Published online Oct 16, 2016. doi: 10.4253/wjge.v8.i18.674
Peer-review started: April 18, 2016
First decision: May 19, 2016
Revised: June 13, 2016
Accepted: August 11, 2016
Article in press: August 15, 2016
Published online: October 16, 2016
Processing time: 182 Days and 16.7 Hours
Plexiform angiomyxoid myofibroblastic tumor (PAMT) is a rare benign mesenchymal tumor of stomach. Rarity of this kind of tumors and scarce review articles may cause underrecognition of this entity and pose a real diagnostic challenge to gastroenterologists, pathologists and surgeons when encountering such patients and differentiating PAMT from other gastric intramural tumors. We report a case of 28-year-old woman, who presented with epigastric pain after meals, iron-deficiency anaemia and weight loss. Upper gastrointestinal endoscopy revealed submucosal tumor-like elevated lesion in the anterior wall of the antrum with intact overlying mucosa. Endoscopic ultrasound showed a 3-cm hypoechoic homogenous mass, originating from the third layer of the gastric wall. Endoscopic ultrasound-guided fine needle aspiration was not informative. Endoscopic buttonhole biopsy was performed to obtain specimens. Following this, the unexpected prolapse of the tumor occurred into the lumen of the stomach, causing gastric outlet obstruction - the biopsy was obtained. Pathomorphological features suggested the diagnosis of PAMT. Gastric resection of the Billroth I type was performed. Diagnosis was confirmed by histological analysis of the surgical specimen.
Core tip: Plexiform angiomyxoid myofibroblastic tumor is a rare benign mesenchymal tumor of stomach. Rarity of this kind of tumors and scarce review articles may cause underrecognition of this entity and pose a real diagnostic challenge, when differentiating between various intramural lesions. Clinical signs and symptoms are nonspecific or absent, radiological features often overlap, upper gastrointestinal endoscopy has a limited role because of intramural location. Endoscopic ultrasound yields opportunity to visualize and biopsy the tumor. Definite diagnosis requires histological and immunohistochemical analysis.
- Citation: Jonaitis L, Kiudelis M, Slepavicius P, Poskienė L, Kupcinskas L. Plexiform angiomyxoid myofibroblastic tumor of stomach: A rare case. World J Gastrointest Endosc 2016; 8(18): 674-678
- URL: https://www.wjgnet.com/1948-5190/full/v8/i18/674.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i18.674
Plexiform angiomyxoid myofibroblastic tumor (PAMT) is a benign mesenchymal tumor of stomach. To date, only 19 immunohistochemically confirmed cases have been reported in the literature. We report a case of 28-year-old woman with submucosal tumor in the anterior wall of the antrum. After repeated biopsies pathomorphological features of the specimen suggested the diagnosis of PAMT. Gastric resection of the Billroth I type was performed and diagnosis of PAMT was confirmed.
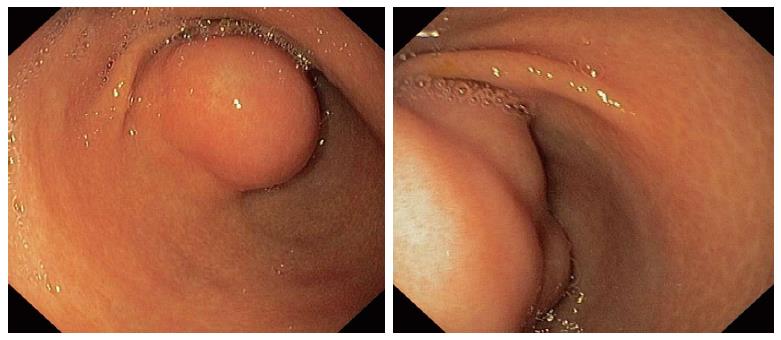
A 28-year-old previously healthy Caucasian female was ivestigated due to epigastric pain, associated with meals, iron-deficiency anaemia and lost 8 kg of weight during the preceding six months. Her previous medical history was unremarkable. Outpatient upper gastrointestinal endoscopy revealed submucosal tumor-like elevated lesion in the anterior wall of the antrum with intact overlying mucosa (Figure 1). Histology from that mucosa showed active chronic Helicobacter pylori-positive gastritis with reactive lymphoid hyperplasia.
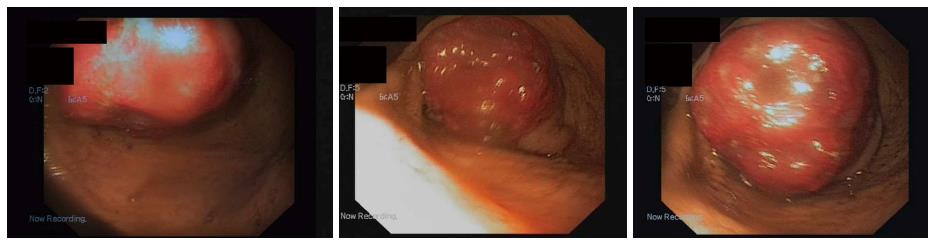
The endoscopic ultrasound was used to assess the tumor: It showed a 3-cm hypoechoic homogenous mass, originating from the third layer of the gastric wall. Endoscopic ultrasound-guided fine needle aspiration was performed to obtain specimens, but histopathological findings were not informative. Therefore endoscopic buttonhole biopsy was performed, but results were not informative again. After this procedure the patient was discharged home, but hospitalized again 7 d later due to vomiting, nausea and discomfort in the upper abdomen. The endoscopy revealed that submucosal mass protruded into the gastric lumen and caused gastric outlet obstruction (Figure 2). The biopsies were taken from protruded mass.
This time microscopic features suggested the diagnosis of PAMT. The partial gastrectomy of the Billroth I type has been performed.
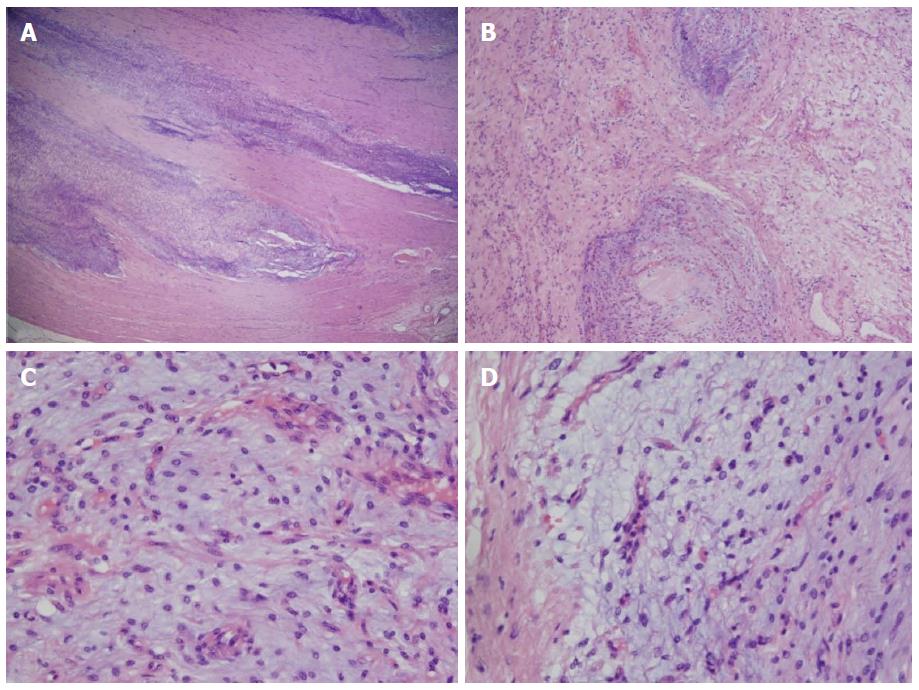
Histological examination of resected tumor confirmed the diagnosis: Microscopically, gastric wall showed involvement of submucosa and muscularis propria by a tumor comprising plexiform islands of monomorphic spindle cells accompanied by abundant myxoid stroma, that was rich in small vessels. The surface of tumor was ulcerated with hyperplastic changes found in adjacent mucosa. On immunohistochemical staining, the tumor cells were positive for smooth muscle actin and negative for desmin, CD34 and S100 protein. Mitoses were rarely seen (< 1/50 HPF). The vascular endothelial Ki-67 labeling index was approximately 40% (Figure 3).
Recovery after operation was complicated by gastroduodenal anastomositis, which was managed successfully with conservative measures.
We plan to make the follow-up investigations (upper gastrointestinal endoscopy and abdominal ultrasound) for the possible recurrence of tumor after 6 mo and then once a year.
PAMT also known as plexiform fibromyxoma of stomach, is an unique benign mesenchymal gastric tumor, originating within the muscularis propria[1-6]. To date, only 19 immunohistochemically confirmed cases have been reported in the medical literature[7-9]. According to the reported cases of PAMT, the estimated frequency of this gastric mesenchymal tumor is less than 1/150 compared with that of gastric gastrointestinal stromal tumor (GIST). The patients’ ages range from 7 to 75 years (mean, 43 years) and approximate male-to-female ratio is 1:1[10-13].
The representative signs and symptoms include abdominal pain and discomfort, nausea, vomiting, and weight loss (caused by pyloric obstruction), hematemesis and anemia (associated with upper gastrointestinal bleeding caused by ulceration), palpable abdominal mass. Cystic degeneration, fistulating abscess formation and perforation were also reported[1,2,12].
Endoscopically, PAMT appears as submucosal mass, which ranges from 1.9 cm to 15 cm (mean, 6.3 cm)[14]. Tumor is always located in gastric antrum (there are presumptions about the possible origin from cells specifically distributed at this location of muscularis propria layer) though it can extend to the pylorus and duodenal bulb[15]. The overlying mucosa is often ulcerated.
On computed tomography (CT) scan, the tumor appears relatively small (found in the gastric antrum). There is strong and heterogeneous internal enhancement effect. Small nodules show a strong enhancement effect in the rim[10]. The tumor also shows areas of low attenuation (because of the presence of myxoid tissue) and foci of vascularity. Magnetic resonance (MR) images demonstrate the myxoid stroma as T2-hyperintense lesion with persistent enhancement after administration of contrast material[3,5].
Histologically, PAMT is characterized by multinodular plexiform growth pattern (except for the extragastric tumor components). Tumor originates within the third layer of stomach wall - the muscularis propria - and may extend into the submucosa and mucosa, causing ulceration (furthermore, gastric content particles may impact into the fistulated tumor, resulting in abscess and pseudocyst formation). Bland-looking spindle tumor cells are separated by an abundant myxoid or fibromyxoid matrix, rich in capillaries. Nuclei of the tumor cells are oval or plump-shaped and cytoplasm is slightly eosinophilic. Nucleoli are small and inconspicious. Mitoses are rare (usually 0-4/50 HPF). The stroma is positive for Alcian blue stain, occasional collagenization may be observed. Immunohistochemical analysis of PAMT cells shows positivity for actin and vimentin, and negativity for CD34, S100P, KIT, DOG1, cytokeratin, neurofilament, epithelial membrane antigen, ALK. Tumor cells are mostly positive for α-smooth muscle actin (up to 80%), while positivity for desmin, CD10 and caldesmon is variable. Ki-67 usually demonstrates a very low proliferation index (1%-2%). Genetic studies show no mutations in KIT and PDGFRA[1,2,6,7,9,14].
Differential diagnoses of PAMT include GIST, which accounts for the majority of intramural gastric tumors, also inflammatory fibroid polyp, plexiform neurofibroma, myxoid leiomyoma, leiomyosarcoma, desmoid fibromatosis, gastric schwannoma, solitary fibrous tumor, inflammatory myofibroblastic tumor[4,8].
As mentioned before, differentiating between various intramural lesions may be difficult - clinical signs and symptoms are nonspecific or absent, radiological features often overlap, upper gastrointestinal endoscopy has a limited role because of intramural location. Endoscopic ultrasound yields opportunity to visualize and biopsy the tumor. Definite diagnosis requires histological and immunohistochemical analysis[11,16]. According to the described cases PAMT has good prognosis, no cases of local recurrence or metastasis had been reported.
As gastric PAMT is so rare in clinical practice, special attention is necessary to recognise this entity and avoid misdiagnosis. Although absence of confirmed recurrences or metastases suggests that PAMT of stomach is benign[12], larger number of cases must be reported and analysed in order to specify it’s clinical significance, outcome and prognosis.
A 28-year-old previously healthy Caucasian female presented with epigastric pain, associated with meals, iron-deficiency anaemia and loss of weight during the preceding six months.
Submucosal tumor-like elevated lesion in the anterior wall of the antrum.
Gastrointestinal stromal tumor, inflammatory fibroid polyp, plexiform neurofibroma, myxoid leiomyoma, leiomyosarcoma, desmoid fibromatosis, gastric schwannoma, solitary fibrous tumor, inflammatory myofibroblastic tumor.
Iron-deficiency anaemia.
The endoscopic ultrasound showed a 3-cm hypoechoic homogenous mass, originating from the third layer of the gastric wall.
Plexiform angiomyxoid myofibroblastic tumor (PAMT).
Partial gastrectomy of the Billroth I type.
PAMT also known as plexiform fibromyxoma of stomach, is an unique benign mesenchymal gastric tumor, originating within the muscularis propria. To date, only 19 immunohistochemically confirmed cases have been reported in the medical literature.
Differentiating between various intramural lesions may be difficult - clinical signs and symptoms are nonspecific or absent, radiological features often overlap, upper gastrointestinal endoscopy has a limited role because of intramural location. Definite diagnosis requires histological and immunohistochemical analysis.
The paper Plexiform angiomyxoid myofibroblastic tumor of stomach: A rare case is an interesting description of a rare condition of tumor. The case presented here shows that surgical intervention under is successful.
Manuscript source: Unsolicited manuscript
Specialty Type: Gastroenterology and hepatology
Country of Origin: Lithuania
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bae YK, Shen H, Tomazic A S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Takahashi Y, Suzuki M, Fukusato T. Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J Gastroenterol. 2010;16:2835-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Lee PW, Yau DT, Lau PP, Chan JK. Plexiform fibromyxoma (plexiform angiomyxoid myofibroblastic tumor) of stomach: an unusual presentation as a fistulating abscess. Int J Surg Pathol. 2014;22:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Kang HC, Menias CO, Gaballah AH, Shroff S, Taggart MW, Garg N, Elsayes KM. Beyond the GIST: mesenchymal tumors of the stomach. Radiographics. 2013;33:1673-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Stanford Medicine. Gastric Plexiform Fibromyxoma. Differential Diagnosis. Available from: http//surgpathcriteria.stanford.edu/gitumors/gastric-plexiform-fibromyxoma/differential-diagnosis.html. |
| 5. | Sakamoto K, Hirakawa M, Atsumi K, Mimori K, Shibata K, Tobo T, Yamamoto H, Honda H. A case of gastric plexiform fibromyxoma: radiological and pathological findings. Jpn J Radiol. 2014;32:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Patho l. 2009;33:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 7. | Wang FH, Chen ZR, Niu HL, Zeng RX, Xia JQ. Plexiform fibromyxoma of stomach: a distinctive benign tumor of gastric antrum. Zhonghua Binglixue Zazhi. 2012;41:190-191. [PubMed] |
| 8. | Sing Y, Subrayan S, Mqadi B, Ramdial PK, Reddy J, Moodley MS, Bux S. Gastric plexiform angiomyxoid myofibroblastic tumor. Pathol Int. 2010;60:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Lu B, Ye W, Liu H. A Rare Gastric Tumor in a Young Woman. Gastric Plexiform Angiomyxoid Myofibroblastic Tumor. Gastroenterology. 2015;149:294-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Ikemura M, Maeda E, Hatao F, Aikou S, Seto Y, Fukayama M. Plexiform angiomyxoid myofibroblastic tumor (PAMT) of the stomach. A case report focusing on its characteristic growth pattern. Int J Clin Exp Pathol. 2014;7:685-689. [PubMed] |
| 11. | Rau TT, Hartmann A, Dietmaier W, Schmitz J, Hohenberger W, Hofstaedter F, Katenkamp K. Plexiform angiomyxoid myofibroblastic tumour: differential diagnosis of gastrointestinal stromal tumour in the stomach. J Clin Pathol. 2008;61:1136-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Schulz T, Drgac J, Chmelar C, Höhler T, Agaimy A, Vieth M. Plexiform angiomyxoid myofibroblastic tumour of the stomach. Pathologe. 2012;33:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kim A, Bae YK, Shin HC, Choi JH. Plexiform angiomyxoid myofibroblastic tumor of the stomach: a case report. J Korean Med Sci. 2011;26:1508-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Li P, Yang S, Wang C, Li Y, Geng M. Presence of smooth muscle cell differentiation in plexiform angiomyxoid myofibroblastic tumor of the stomach: a case report. Int J Clin Exp Pathol. 2014;7:823-827. [PubMed] |
| 15. | Banerjee N, Gupta S, Dash S, Ghosh S. Plexiform angiomyxoid myofibroblastic tumour of the duodenum: a rare entity. BMJ Case Rep. 2015;2015:pii: bcr2015210004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Wang LM, Chetty R. Selected unusual tumors of the stomach: a review. Int J Surg Pathol. 2012;20:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |