer scar. Such lesions are now included in the expanded indications for ESD[1].
Perforation is an important procedural complication of ESD, reported to occur at an incidence of 3.6% to 8.7%. Most cases of intraoperative perforation can be closed by clipping[2-4]. In contrast to intraoperative perforation diagnosed during endoscopic treatment, delayed perforation detected after ESD is often associated with peritonitis at time of diagnosis and frequently requires emergency treatment, including surgical intervention[5]. Few studies have reported on delayed perforation, and its management remains controversial. We describe our experience with 4 patients (4 lesions) who underwent emergency surgery for delayed perforation that developed after ESD in our hospital.
A total of 1984 consecutive lesions of early gastric cancer treated by endoscopic resection between September 2002 and March 2015 were studied. Informed consent was obtained from all patients in accordance with our institutional protocol.
We defined delayed perforation as the abrupt onset of abdominal pain and signs and symptoms of peritoneal irritation accompanied by the presence of free air on chest and abdominal radiography or abdominal computed tomography (CT) in a patient who showed no evidence of perforation during ESD or free air immediately after ESD, as proposed by Hanaoka et al[5].
The circumference of the lesion was marked with a needle knife. After injecting glycerol solution into the submucosa, an initial cut was made with a needle knife outside the marking. An IT Knife (Olympus Medical Systems, Tokyo, Japan) was inserted into this cut and operated to cut around the lesion[6]. The marked lesion was separated from the surrounding normal mucosa. Then, the submucosal layer was dissected using the IT Knife, and the lesion was finally removed. An IT Knife was used to perform ESD until the end of March 2007, and an IT Knife2 (Olympus Medical Systems) was used from April 2007 onward[7].
We have described our experience with 4 patients (0.25%) who underwent surgery for delayed perforation that developed after ESD. The clinicopathological features and clinical outcomes of the patients with delayed perforation are summarized in cases 1 to 4 of Table 1. Among the 4 patients, 1 lesion was resected in 3 patients, and 2 lesions were resected in the other patient. The lesions were located the lower third of the stomach in 3 patients and the upper third of the remnant stomach in 1 patient. The diameters of resected specimens were large, exceeding 50 mm in 3 of the 4 patients; the longest diameter was 102 mm. In 1 of these patients, the ulcer floor had fused together after two adjacent lesions had been resected, and the resected specimen was 80 mm in diameter. The procedure time was longer than 90 min in all 4 patients, and the longest time was 240 min.
| Case No. | Age | Sex | Location | Tumor size (mm) | Resected specimen size (mm) | Depth of tumor | Scar in tumor | Histological type | Time required for ESD (h) | Device | Time until peritonitis (h) | Size of perforation (mm) | Treatment of perforation | Hospital stay (d) |
| 1 | 89 | Male | L, Lc | 84 × 50 | 102 × 73 | SM1 | Absent | Diff. | 4 | IT | > 24 | 3 | Surgery | 23 |
| 2 | 74 | Male | L, Gc | 17 × 10, 20 × 17 | 80 × 45 (2 lesions) | M | Absent | Diff. | 2.4 | IT2 | 10 | - | Surgery | 15 |
| 3 | 63 | Male | R, P | 15 × 12 | 28 × 28 | M | Absent | Diff. | 1.5 | IT2 | 15 | - | Surgery | 30 |
| 4 | 83 | Female | L, Lc | 37 × 15 | 53 × 30 | SM2 | Present | Diff. | 2.5 | IT2 | 11 | 2 | Surgery | 23 |
| 5[4] | 50 | Female | U, Lc | 20 | 50 | M | Present | Diff. | 3.5 | IT2 | 24 | 20 | Surgery | 16 |
| 6[4] | 60 | Male | M, A | 18 | 32 | SM | Absent | Diff. | 2 | IT | 19 | - | Surgery | 14 |
| 7[4] | 70 | Male | U, A | 15 | 45 | M | Absent | Diff. | 3 | IT | 21 | - | Conservative | 15 |
| 8[4] | 61 | Male | U, P | 50 | 85 | SM | Absent | Diff. | 9 | IT | 15 | - | Surgery | 33 |
| 9[4] | 64 | Female | U, Lc | 12 | 50 | M | Absent | Diff. | 2.2 | IT | 23 | - | Surgery | 20 |
| 10[4] | 64 | Male | U, P | 15 | 45 | M | Present | Diff. | 1.5 | IT2 | 10 | - | Surgery | 12 |
| 11[13] | 70 | Female | R, Lc | 5 | 30 | M | Absent | Diff. | 2 | TT | > 24 | - | Conservative | 21 |
| 12[14] | 60 | Female | U, P | 4 | 19 | M | Absent | Signet | 1.1 | IT2 | > 24 | 2 | Endo clips | 12 |
| 13[8] | 70 | Female | L, Gc | 26 | 38 | M | Absent | Diff. | 0.5 | IT | - | 3 | Endo clips | 13 |
| 14[15] | 60 | Male | M, Gc | 6 × 4 | 18 × 17 | M | Absent | Diff. | 0.4 | - | 10 | 1 | Surgery | 10 |
| 15[16] | 64 | Male | L, A | 18 × 15 | 40 × 38 | SM2 | Present | Diff. | - | - | > 24 (49 d) | 8 | Surgery | - |
| 16[9] | 59 | Female | L, A | 10 | - | M | Present | Diff. | 0.4 | - | > 24 | 20 | Conservative | 33 |
All cases of delayed perforation occurring in our hospital developed within 24 h in all except 1 patient. Because all patients had peritonitis at the time of detection of delayed perforation, emergency surgery was required. However, none of the 4 patients died of delayed perforation.
The patient was an 89-year-old man with a superficial and depressed type (0-IIc) differentiated adenocarcinoma, 84 mm × 50 mm, arising in the posterior wall of the lesser curvature at the gastric angle. The tumor invaded the first layer of the submucosa (SM1). ESD was performed using an IT Knife, and the procedure time was 4.0 h. The resected specimen measured 102 mm × 73 mm (Table 1).
In the early morning 2 d after ESD, the patient had dyspnea and abdominal distension. Abdominal CT showed the presence of free air, and emergency surgery was performed on the same day. A perforation was found at the site resected by ESD. Omental implantation was performed at the site. Delayed perforation was apparently caused by the transfer of heat generated by extensive resection and prolonged local dissection to the muscular layer.
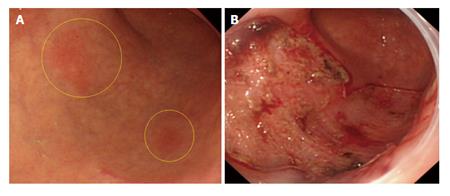
The patient was a 74-year-old man with 2 adjacent 0-IIc lesions (20 mm × 17 mm and 17 mm × 10 mm) arising in the anterior and posterior walls of the greater curvature at the gastric angle (Table 1 case No. 2). ESD was performed with the use of an IT Knife2 (Figure 1A). The time required for ESD was 2.4 h. The ulcers had fused together to form a single ulcer on the resected surface. The resected specimen measured 80 mm × 45 mm (Figure 1B) (Table 1).
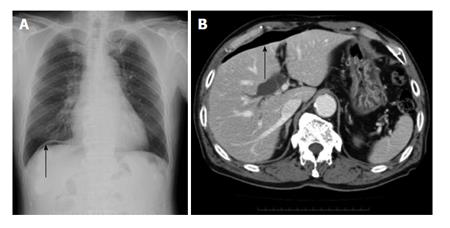
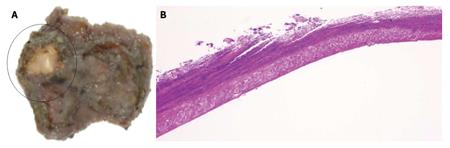
The patient had fever and abdominal pain at night on the day of ESD. Chest radiography and abdominal CT on the day after ESD showed the presence of free air, and emergency surgery was performed on the same day (Figure 2). Although there was no distinct evidence of perforation, the muscular layer had become thin. The site was therefore partially resected. Pathological examination showed no distinct signs of perforation. However, the muscular layer had become necrotic (Figure 3). Delayed perforation was most likely ascribed to the transmission of heat resulting from the extensive hemostatic procedure to the muscular layer.
The patient was a 63-year-old man who had previously undergone distal gastrectomy with Billroth I reconstruction for gastric cancer. A superficial and elevated type (0-IIa) lesion, measuring 15 mm × 12 mm, had arisen in the posterior wall of the greater curvature in the remnant stomach. ESD was performed using an IT Knife2. The time required for ESD was 1.5 h. The resected specimen measured 30 mm × 24 mm (Table 1).
The patient vomited during the night of the day of ESD. In the early morning of the next day, fever and abdominal pain developed. Abdominal CT showed free air, and emergency surgery was performed on the same day. A perforation was noted at the treatment site. Omental implantation and antecolic Roux-en-Y reconstruction were performed. Delayed perforation was apparently caused by direct exposure of the muscular layer of the remnant stomach to acid and bile after surgery.
The patient was an 83-year-old woman with a tumor, 37 mm × 15 mm, in the lesser curvature of the antrum. ESD was performed with an IT Knife2 for local recurrent lesions with an ulcer scar that had developed after ESD. The ESD procedure time was 2.5 h, and the resected specimen measured 53 mm × 30 mm.
Abdominal pain developed during the night of the day of ESD. On the following day, abdominal CT revealed the presence of free air, and emergency surgery was performed on the same day. A perforation was found at the site of treatment. Because the resected lesions were strongly suspected to invade the submucosa, distal gastrectomy with Billroth I reconstruction was performed. Delayed perforation was apparently attributed to the transmission of heat generated by the prolonged local dissection procedure, necessitated by the presence of an ulcer scar, to the muscular layer of the stomach (Table 1).
Perforation can be classified into 2 types according to the time of onset: Intraoperative perforation, which occurs during ESD, and delayed perforation, which is detected after treatment with no evidence of free air during ESD or on abdominal radiographs obtained immediately after surgery.
There are several possible causes of the delayed perforation that occurred in our hospital: (1) the transmission of heat generated by the prolonged local dissection procedure to the muscular layer of the stomach; (2) direct exposure of the muscular layer to acid and bile in the postoperative remnant stomach; and (3) ischemic changes of the mucosa caused by excessive hemostatic procedures. Patient 1 and patient 2 were treated when we had relatively little experience, shortly after the introduction of ESD. Delayed perforation in these patients was suggested to have been caused by the transmission of excessive heat caused by prolonged dissection to the muscular layer.
It is difficult to predict the risk of delayed perforation occurring after ESD because the incidence is low and unknown risk factors are most likely involved. Hanaoka et al[5] proposed that delayed perforation is most likely to occur at sites of lesions involving the lesser curvature of the stomach, which is anatomically susceptible to decreased blood flow. Two of the 4 patients in our study had lesions located in the lesser curvature of the stomach.
As for the treatment of perforations, most intraoperative perforations can be closed by clipping the perforation site and then be followed up conservatively[3]. In contrast, delayed perforations are already associated with peritonitis at the time of detection, and surgical intervention is generally required.
Table 1 summarizes the clinical and histopathological characteristics and the clinical courses of 16 patients (8 men and 8 women) with delayed perforation, including the 4 patients in the present study as well as those reported previously. The median age was 64 years (range, 50-89). Lesions were located in the upper third of the stomach in 6 patients, the middle third in 2 patients, the lower third in 6 patients, and the remnant stomach in 2 patients. Lesions were located along the lesser curvature in 4 patients. The median specimen diameter was 45 mm (range, 18-102). The depth of invasion was intramucosal in 11 patients and submucosal in 5. The median ESD procedure time was 2.0 h (range, 0.4-9.0). Delayed perforation most frequently occurred in patients with a long resected specimen diameter, a deep depth of invasion, and a prolonged ESD procedure time.
Delayed perforation was treated by surgery in 11 patients and conservative therapy including closure with an endoclip and follow-up in 5. The median hospital stay was 16 d (range, 10-33) in the patients who underwent surgery and 21 d (range, 15-33) in the patients who were followed up. The hospital stay thus tended to be longer in the conservatively treated patients. In previous studies, some patients with delayed perforation had minimal abdominal symptoms at the time of diagnosis. In other patients, a small perforation several millimeters in diameter was detected by chance on follow-up endoscopy performed the day after ESD. The perforation was closed by clipping. Patients with localized peritonitis who responded to conservative therapy have also been reported[4]. However, an intraperitoneal abscess developed in some patients who were followed up conservatively, and drainage was required. Long-term hospitalization was also necessary in some patients[8].
Increased intragastric pressure has been reported to reduce mucosal blood flow and cause ischemic changes[9,10]. Therefore, one of the solutions to prevent delayed perforation would be insertion of a nasogastric tube to achieve decompression of the gastric lumen.
Similar to our patients, delayed perforation may extensively involve the ulcer floor, and the muscular layer may already be necrotic. Closure of a perforation by endoscopic clipping may therefore be challenging. Moreover, insufflation at the time of endoscope insertion can increase the size of the perforation and thus have a negative effect. Even if the perforation site can be successfully closed by endoscopic clipping, re-perforation accompanied by the intraperitoneal leakage of gastric juice or bile has been reported in postoperative patients with a remnant stomach not surrounded by the greater omentum[3]. Therefore, if delayed perforation is diagnosed on the basis of postoperative abdominal findings and the presence of free air on plain radiographs, surgeons should immediately be consulted about the need for surgical intervention. Performing surgery before the exacerbation of peritonitis will also most likely contribute to a better postoperative course.
Delayed perforation is a serious complication of ESD for early gastric cancer[11,12]. A diagnosis of delayed perforation requires prompt action, including surgical intervention when required.
Among 1984 lesions of early gastric cancer treated in the authors’ hospital by endoscopic submucosal dissection (ESD) in 1588 patients from September 2002 through March 2015, delayed perforation developed in 4 patients.
Gastrointestinal perforation.
Chest radiography and abdominal computed tomography (CT) on the day after ESD showed the presence of free air. They diagnosis delayed perforation.
At the ulcer floor, the muscular layer was exposed, and all layers had become necrosis.
Chest radiography and abdominal CT on the day after ESD showed the presence of free air, and emergency surgery was performed on the same day.
Few studies have reported on delayed perforation, and its management remains controversial.
Delayed perforation is a serious complication of ESD for early gastric cancer. A diagnosis of delayed perforation requires prompt action, including surgical intervention when required.
The authors have reported good study for “Delayed perforation after endoscopic submucosal dissection for early gastric cancer” and have submitted a well-written manuscript.
P- Reviewer: Park WS S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 2. | Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63:602-605. [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Sekiguchi M, Suzuki H, Oda I, Yoshinaga S, Nonaka S, Saka M, Katai H, Taniguchi H, Kushima R, Saito Y. Dehiscence following successful endoscopic closure of gastric perforation during endoscopic submucosal dissection. World J Gastroenterol. 2012;18:4224-4227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Ikezawa K, Michida T, Iwahashi K, Maeda K, Naito M, Ito T, Katayama K. Delayed perforation occurring after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2012;15:111-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, Chatani R, Hanafusa M, Tsujii Y, Kanzaki H. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 314] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Ono H, Hasuike N, Inui T, Takizawa K, Ikehara H, Yamaguchi Y, Otake Y, Matsubayashi H. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer. 2008;11:47-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Sumie H, Rikitake Y, Matsuo T, Mukasa M, Yoshida H, Ushijima T, Kizaki J, Nagata S, Noda T, Maeyama Y. Successful conservative management of a case of panperitonitis and intra-abdominal abscess in a patient with delayed perforation after ESD for early gastric cancer. Jpn J Clin Exp Med. 2014;91:105-110. [Cited in This Article: ] |
| 9. | Stadaas J, Aune S, Haffner JF. Effects of proximal gastric vagotomy on intragastric pressure and adaptation in pigs. Scand J Gastroenterol. 1974;9:479-485. [PubMed] [Cited in This Article: ] |
| 10. | Saul SH, Dekker A, Watson CG. Acute gastric dilatation with infarction and perforation. Report of fatal outcome in patient with anorexia nervosa. Gut. 1981;22:978-983. [PubMed] [Cited in This Article: ] |
| 11. | Takizawa K, Hasuike N, Ikehara H, Inui T, Ono H. Management and prevention during endoscopic submucosal dissection (ESD). Endosc Dig. 2008;20:373-378. [Cited in This Article: ] |
| 12. | Onozato Y, Iizuka H, Sagawa T, Yoshimura S, Sakamoto I, Arai H, Ishihara H, Tomizawa N, Ogawa T, Takayama H. A case report of delayed perforation dne to endoscopic submucosal dissection (ESD) for early gastric cancer. Progr Dig Endosc. 2006;68:114-115. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Hirasawa T, Yamamoto Y, Okada K, Hayashi Y, Nego M, Kishihara T, Yshimoto K, Ishiyama A, Ueki N, Ogawa T. A case of the delayed perforation due to endoscopic submucosal dissection for the early gastric cancer of the residual stomach. Progr Dig Endosc. 2009;74:52-53. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Akamatsu M, Yokoyama N, Maeda C, Katayanagi N, Nagahama M, Nshimaki T. A Patient of Late Gastric Perforation Caused by Gastric Endoscopoc Submucosal Dessection Repaired with SILS Technique. J Japanese College Surg. 2012;37:951-954. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Kato K, Tominaga K, Nagami Y, Machida H, Okazaki H, Tanigawa W, Watanabe T, Fujiwara Y, Ohsawa M, Arakawa T. A Patient of Delayed Perforation of a Gastric Ulcer Induced by Endoscopic Submucosal Dissection for Early Gastric Cancer. Gastroenterol Endosc. 2011;53:3280-3285. [DOI] [Cited in This Article: ] |
| 16. | Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, Kida M, Oida M, Saigenji K. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |