Published online Jun 25, 2015. doi: 10.4253/wjge.v7.i7.688
Peer-review started: October 6, 2014
First decision: October 28, 2014
Revised: February 7, 2015
Accepted: March 18, 2015
Article in press: March 20, 2015
Published online: June 25, 2015
The prognosis of rectal cancer (RC) is strictly related to both T and N stage of the disease at the time of diagnosis. RC staging is crucial for choosing the best multimodal therapy: patients with high risk locally advanced RC (LARC) undergo surgery after neoadjuvant chemotherapy and radiotherapy (NAT); those with low risk LARC are operated on after a preoperative short-course radiation therapy; finally, surgery alone is recommended only for early RC. Several imaging methods are used for staging patients with RC: computerized tomography, magnetic resonance imaging, positron emission tomography, and endoscopic ultrasound (EUS). EUS is highly accurate for the loco-regional staging of RC, since it is capable to evaluate precisely the mural infiltration of the tumor (T), especially in early RC. On the other hand, EUS is less accurate in restaging RC after NAT and before surgery. Finally, EUS is indicated for follow-up of patients operated on for RC, where there is a need for the surveillance of the anastomosis. The aim of this review is to highlight the impact of EUS on the management of patients with RC, evaluating its role in both preoperative staging and follow-up of patients after surgery.
Core tip: In the era of tailored management of patients with rectal cancer (RC), endoscopic ultrasonography (EUS) has become crucial for the appropriate preoperative staging of these patients. This review highlights the impact of EUS on the management of patients with RC, evaluating its role in both preoperative staging of RC and follow-up of patients after surgery. Finally, possible new application are discussed, on the basis of the technologic innovation and the evolution of the therapeutic strategies.
- Citation: Marone P, Bellis M, D’Angelo V, Delrio P, Passananti V, Girolamo ED, Rossi GB, Rega D, Tracey MC, Tempesta AM. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J Gastrointest Endosc 2015; 7(7): 688-701
- URL: https://www.wjgnet.com/1948-5190/full/v7/i7/688.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i7.688
Every year approximately 40000 patents are diagnosed with rectal cancer (RC), and the incidence of RC in the European Union is 15-25/100000 per year, with an estimated mortality of 4-10/100000 per year[1]. The prognosis of RC is strictly related to both T and N stage of the disease at the time of diagnosis[2]. This is traditionally staged according to local invasion depth (T stage), lymph node involvement (N stage), and presence of distant metastases (M stage) (Table 1)[3,4]. Staging RC is crucial for choosing the best multimodal therapy (Table 2)[2]: patients with high risk locally advanced RC (LARC) undergo surgery after neoadjuvant chemotherapy plus radiotherapy (NAT); those with low risk LARC are operated on after a preoperative short-course radiation therapy. The latter is used as a valid alternative to NAT in elderly patients, or for patients unfit for preoperative chemotherapy because of severe comorbidities. Finally, surgery alone is recommended only for early RC. Total mesorectal excision (TME) is the standard surgical approach, with or without sphincter preservation. Extended abdomino-perineal resection is performed in distal RC which requires sphincter demolition. Local excision is performed in small T1 cancers with favorable histology by means of trans anal endoscopic microsurgery (TEM) or trans anal minimally invasive surgery. Local excision is also performed in selected patients showing complete clinical response after NAT. Therefore, precise staging of patients has a pivotal role for the selection of different therapeutic options and team work among the members of the multidisciplinary team is mandatory to improve patients outcome[2].
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: Intraepithelial or invasion of lamina propria |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades through the muscularis propria into pericolorectal tissues |
| T4a | Tumor penetrates to the surface of the visceral peritoneum |
| T4b | Tumor directly invades or is adherent to other organs or structures |
| Regional lymph nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional nodal metastasis |
| N1 | Metastasis in 1-3 regional lymph nodes |
| N1a | Metastasis in one regional lymph node |
| N1b | Metastasis in 2-3 regional lymph nodes |
| N1c | Tumor deposit(s) in the subserosa, mesentery, or non-peritonealized pericolic or perirectal tissues without regional nodal metastasis |
| N2 | Metastasis in 4 or more regional lymph nodes |
| N2a | Metastasis in 4-6 regional lymph nodes |
| N2b | Metastasis in 7 or more regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confined to one organ or site (i.e., liver, lung, ovary, non-regional node) |
| M1b | Metastases in more than one organ/site or the peritoneum |
| cT1 cT2 cN0 cCRM- | Surgery alone |
| Any cT cN+ cT2 cT3 cN0 cCRM+ | CRT |
| cT2 cT3 cN0 cCRM- | SCRT |
Several imaging methods are used for staging patients with RC: computerized tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) and endoscopic ultrasound (EUS)[1]. The latter has a high accuracy for loco-regional staging of RC, since it is capable to evaluate precisely the mural infiltration of the tumor (T), especially in the early RC. On the other hand, EUS is less accurate in restaging RC after NAT and before surgery. Recently, EUS has been used in clinical trials where patients have been selected for less invasive therapies: polypectomy for T1 RC; TEM for T1/T2-N0 cancers, and NAT + TEM for T2N0 tumors. Finally, EUS is indicated for following-up patients operated on for RC, where there is a need for surveillance of the colorectal anastomosis, which is at risk for local recurrences[2,5-7].
This review evaluates the role of EUS in the loco-regional staging of patients with RC, analyzing both accuracy and limits of this imaging method, which is part of the multidisciplinary approach for patients with RC. In particular, the aim of the review is to highlight the impact of EUS on the management of patients with RC, evaluating its role in both preoperative staging and follow-up after surgery. Finally, possible new applications are discussed on the basis of the technological innovation and the evolution of the therapeutic strategies[7-10] (Figure 1).
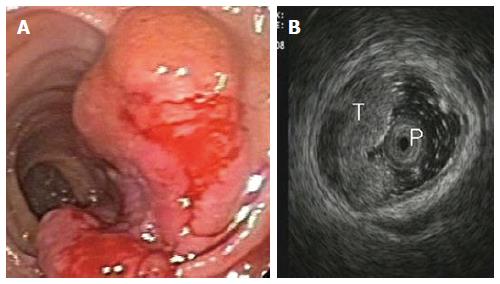
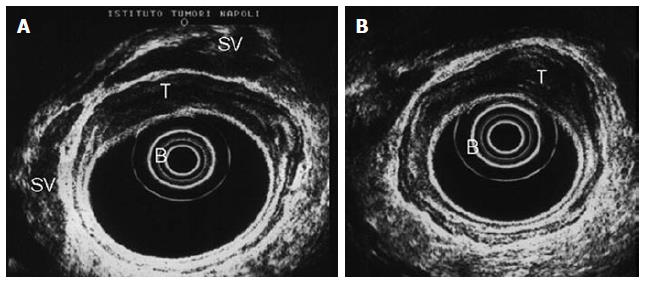
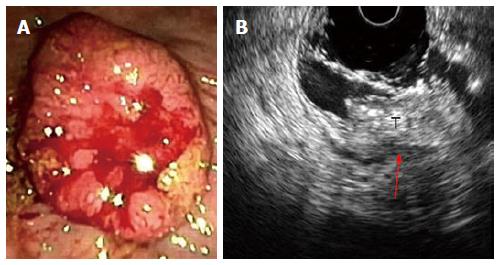
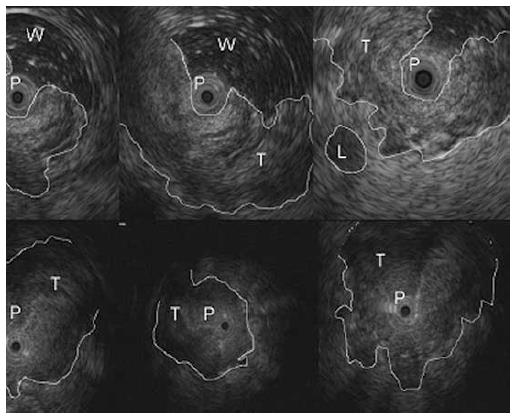
At the time of EUS, RC usually appear as a hypoechoic mass, with loss of the normal echo-layers of the wall, which is inhomogeneous and irregular because of the fusion of the layers infiltrated by the tumor[5,9-11]. According to the infiltration depth, there are four different echoendoscopic T stages (uT) (Table 3, Figures 1-5). In patients with RC, EUS assesses the tumor penetration depth into the rectal wall, with an overall accuracy for T stage of about 84%, ranging from 63% to 96%, while the reported accuracy of CT and MRI are 65%-75% and 75%-85%, respectively (Table 4)[12-45]. In a systematic review of 31 articles published over a period of 20 years, Skandarajah et al[46] reported that EUS has an overall accuracy of 82% for T stage and it is useful for discriminating early superficial RC. In another review of 42 studies, which analyzed the accuracy of EUS in patients with RC, confirmed by pathological exam of the surgical specimen, Puli et al[47,48] concluded that EUS has a sensitivity of 81%-96% and a specificity of 91%-98%, showing a higher sensitivity for LARC (95%), compared with early cancer (88%). In a multicenter, prospective, study conducted in 384 hospitals in Germany over a 8-year period, Marusch et al[49] analyzed the diagnostic accuracy of rectal EUS in the clinical staging of 7000 patients with RC who had not received NAT. This allowed uT vs pT comparison, which showed a uT-pT correspondence of 65%. The latter was related to the hospital volume, with uT-pT correspondence of 63% for hospitals undertaking ≤ 10 EUS/year, 65 % for those performing 11 - 30 EUS/year, and 73% for hospitals where more than 30 EUS/year were performed. Furthermore, the poorest uT-pT correspondence was found for T2 and T4 RC, with understaging occurring in 18 % of cases and overstaging in 17 % of patients[49]. These results were similar to those of a previous multicenter, prospective, study conducted by the same authors who reported that EUS had overall accuracy of 63% for T staging of RC. The diagnostic accuracy was 51% for pT1 RC, 58% for pT2 lesions, 73% for pT3 tumors, and 44% for pT4 cancers, with overstaging in 24% of cases and understaging in 13% of patients[33]. According to the results of both studies, EUS staging of RC in clinical practice does not have the same accuracy reported in the literature and the authors believe that EUS is a useful tool for guiding the therapeutic strategy of RC only when it is performed by experts[33,49]. Lower accuracy of EUS was also reported in a series of 545 patients with RC, where this method showed an overall accuracy of 69% for T stage and 64% for N stage[32]. A possible limitation of this study was the exclusion from the analysis of those patient who underwent NAT. This could have affected the accuracy of EUS for T stage, especially for T3 RC which is usually visualized the best at the time of EUS. Another pitfall of the study could be the different experience of the operators, which influenced the accuracy of EUS, as highlighted by Marusch et al[33,49]. Indeed, Kauer et al[34] observed that there is a high inter-observer variability (61%-77%), according to the experience of the operator. These authors reported that EUS has an overall accuracy of 69% for T staging of RC, with T3 tumors better (86%) staged and T4 cancer the least (36%) accurately classified. Differentiating T1 from T2 was difficult in this retrospective series, where overstaging (19%) was much more frequent than understaging (12%)[34].
| uT1 = tumor invasion limited to the mucosa and the submucosa; this is further divided into T1m, if the tumor infiltrates the mucosa, with normal muscolaris mucosa, and T1sm, when there is submucosal invasion (Figures 1 and 7) |
| uT2 = tumor infiltration of the muscolaris propria, with the tumor mass extended through the first 4 layers of the rectal wall. The outer layer corresponding to the muscolaris propria is smooth, meaning that the tumor is still limited to the rectal wall (Figure 2) |
| uT3 = tumor invasion of the perirectal fat, with an irregular 4th layer, which means that the tumor has spread outside the rectal wall (Figures 3 and 4) |
| uT4 = tumor infiltration of adjacent structures and organs, which are strictly connected to the rectal hypoechoic mass (Figure 5) |
| Ref. | Ptsno. | TStage | NStage | P/R | Tipe of EUS probe |
| Saitoh et al[13] | 88 | 90% | 75% | - | Flexible, radial, (7 MHz) Rigid, radial (5-7.5 MHz) |
| Feifel et al[14] | 79 | 89% | - | P | Rigid, linear (3-7 MHz) |
| Yamashita et al[15] | 122 | 78% | - | R | Rigid, linear (5.5-7 MHz) |
| Beynon et al[16] | 100 | 93% | 83% | - | Rigid |
| Rifkin et al[17] | 102 | 72% | 81% | Rigid, radial (7 MHz) | |
| Hildebrandt et al[18] | 113 | - | 78% | P | Rigid, radial (7 MHz) |
| Tio et al[19] | 91 | 88% | - | - | Rigid |
| Katsura et al[20] | 120 | 92% | - | Rigid, radial, (7 MHz) | |
| Glaser et al[21] | 154 | 86% | 81% | P | Rigid, radial (7 MHz) |
| Herzog et al[22] | 118 | 89% | 80% | P | Rigid, radial (7 MHz) |
| Cho et al[23] | 76 | 82% | 70% | P | Flexible, radial (7 MHz) |
| Thaler et al[24] | 36 | 88% | 80% | P | Rotating wall transducer IR 1510 AKTM (Kretz) (5, 7.5, 10 MHz) |
| Nielson et al[25] | 100 | 85% | - | - | Probe (7 MHz) |
| Sailer et al[26] | 160 | 77% | 83% | P | Rigid |
| Nishimori et al[27] | 70 | 76% | 69% | Flexible | |
| Norton et al[28] | 121 | 92% | 65% | P | Flexible, radial (7.5-12 MHz) |
| Kim et al[29] | 89 | 81% | 63% | Rotating transducer (7.5 MHz) | |
| Marone et al[30] | 63 | 81% | 70% | R | Flexible, radial (7.5-12 MHz) |
| Akasu et al[31] | 154 | 96% | 72% | R | Flexible, radial (7.5-12 MHz |
| Garcia-Aquilar et al[32] | 545 | 69% | 64% | P | Rigid, radial (7-10 MHz) |
| Harewood et al[12] | 80 | 91% | 82% | P | Flexible, radial (7.5-12 MHz) |
| Marusch et al[33] | 422 | 63% | - | P | Rigid |
| Kauer et al[34] | 458 | 69% | 68% | R | Probe (7.5-10 MHz ) |
| Vila et al[35] | 120 | 83% | 72% | P | Flexible, radial |
| Landman et al[36] | 938 | - | 70% | P | Probe (10 MHz) |
| Halefoglu et al[37] | 34 | 85% | 76% | P | Probe (7-10 MHz) |
| Lin et al[38] | 192 | 86% | 78% | P | Flexible, radial (7.5-12 MHz) |
| Fernández-Esparrach et al[39] | 90 | 95% | 65% | P | Flexible, radial (5-20 MHz) |
| Ünsal et al[40] | 31 | 80% | 70% | R | Radial |
| Zhu et al[41] | 110 | 91% | 85% | - | Rigid, radial (5-10 MHz) |
| 4976 | |||||
| Mean | 84 | 74 | |||
| Range | 63-96 | 63-85 |
Superficial RC limited to the mucosa can be resected endoscopically. Whenever a trans anal resection is planned, it is recommended to perform a preoperative EUS staging of the tumor, as suggested by Kneist et al[50]. These authors evaluated the accuracy of EUS in 552 patients undergoing trans anal excision of RC and they reported that EUS has a sensitivity of 95% and a positive predictive value of 93% in staging early RC[50]. Similarly, Glancy et al[51] demonstrated that EUS has an overall accuracy of 95% in staging early superficial RC suitable for local treatment. This high accuracy rate was confirmed by Zorcolo et al[52], who reported that EUS allows a precise distinction between early and advanced RC, with sensitivity of 96%, specificity of 85%, and overall accuracy of 94%. The latter is lower in our personal series, where we reported that EUS has an accuracy rate of 81% in differentiating early (T1) from advanced RC (T2), with the same occurrence of overstaging and understaging (9%)[30]. Finally, a recent meta-analysis analyzed the results of 11 studies, which discussed the efficacy of preoperative EUS in staging patients with early RC: the sensitivity of EUS in diagnosing T0 was 97%, with a specificity of 96%[48]. These data support the conclusion that EUS accurately diagnoses T0 RC, helping physicians to choose endoscopic treatment for patients with early RC.
Several studies have shown that EUS accuracy for T stage is strictly related to the depth of infiltration and the accuracy is lower for T2 stage than for early (T1) or advanced (T3-4) RC (Table 5)[31,30,38,39,41]. These assumptions are supported by the results of another meta-analysis which examined 42 studies, with a total number of 5039 patients: the pooled sensitivity and specificity of EUS for T1 stage was 88% and 98%, respectively; for T2 stage, EUS had pooled sensitivity and specificity of 80% and 96%, respectively; for T3 stage, the pooled sensitivity and specificity of EUS were 96% and 91%, respectively; finally, for T4 stage, EUS had pooled sensitivity of 95% and specificity of 98%, respectively. The authors of this meta-analysis concluded that EUS should be the imaging method of choice for T staging of RC[47]. Despite the high accuracy that EUS has for T stage, this imaging method is not capable of differentiating peri-tumoral inflammation and edema from neoplastic infiltration. One of the mayor limits of EUS, is overstaging T2-T3 RC, with the risk of overtreatment[30,32,53-59]. In T3 stage cancer infiltrates the rectal wall up to the perirectal fat, with different penetration depth. The precise evaluation of the infiltration depth into the perirectal fat is an important prognostic factor for T3 RC. Harewood et al[56] demonstrated that T3 RC are not all equal, with minimally invasive disease carrying a more favorable prognosis. In a series of 42 patients with T3 RC, who underwent surgery without receiving NAT, EUS overstaged the minimally invasive (invasion < 2 mm beyond muscolaris propria at EUS) T3 cancer in 50% of cases, in comparison with advanced (invasion > 2 mm beyond muscolaris propria at EUS) T3 RC. These were overstaged only in 4% of cases. The reported EUS accuracy for differentiating T1/T2 and T3/T4 was 88%, with an overall accuracy of 76% for T stage and 63% for N stage. Since the overstaging rate of minimally invasive T3 RC was high, the authors recommend to exclude these patients from NAT, which should be used only for patients with advanced T3 RC[56]. These data highlight the importance of proper measurement of the infiltration depth of RC at EUS, because this information is crucial for establishing the prognosis and guiding the multimodal therapy. According to Esclapez et al[57], an ultrasonographic maximum tumor thickness cutoff point of 19 mm could be useful to classify patients preoperatively and select them for primary surgery or NAT. Indeed, these authors showed that tumor thickness of more than 19 mm in uT3 RC was associated with a higher rate of postoperative recurrence[57].
In approximately 14% of RC there is a stricture that cannot be traversed by the echoendoscope, leading to inaccurate staging and potential errors because EUS evaluates only the distal portion of the cancer[5,50,51]. The presence of a stricture is a limitation for staging RC at EUS: this determines not only inaccurate T staging, but also incomplete N staging because perirectal lymph nodes cannot be examined. Moreover, a stricture often does not permit perpendicular position of the ultrasonographic beam and an adequate focal distance of the probe from the tumor leading to misstaging. All these pitfalls can lead to an incorrect staging of the tumor, which can then affect the therapeutic strategy[5]. Marone et al[60] reported that EUS has an overall accuracy of 83% in a series of 127 patients with RC, who underwent surgery without receiving NAT. When the T stages were analyzed separately, EUS showed an accuracy of 76% for T1, 72% for T2, 91% for T3 and 67% for T4 stages. Overall, EUS misstaged T in 16% of cases, with 11% of overstaging and 5% of understaging errors. The presence of a stricture lowered the accuracy rate of EUS for T stage from 93% to 56%; similarly the distance of RC from the anal verge affected the accuracy of EUS for T stage, which decreased from 92% for tumors located > 5 cm from the anal verge to 67% for cancer sites < 5 cm from the anus[60]. Therefore, the presence of a stricture and tumor distance of less than 5 cm from the anal verge are two factors limiting the accuracy of EUS in staging RC.
Recently, the capability of EUS in assessing MRF and predicting the circumferential resection margin (CRM) of RC has been evaluated by Granero-Castro et al[61]. In a series of 76 patients with mid-low RC, preoperative staging was performed by means of both MRI and EUS and the patients underwent surgery without receiving NAT. A comparison between preoperative (EUS and MRI) CRM status and pathologic examination after TME surgery was eventually made: overall accuracy of EUS and MRI in assessing CRM status was 84% and 92%, respectively, with similar negative predictive values (97%). When focusing on low RC, the overall accuracy of EUS increased to 87%, whereas MRI lowered its accuracy rate to 87%, with a negative predictive value of 96% for both imaging methods. These data suggest that EUS should be used together with MRI for predicting CRM involvement in low anterior RC.
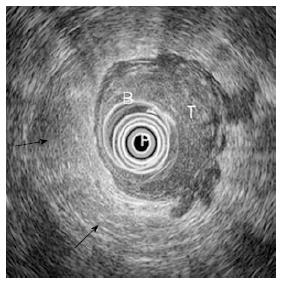
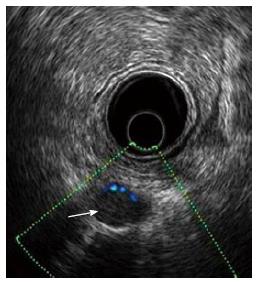
EUS allows the assessment of perirectal lymph nodes for metastatic infiltration: these are metastatic when they appear as roundish or oval, homogeneous echo-poor nodules with a short axis of at least 5 mm (Figure 6)[5,7,9,10]. According to the number of metastatic lymph nodes, there are two different N (uN) echoendoscopic stages (Table 6).
| uN1 = 1-3 positive nodes |
| uN2 = More than 4 metastatic lymph nodes |
The incidence of malignant metastatic lymph nodes in patients with RC is strictly related to T stage and varies from 6%-11% for T1, 10%-35% for T2 and 26%-65% for T4 RC[3,5,7,8]. Determination of lymph nodes involvement during EUS is difficult and less precise, with a variable accuracy of 63%-85% (Table 4)[12-45]. Kauer et al[34] reported that EUS has an overall accuracy of 68% in diagnosing metastatic lymph nodes associated to RC, with a sensitivity of 52% and a specificity of 82%. A recent meta-analysis of 35 published studies evaluated the accuracy of EUS in diagnosing metastatic lymph nodes of patients with RC[7]. EUS showed sensitivity of 73% and specificity of 76% for N staging and the data analyzed supported the hypothesis that EUS is more accurate in excluding nodal invasion, rather than diagnosing it. Indeed, determination of nodal invasion is less accurate because of difficulty in discriminating between inflammatory and metastatic nodes, which leads to false positive diagnosis and possible overtreatment. The size of lymph nodes could be indicative of neoplastic invasion: nodes greater than 5 mm can be metastatic in 50%-70% of cases, whereas those smaller than 4 mm harbor malignancy in less than 20% of cases[16]. These data have been partly confirmed by Akasu et al[59], which observed that the incidence of nodal metastases is strictly related to the size of the lymph node in patients with RC: 9.5% for nodes less than 2 mm; 47% when the lymph node measures 3-5 mm and 87% for nodes larger than 6 mm. However, despite this correlation between size of the node and incidence of metastatic invasion, there are several reports of metastatic lymph nodes smaller than 5 mm in patients with RC, with an overall incidence of 18%[20,62-64]. There is a clear correlation between T stage of RC and risk of metastatic invasion of perirectal lymph nodes. The more advanced the RC, the higher the risk of metastatic lymph nodes: less than 5% with T1m and more than 80% with T3 RC[65,66]. The latter results were confirmed by Landmann et al[36], who reported that the accuracy of EUS for N staging decreases from 84% in pT3 RC to 48% in pT1 cancers. The low detection rate of metastatic lymph nodes in T1 RC is probably explained by the fact that in these cancers possible metastatic nodes are small, with a size variable from 0.3 to 3.3 mm. Therefore, EUS can misstage early RC where the presence of neoplastic invasion is possible even in small lymph nodes: this exposes a patient who undergoes local excision to pelvic recurrence because of misstaged early cancer. To avoid this, it was proposed to decrease the dimensional cut off of 5 mm to 3 mm, with increased sensitivity, but reduced specifity and overall accuracy for N staging at EUS. Indeed, with a 5 mm cut off, EUS has an overall accuracy of 89% for N stage in T1 RC, with sensitivity of 39% and specificity of 89%. On the other hand, reducing the cut off to 3 mm, EUS shows an increased (75%) sensitivity for N stage in T1 RC, with significantly reduced specificity (49%) and overall accuracy (53%)[31,36,45]. These data confirm that the size of the lymph node cannot be the only parameter to be used for assessing neoplastic nodal invasion in patients with RC[36,45,59,67].
EUS accuracy for N staging can be ameliorated associating other parameters to the dimensional criterion used for defining malignant lymph nodes. These ultrasound features include lymph node short axis size, echogenicity, shape, and border. Among them, those which better correlate with malignancy are: enlarged node (≥ 1 cm in short axis), hypoechoic appearance, round shape, and smooth border[11]. The presence of two or more features is associated with EUS sensitivity of 77%, specificity of 29%, and accuracy of 54%. Three or more features give EUS a sensitivity of 68%, and a specificity of 52%, with an accuracy of 61%. Finally, with four or more features EUS shows sensitivity of 23%, specificity of 100% and accuracy of 61%. Simultaneous presence of all these features in a lymph node is related to 100% of positive predictive value, but this is a rare occurrence (less than 25% of cases)[65]. Despite all the efforts to find the right criteria, determination of lymph nodes involvement during EUS is less accurate and useful than T staging. The most important limitation is the difficulty in both discriminating between inflammatory and metastatic lymph nodes and recognizing small metastatic nodes. These limitations can be overcome by EUS-guided FNA, which allow sampling of the suspicious perirectal nodes, leading to correct N staging. However, even with EUS-guided FNA, the overall accuracy of EUS for N stage remains low, because distant metastatic lymph nodes are undetectable by EUS, since they are out of the scanning area. Indeed, incomplete evaluation of the iliac nodes is the most frequent cause of incorrect staging of patients with RC, leading to mistreatment in 6% of cases[16,45,62,63,66-70]. Recently, Kim et al[64] suggested that tridimensional EUS could obviate the low accuracy of EUS for N staging. However, these results need to be confirmed.
Sometimes, EUS staging of RC can be incorrect and the cancer is misstaged because of overstaging rather than understaging. At EUS, hypoechoic fibrosis and/or inflammation cannot be differentiated by the hypoechoic mass of the tumor leading to overstaging. On the other hand, understaging occurs when the microscopic neoplastic invasion into the next layer is undetectable during EUS, especially when an entire layer is distended by the invading tumor which abuts into the adjacent layer, without showing clear infiltration. Moreover, a stricture which cannot be traversed limits the accuracy of EUS, while location, shape and size of the tumor can alter the direction of scanning and result in overstaging. Similarly, the T stage can influence the results of EUS staging, as in the case of T2 cancer for which EUS staging is less accurate. Finally, EUS is operator dependent and there is a substantial difference in accuracy between novice and experienced endoscopists, since the latter have learned over the time how to avoid technical problems, like oblique scans, overfilling of the balloon and inadequate water filling of the rectum[5].
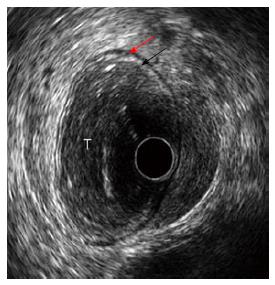
Dedicated echoendoscopes have some limitations due to the fact that combining endoscopy and ultrasonography in one instrument increases the diameter of such scopes (12-13 mm). Because of the large diameter, complete passage of severe strictures is often impossible. Furthermore, conventional EUS often requires a second examination, separate from the previous routine endoscopy. The miniature ultrasonic probes (diameters about 2 mm; frequencies 12-20-30 MHz) can be passed through the working channel of standard endoscopes to provide high frequency ultrasound images (Figures 1, 5 and 7). These miniprobes allow simultaneous endoscopic and ultrasonographic evaluation of the lesions, complete assessment of strictures that cannot be traversed by conventional echoendoscopes and accurate staging of superficial lesions[8]. The rarity of lymph node metastases in T1m or T1sm 1 RC supports the indication for endoscopic resection of these lesions, which require accurate preoperative staging. This has been performed by Harada et al[71], using a 15-MHz ultrasound miniprobe in 35 patients with submucosal invasive colorectal cancer. The accuracy of miniprobes was low (37%) in categorizing the different depth of submucosal invasion, while it was high (86%) in differentiating between mucosal/superficial submucosal infiltration (M and SM) and deep submucosal invasion (SM2, SM3, MP, and S)[71]. These data support the indication for ultrasonographic staging of early colorectal via miniprobes in order to plan endoscopic resection. In a prospective study of 131 consecutive patients with adenocarcinoma or broad-based polyps of colorectum, EUS accuracy for T staging with miniprobes was 96%, with 4% of overstaging and 2% of understaging[72]. The overall accuracy of N staging using miniature ultrasonographic probes was 87% (sensitivity 95%, specificity 71%, positive predictive value 87%, negative predictive value 88%). These data confirm that miniprobe ultrasonography has a high overall accuracy for both T and N staging of colorectal cancer and it may be useful for selecting patients fit for local resection. Finally, Gall et al[73] conducted a meta-analysis of ten studies with a total of 642 patients to evaluate the accuracy of miniprobe EUS in staging RC. The pooled sensitivity and specificity were respectively 91% and 98% for T1 cancers, 78% and 94% for T2 tumors, 97% and 90% for T3/T4 RC. Eight percent of T1/T2 cancers were upstaged to T3/T4 tumors and 5% of T3/T4 RC were downstaged. Finally, the pooled sensitivity and specificity for N staging were 63% and 82%, respectively. These data confirm that miniprobe EUS is highly effective for clinical staging of RC, allowing identification of those patients who may be suitable for nonsurgical treatments.
According to a recent study, EUS-FNA is useful for assessing primary and metastatic rectal cancers. In this setting, EUS-FNA had sensitivity, specificity, positive and negative predictive values of 89%, 79%, 89% and 79% respectively. This technique improves staging of suspected nodal or distant metastases, but it is indicated only when cytologic results will change the therapeutic strategy[74,75]. This is the conclusion of Harewood et al[12], who reported that standard EUS modified therapeutic strategy of LARC in 25 patients, while only in 1 case EUS-guided FNA was crucial for choosing the correct therapy. According to Shami et al[76], EUS-guided FNA has a clinical impact of 19% on staging and subsequent management of patients with RC. In this cancer the incidence of lymph node metastases is strictly related to T stage, with a higher risk of nodal metastasis with more advanced T stages. Peritumoral lymph nodes are highly predictive of cancer invasion: the majority of perirectal nodes detected by EUS are metastatic in patients with RC. This is the explanation for the low clinical impact of EUS-guided FNA in staging patients with RC. Moreover, T3 RC is an indication for NAT, independently from N stage, which has no influence on the therapeutic strategy of patients with LARC[12,75]. EUS-guided FNA seems to offer the most potential for the management of T1-2 stage disease, where the presence of metastatic perirectal lymph nodes modifies the therapeutic strategy. Therefore, its use should be confined to this subgroup of patients[12,67,75,77]. This indication is confirmed by Levy and colleagues who evaluated the role of EUS guided FNA in N staging of 32 patients with RC and suspicious iliac lymph nodes[70]. In approximately 50% of cases, the sampled nodes were positive for neoplastic invasion and determined a change in the therapeutic strategy. Of note, CT scan did not detect half of the lymph nodes which were malignant at EUS–guided FNA. These data support the need to properly investigate the iliac lymph nodes during staging of patients with RC.
In RC, EUS has been compared with digital examination, CT scan and MRI. EUS is superior to rectal digit examination, showing a higher accuracy (91%-92% vs 52%-60%). CT scan is unable to correctly define the single layers of the rectal wall and therefore is not indicated for T staging of RC, while it is crucial for diagnosing distant metastases[77]. EUS is more accurate than CT scan in loco-regional staging of RC, showing an accuracy rate of 87% for T stage and 62% for N stage, compared to that of CT scan (76% for T stage and 62% for N stage)[6,63,77]. Similarly, EUS was considered more precise (85% vs 77%) than MRI in determining the T stage of RC[77]. However, recent technology has allowed MRI to define the status of MRF and subsequently delineate the possible threatened CRM, making this imaging method crucial for loco-regional staging of RC[44]. A systematic review of 31 articles published over a 20-year period evaluated the role EUS and MRI in loco-regional staging of RC[46]. While EUS is more useful for staging early RC, with an overall accuracy of 82%, MRI is indicated for staging advanced disease, providing a better definition of both the mesorectum and the MRF. The latter is crucial for choosing the best therapeutic strategy. In another systematic review, Kwok et al[78] evaluated the role of CT scan, EUS and MRI in the preoperative staging of RC. In determining T stage, EUS was more accurate than CT scan and MRI. The latter, with the adjunct of an endorectal coil, has the same accuracy of EUS for T stage, while it is more precise in determining nodal metastases. Both EUS and MRI with an endorectal coil are limited by the presence of strictures when staging RC. An MRI with a pelvic phased-array coil is not invasive, has a high spatial resolution and appears to be a promising image method for loco-regional staging of RC[37,79]. Yimei et al[80] evaluated the reference value to surgeons of both EUS and MRI, reporting that EUS has higher sensitivity (P = 0.044) and specificity (P = 0.039) than MRI, showing elevated accuracy for early stage RC. This makes EUS staging crucial for the identification of those patients who are suitable for less invasive surgery. On the other hand, MRI is useful for the proper diagnosis of LARC which need to undergo multimodal treatment. MRI has been preferred to EUS because it is better tolerated, can be used in stenotic tumors and it can define the infiltration depth of MRF and assess the CRM. The latter is a crucial information for choosing the best therapeutic strategy. However, Cesmeli et al[81] point out that EUS is still important in the preoperative staging of early RC, because of its ability to delineate the different layer of the rectal wall, allowing the selection of those patients suitable for local excision. EUS can also improve N staging by performing FNA, whenever N stage can change the therapeutic strategy[81]. In a series of 49 patients, EUS and MRI showed similar accuracy (88%), in predicting pathologic CRM of low RC[82]. Therefore, EUS and MRI are complementary and should be both used for preoperative staging of patients with RC. The fact that staging accuracy is improved by combination of MRI and EUS is supported by the results of a recent study in which the authors compared feasibility and accuracy of both 1.5 Tesla MRI and three-dimensional (3D) EUS for staging patients with RC before and after preoperative chemotherapy[83]. The stage accuracy by MRI, 3D-EUS and the combination of MRI and 3D-EUS was 65%, 70% and 74%, respectively, before chemotherapy and 65%, 78% and 83%, respectively, after chemotherapy. The post chemotherapy staging by MRI alone was improved by a combination of MRI assessment of the lymph nodes and 3D-EUS assessment of the perirectal tissue penetration (P= 0.046). These results confirmed that staging accuracy is improved by combining MRI with EUS.
According to the data of the literature, EUS and MRI are superior for T- staging, while CT and PET/CT are the main stay for metastatic work-up. EUS is superior in staging early cancers and defining the infiltration of the anal sphincter, while MRI is excellent for staging T4 and clarifying both the MRF status and the infiltration of the elevator muscle; CT and EUS are complementary, rather than competitive in loco-regional and distant staging of RC[84-86]. Therefore, the best approach for RC is the combination of all different imaging methods, which are complementary: they should be utilized according to the clinical condition of the patient, the availability of each single test and the personal preference. Cost-benefit studies have demonstrated that the most cost-effective association of imaging methods is EUS plus CT scan[87].
Loco-regional staging of RC after NAT is affected by local effects of the treatment which determines peritumoral inflammation, edema, necrosis, and fibrosis of the neoplastic tissue. This reduces the accuracy of EUS, leading to overstaging errors (Table 7)[88-90]. EUS staging of RC after NAT is inaccurate, as shown by Vanagunas and colleagues in a series of 82 patients with LARC[90]. After NAT, EUS correctly predicted complete response to chemoradiation in only 63% of cases and its overall accuracy for pathologic T-stage was 48%, with 14% of understaging and 38% of overstaging. These data suggest that EUS staging of RC after NAT is inaccurate, and its routine use for restaging patients should be discouraged. Similary, Marone et al[91] and Maor et al[92], demonstrated that EUS restaging of LARC after NAT has low accuracy. Both studies compared two groups of patients with LARC: one operated on without receiving NAT and another one who underwent surgery after NAT. The results of the studies were similar, showing that EUS restaging of LARC after NAT has low accuracy (60%-70%) and is able to predict a complete response in only 50% of cases. Further confirmation of this low accuracy came from a study where the authors compared sensitivity and specificity of EUS and MRI, in patients with LARC after NAT[93]. Both EUS and MRI had low accuracy (46% vs 44%) for T stage of LARC after NAT. Better accuracy of EUS restaging was reported by Radovanovic and colleagues who demonstrated that EUS has an accuracy of 75% for T stage after NAT, with 18% of overstaging and 7% of understaging[94]. The majority of overstaging occurred in patients with uT3 tumors, eventually found to have pT0-pT2 RC. EUS was able to correctly stage only one of the patients who had complete response after NAT. Despite the fact that EUS restaging accuracy for LARC was higher, the results of this study confirm that EUS is not useful after NAT.
After surgery, local recurrence of LARC has an incidence of about 25%, which decreases to 10%, if NAT has been administered before surgery (10%). The risk of local recurrence is strictly related to T stage and it is higher for more advanced T stages, occurring mostly in the first two postoperative years[95-97]. Early identification of local recurrence and its immediate treatment could potentially improve patients survival. EUS has a high sensitivity, but low specificity in defining local recurrences. A limitation of EUS is its inability to clearly differentiate postoperative changes and benign lesions from cancer recurrence[95-97].
EUS-guided FNA increases the specificity of EUS (57% vs 97%). To date, there are no guidelines which define the role of EUS in the follow-up of patients operated on for RC, since there are no clear data that echoendoscopic follow-up and/or EUS-guided FNA influence patients survival after surgery for RC[95-97].
The recent development of new technology for EUS generates novel applications for echoendoscopic diagnosis and staging of gastrointestinal tumors. Tridimensional EUS (3D-EUS) seems to improve the spatial visualization of RC allowing better evaluation of tumor resectability[98]. 3D-EUS is more accurate than 2D-EUS and CT scan in T staging of RC, for which the three imaging methods have an accuracy of 78%, 69% and 57%, respectively[64]. 3D-EUS visualization of the outer margin of the rectal wall is well related to neoplastic infiltration and metastatic nodal invasion diagnosed by pathological examination of the surgical specimen. Some data suggest that 3D-EUS allows correct visualization of MRF, which was not well delineated by 2D-EUS. Proper measurements of the tumoral area before and after NAT could be a useful criterion for evaluating the response of RC to NAT[98-100].
Elastography is a new technique which has been recently added to the armamentarium of EUS and allows measurement of tissue elasticity useful to differentiate normal from tumoral tissue. Preliminary data have shown that simultaneous elastography during EUS improves its accuracy for T staging of RC[98]. Finally, EUS with contrast medium administration (contrast harmonic EUS or CH-EUS) and simultaneous Doppler visualization allows the study of tumoral vascularization and irroration. These data are useful for the evaluation of both tumoral response to NAT and efficacy of anti-angiogenic treatments, because this combination of techniques shows accurately those changes in the vascular pattern of RC which reflect its response to therapy. Miyata et al[101] evaluated the micro-vascularization of lymph nodes by means of CH-EUS in order to differentiate benign from malignant nodes: sensitivity, specificity, and accuracy of CH-EUS for malignant lesions were 95%, 97%, and 97%, respectively. These data show that CH-EUS is accurate in detecting minimal changes of tumoral vascularization in lymph nodes which harbor neoplastic invasion. This information could address the correct use of FNA-guided EUS, whenever it is needed[101,102]. To date, there are still little data on the clinical application of simultaneous use of these new methods together with standard EUS. Therefore, further clinical trials are needed for the evaluation of indication, accuracy, clinical impact and limitation of CH-EUS and Doppler-EUS.
Prognosis of patients with RC is strictly dependent from the stage of the disease at the time of diagnosis. Multidisciplinary approach to patients with RC is the standard of care in order to reduce local recurrences and improve survival outcomes. A strong cooperation among members of a multidisciplinary team is mandatory to improve patients outcomes, because the latter are strictly dependent from the chosen therapeutic strategy. This is the results of an accurate loco-regional staging, especially if metastatic disease has been excluded. CT scan, MRI, PET are the imaging method used for staging RC and give information on both loco-regional and distant disease. In the last decades, EUS has been used in combination with these imaging methods for staging RC in order to better define both the T stage and the involvement of loco-regional lymph nodes. EUS has significant clinical impact on patients with RC, allowing to identify those who are candidate for local excision and/or direct surgery, without receiving NAT. LARC is well defined by EUS, even if the identification of both MRF and possible threatened CRM is more precisely obtained by MRI. The latter lacks accuracy for mid - low anterior RC, which could be better staged by EUS, as recent data suggested. Therefore, EUS and MRI are complementary and they should be used simultaneously, with a significant increase of the overall accuracy for the T stage of RC. EUS is superior in identifying early cancers and infiltration of anal sphincter, while MRI is excellent in recognizing T4, in relationship to MRF infiltration of the elevator muscle. While EUS and MRI are superior for T- staging, CT and PET/CT are the main stay for metastatic work-up.
Restaging after NAT is mandatory for establishing a correct prognosis of patients with RC and choosing the most effective treatment. This should be tailored according to the results of NAT, whose experimental drugs can be tested in clinical trials and evaluated by means of restaging RC. The latter is not performed by means of EUS because this imaging method has low accuracy in restaging RC, due to the difficulty in differentiating inflammation and tissue fibrosis from actual residual cancer.
EUS has low sensitivity, but high specificity in diagnosing local recurrences in patients operated on for RC, because it is unable to differentiate perianastomotic surgical changes from recurrent cancer. In this case, EUS-guided FNA increases specificity, but its use in clinical practice has not been standardized. Probably, high resolution images and guided FNA are the best combination for improving EUS accuracy in naive and recurrent RC.
Technological improvements, like elastography, contrast medium administration, high ultrasonographic frequencies and 3D, will certainly improve EUS accuracy and broaden its clinic use; however there is a need for further studies which should confirm the potential of these new technologies.
In conclusion, accurate EUS staging is crucial for the best treatment of each single patient with RC and especially LARC, because patients can be understaged or overstaged, with subsequent mistreatments.
P- Reviewer: Gurkan A, Mentes O, M'Koma AE, Sieg A S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Glimelius B, Påhlman L, Cervantes A. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v82-v86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Avallone A, Aloj L, Delrio P, Pecori B, Leone A, Tatangelo F, Perri F, Petrillo A, Scott N, Budillon A. Multidisciplinary approach to rectal cancer: are we ready for selective treatment strategies? Anticancer Agents Med Chem. 2013;13:852-860. [PubMed] [Cited in This Article: ] |
| 3. | Greene FL, Page DL, Fleming ID, editors . AJCC Cancer Staging Manual. NewYork, NY: Springer 2010; . [Cited in This Article: ] |
| 4. | Sobin LH, Wittekind C, editors . TNM: Classification of Malignant Tumours. NewYork, NY: Wiley-Liss 2002; . [Cited in This Article: ] |
| 5. | Marone P. Ecoendoscopia: I tumori del retto: stadiazione con US endorettale, valutazione dopo radio-chemioterapia neoadiuvante, identificazione della recidiva. In Catalano O, Siani S eds. Ecografia in oncologia: testo atlante di ultrasonografia diagnostica ed interventistica dei tumori. Springer Italia. 2007;313-318. [Cited in This Article: ] |
| 6. | Rösch T. Endosonography of the colon and rectum. Philadelphia: W.B Saunders Company 1999; 271-277. [Cited in This Article: ] |
| 7. | Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Menzel J, Domschke W. Gastrointestinal miniprobe sonography: the current status. Am J Gastroenterol. 2000;95:605-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Thomas J, Savides S. Endoscopic Ultrasound Staging of Rectal Cancer. In Van Dam J and Sivak M eds, Gastrointestinal Endosonography, Philadelphia: W.B Saunders 1999; 279-289. [Cited in This Article: ] |
| 10. | Caletti G. The gut wall. In Van Dam J and Sivak M eds, Gastrointestinal Endosonography, Philadelphia: W.B Saunders 1999; 103-114. [Cited in This Article: ] |
| 11. | Gleeson FC. EUS in rectal cancer: anorectal anatomy. In: Hawes RH, Fockens P, Varadarajulu S eds, Endosonography 3rd edition, Philadelphia: WB Saunders 2015; 260-268. [Cited in This Article: ] |
| 12. | Harewood GC, Wiersema MJ, Nelson H, Maccarty RL, Olson JE, Clain JE, Ahlquist DA, Jondal ML. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123:24-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Saitoh N, Okui K, Sarashina H, Suzuki M, Arai T, Nunomura M. Evaluation of echographic diagnosis of rectal cancer using intrarectal ultrasonic examination. Dis Colon Rectum. 1986;29:234-242. [PubMed] [Cited in This Article: ] |
| 14. | Feifel G, Hildebrandt U, Dhom G. Assessment of depth of invasion in rectal cancer by endosonography. Endoscopy. 1987;19:64-67. [PubMed] [Cited in This Article: ] |
| 15. | Yamashita Y, Machi J, Shirouzu K, Morotomi T, Isomoto H, Kakegawa T. Evaluation of endorectal ultrasound for the assessment of wall invasion of rectal cancer. Report of a case. Dis Colon Rectum. 1988;31:617-623. [PubMed] [Cited in This Article: ] |
| 16. | Beynon J, Mortensen NJ, Rigby HS. Rectal endosonography, a new technique for the preoperative staging of rectal carcinoma. Eur J Surg Oncol. 1988;14:297-309. [PubMed] [Cited in This Article: ] |
| 17. | Rifkin MD, Ehrlich SM, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology. 1989;170:319-322. [PubMed] [Cited in This Article: ] |
| 18. | Hildebrandt U, Klein T, Feifel G, Schwarz HP, Koch B, Schmitt RM. Endosonography of pararectal lymph nodes. In vitro and in vivo evaluation. Dis Colon Rectum. 1990;33:863-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Tio TL, Coene PP, van Delden OM, Tytgat GN. Colorectal carcinoma: preoperative TNM classification with endosonography. Radiology. 1991;179:165-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 20. | Katsura Y, Yamada K, Ishizawa T, Yoshinaka H, Shimazu H. Endorectal ultrasonography for the assessment of wall invasion and lymph node metastasis in rectal cancer. Dis Colon Rectum. 1992;35:362-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Glaser F, Kuntz C, Schlag P, Herfarth C. Endorectal ultrasound for control of preoperative radiotherapy of rectal cancer. Ann Surg. 1993;217:64-71. [PubMed] [Cited in This Article: ] |
| 22. | Herzog U, von Flüe M, Tondelli P, Schuppisser JP. How accurate is endorectal ultrasound in the preoperative staging of rectal cancer? Dis Colon Rectum. 1993;36:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 212] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Cho E, Nakajima M, Yasuda K, Ashihara T, Kawai K. Endoscopic ultrasonography in the diagnosis of colorectal cancer invasion. Gastrointest Endosc. 1993;39:521-527. [PubMed] [Cited in This Article: ] |
| 24. | Thaler W, Watzka S, Martin F, La Guardia G, Psenner K, Bonatti G, Fichtel G, Egarter-Vigl E, Marzoli GP. Preoperative staging of rectal cancer by endoluminal ultrasound vs. magnetic resonance imaging. Preliminary results of a prospective, comparative study. Dis Colon Rectum. 1994;37:1189-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Nielsen MB, Qvitzau S, Pedersen JF, Christiansen J. Endosonography for preoperative staging of rectal tumours. Acta Radiol. 1996;37:799-803. [PubMed] [Cited in This Article: ] |
| 26. | Sailer M, Leppert R, Kraemer M, Fuchs KH, Thiede A. The value of endorectal ultrasound in the assessment of adenomas, T1- and T2-carcinomas. Int J Colorectal Dis. 1997;12:214-219. [PubMed] [Cited in This Article: ] |
| 27. | Nishimori H, Sasaki K, Hirata K, Hirata K, Natori H. The value of endoscopic ultrasonography in preoperative evaluation of rectal cancer. Int Surg. 1998;83:157-160. [PubMed] [Cited in This Article: ] |
| 28. | Norton SA, Thomas MG. Staging of rectosigmoid neoplasia with colonoscopic endoluminal ultrasonography. Br J Surg. 1999;86:942-946. [PubMed] [Cited in This Article: ] |
| 29. | Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999;42:770-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 199] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Marone P, Petrulio F, de Bellis M, Battista Rossi G, Tempesta A. Role of endoscopic ultrasonography in the staging of rectal cancer: a retrospective study of 63 patients. J Clin Gastroenterol. 2000;30:420-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Akasu T, Kondo H, Moriya Y, Sugihara K, Gotoda T, Fujita S, Muto T, Kakizoe T. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24:1061-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Garcia-Aguilar J, Pollack J, Lee SH, Hernandez de Anda E, Mellgren A, Wong WD, Finne CO, Rothenberger DA, Madoff RD. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Marusch F, Koch A, Schmidt U, Zippel R, Kuhn R, Wolff S, Pross M, Wierth A, Gastinger I, Lippert H. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34:385-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Kauer WK, Prantl L, Dittler HJ, Siewert JR. The value of endosonographic rectal carcinoma staging in routine diagnostics: a 10-year analysis. Surg Endosc. 2004;18:1075-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Vila JJ, Jiménez FJ, Irisarri R, Martínez A, Amorena E, Borda F. Rectal cancer staging with endoscopic ultrasonography: correlation with pathological staging. Rev Esp Enferm Dig. 2007;99:132-137. [PubMed] [Cited in This Article: ] |
| 36. | Landmann RG, Wong WD, Hoepfl J, Shia J, Guillem JG, Temple LK, Paty PB, Weiser MR. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50:1520-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Halefoglu AM, Yildirim S, Avlanmis O, Sakiz D, Baykan A. Endorectal ultrasonography versus phased-array magnetic resonance imaging for preoperative staging of rectal cancer. World J Gastroenterol. 2008;14:3504-3510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Lin S, Luo G, Gao X, Shan H, Li Y, Zhang R, Li J, He L, Wang G, Xu G. Application of endoscopic sonography in preoperative staging of rectal cancer: six-year experience. J Ultrasound Med. 2011;30:1051-1057. [PubMed] [Cited in This Article: ] |
| 39. | Fernández-Esparrach G, Ayuso-Colella JR, Sendino O, Pagés M, Cuatrecasas M, Pellisé M, Maurel J, Ayuso-Colella C, González-Suárez B, Llach J. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Ünsal B, Alper E, Baydar B, Arabul M, Aslan F, Çelık M, Buyraç Z, Akça S. The efficacy of endoscopic ultrasonography in local staging of rectal tumors. Turk J Gastroenterol. 2012;23:530-534. [PubMed] [Cited in This Article: ] |
| 41. | Zhu J, Huang PT, Ding KF, Zhang X, Liu CM, Liu XM, Li BZ, Cai SR, Zheng S. Clinical value of radial endorectal ultrasound in the assessment of preoperative staging of rectal carcinoma. Zhonghua Zhongliu Zazhi. 2013;35:148-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 42. | Golfieri R, Giampalma E, Leo P, Colecchia A, Selleri S, Poggioli G, Gandolfi L, Gozzetti G, Trebbi F, Russo A. Comparison of magnetic resonance (0,5 T), computed tomography, and endorectal ultrasonography in the preoperative staging of neoplasms of the rectum-sigma. Correlation with surgical and anatomopathologic findings. Radiol Med. 1993;85:773-783. [PubMed] [Cited in This Article: ] |
| 43. | Kulig J, Richter P, Gurda-Duda A, Gach T, Klek S. The role and value of endorectal ultrasonography in diagnosing T1 rectal tumors. Ultrasound Med Biol. 2006;32:469-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Ho ML, Liu J, Narra V. Magnetic resonance imaging of rectal cancer. Clin Colon Rectal Surg. 2008;21:178-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Cârţână ET, Pârvu D, Săftoiu A. Endoscopic ultrasound: current role and future perspectives in managing rectal cancer patients. J Gastrointestin Liver Dis. 2011;20:407-413. [PubMed] [Cited in This Article: ] |
| 46. | Skandarajah AR, Tjandra JJ. Preoperative loco-regional imaging in rectal cancer. ANZ J Surg. 2006;76:497-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR. Can endoscopic ultrasound predict early rectal cancers that can be resected endoscopically? A meta-analysis and systematic review. Dig Dis Sci. 2010;55:1221-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Marusch F, Ptok H, Sahm M, Schmidt U, Ridwelski K, Gastinger I, Lippert H. Endorectal ultrasound in rectal carcinoma--do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43:425-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Kneist W, Terzic A, Burghardt J, Heintz A, Junginger T. Selection of patients with rectal tumors for local excision based on preoperative diagnosis. Results of a consecutive evaluation study of 552 patients. Chirurg. 2004;75:168-175. [PubMed] [Cited in This Article: ] |
| 51. | Glancy DG, Pullyblank AM, Thomas MG. The role of colonoscopic endoanal ultrasound scanning (EUS) in selecting patients suitable for resection by transanal endoscopic microsurgery (TEM). Colorectal Dis. 2005;7:148-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23:1384-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Rösch T. Ecografia endoscopica. Classen M, editor. Roma: Verducci Editori 2004; 199-220. [Cited in This Article: ] |
| 54. | Hulsmans FJ, Tio TL, Fockens P, Bosma A, Tytgat GN. Assessment of tumor infiltration depth in rectal cancer with transrectal sonography: caution is necessary. Radiology. 1994;190:715-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 131] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Maier AG, Barton PP, Neuhold NR, Herbst F, Teleky BK, Lechner GL. Peritumoral tissue reaction at transrectal US as a possible cause of overstaging in rectal cancer: histopathologic correlation. Radiology. 1997;203:785-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Harewood GC, Kumar KS, Clain JE, Levy MJ, Nelson H. Clinical implications of quantification of mesorectal tumor invasion by endoscopic ultrasound: All T3 rectal cancers are not equal. J Gastroenterol Hepatol. 2004;19:750-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Esclapez P, Garcia-Granero E, Flor B, García-Botello S, Cervantes A, Navarro S, Lledó S. Prognostic heterogeneity of endosonographic T3 rectal cancer. Dis Colon Rectum. 2009;52:685-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | McClave SA, Jones WF, Woolfolk GM, Schrodt GR, Wiersema MJ. Mistakes on EUS staging of colorectal carcinoma: error in interpretation or deception from innate pathologic features? Gastrointest Endosc. 2000;51:682-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Akasu T, Sugihara K, Moriya Y, Fujita S. Limitations and pitfalls of transrectal ultrasonography for staging of rectal cancer. Dis Colon Rectum. 1997;40:S10-S15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Marone P, de Bellis M, Rossi GB, Avallone A, Delrio P, Tatangelo F, Di Nardo G, Sannino S, Cesario S, Voltura C. Staging errors in the preoperative staging and restaging of patients with rectal cancer. Digestive Liver Diseases. 2007;39:S280. [Cited in This Article: ] |
| 61. | Granero-Castro P, Muñoz E, Frasson M, García-Granero A, Esclapez P, Campos S, Flor-Lorente B, Garcia-Granero E. Evaluation of mesorectal fascia in mid and low anterior rectal cancer using endorectal ultrasound is feasible and reliable: a comparison with MRI findings. Dis Colon Rectum. 2014;57:709-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Bhutani MS. Recent developments in the role of endoscopic ultrasonography in diseases of the colon and rectum. Curr Opin Gastroenterol. 2007;23:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Savides TJ, Master SS. EUS in rectal cancer. Gastrointest Endosc. 2002;56:S12-S18. [PubMed] [Cited in This Article: ] |
| 64. | Kim JC, Kim HC, Yu CS, Han KR, Kim JR, Lee KH, Jang SJ, Lee SS, Ha HK. Efficacy of 3-dimensional endorectal ultrasonography compared with conventional ultrasonography and computed tomography in preoperative rectal cancer staging. Am J Surg. 2006;192:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Catalano MF, Sivak MV, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 299] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 66. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 218] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Gleeson FC, Clain JE, Papachristou GI, Rajan E, Topazian MD, Wang KK, Levy MJ. Prospective assessment of EUS criteria for lymphadenopathy associated with rectal cancer. Gastrointest Endosc. 2009;69:896-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Krajewski KM, Kane RA. Ultrasound staging of rectal cancer. Semin Ultrasound CT MR. 2008;29:427-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Moriya Y, Sugihara K, Akasu T, Fujita S. Importance of extended lymphadenectomy with lateral node dissection for advanced lower rectal cancer. World J Surg. 1997;21:728-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Levy M, Alberts SR, Clain JE, Jonathan E. Clain, Amy C. Clayton, Elizabeth Rajan, Mark D Topazian, Kenneth K. Wang, Maurits J. Wiersema. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) detection of malignant iliac nodes in rectal cancer. Gastrointest Endosc. 2006;63:AB97. [Cited in This Article: ] |
| 71. | Harada N, Hamada S, Kubo H, Oda S, Chijiiwa Y, Kabemura T, Maruoka A, Akahoshi K, Yao T, Nawata H. Preoperative evaluation of submucosal invasive colorectal cancer using a 15-MHz ultrasound miniprobe. Endoscopy. 2001;33:237-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Hurlstone DP, Brown S, Cross SS, Shorthouse AJ, Sanders DS. Endoscopic ultrasound miniprobe staging of colorectal cancer: can management be modified? Endoscopy. 2005;37:710-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Gall TM, Markar SR, Jackson D, Haji A, Faiz O. Mini-probe ultrasonography for the staging of colon cancer: a systematic review and meta-analysis. Colorectal Dis. 2014;16:O1-O8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Knight CS, Eloubeidi MA, Crowe R, Jhala NC, Jhala DN, Chhieng DC, Eltoum IA. Utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of colorectal carcinoma. Diagn Cytopathol. 2013;41:1031-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Wiersema MJ, Harewood GC. Endoscopic ultrasound for rectal cancer. Gastroenterol Clin North Am. 2002;31:1093-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Shami VM, Parmar KS, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum. 2004;47:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 79. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [PubMed] [Cited in This Article: ] |
| 80. | Yimei J, Ren Z, Lu X, Huan Z. A comparison between the reference values of MRI and EUS and their usefulness to surgeons in rectal cancer. Eur Rev Med Pharmacol Sci. 2012;16:2069-2077. [PubMed] [Cited in This Article: ] |
| 81. | Cesmeli E. Anorectal staging: is EUS necessary? Minerva Med. 2014;105:423-436. [PubMed] [Cited in This Article: ] |
| 82. | Granero-Castro P, Muñoz E, Frasson M, García-Granero A, Esclapez P, Campos S, Flor-Lorente B, Garcia-Granero E. Evaluation of mesorectal fascia in mid and low anterior rectal cancer using endorectal ultrasound is feasible and reliable: a comparison with MRI findings. Dis Colon Rectum. 2014;57:709-14. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Swartling T, Kälebo P, Derwinger K, Gustavsson B, Kurlberg G. Stage and size using magnetic resonance imaging and endosonography in neoadjuvantly-treated rectal cancer. World J Gastroenterol. 2013;19:3263-3271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Aljebreen AM, Azzam NA, Alzubaidi AM, Alsharqawi MS, Altraiki TA, Alharbi OR, Almadi MA. The accuracy of multi-detector row computerized tomography in staging rectal cancer compared to endoscopic ultrasound. Saudi J Gastroenterol. 2013;19:108-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Liang TY, Anil G, Ang BW. Imaging paradigms in assessment of rectal carcinoma: loco-regional and distant staging. Cancer Imaging. 2012;12:290-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Samdani T, Garcia-Aguilar J. Imaging in rectal cancer: magnetic resonance imaging versus endorectal ultrasonography. Surg Oncol Clin N Am. 2014;23:59-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Harewood GC, Wiersema MJ. Cost-effectiveness of endoscopic ultrasonography in the evaluation of proximal rectal cancer. Am J Gastroenterol. 2002;97:874-882. [PubMed] [Cited in This Article: ] |
| 88. | Edelman BR, Weiser MR. Endorectal ultrasound: its role in the diagnosis and treatment of rectal cancer. Clin Colon Rectal Surg. 2008;21:167-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Napoleon B, Pujol B, Berger F, Valette PJ, Gerard JP, Souquet JC. Accuracy of endosonography in the staging of rectal cancer treated by radiotherapy. Br J Surg. 1991;78:785-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 118] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99:109-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Marone P, de Bellis M, Avallone A, Delrio P, di Nardo G, D’Angelo V, Tatangelo F, Pecori B, Di Girolamo E, Iaffaioli V. Accuracy of endoscopic ultrasound in staging and restaging patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Clin Res Hepatol Gastroenterol. 2011;35:666-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Maor Y, Nadler M, Barshack I, Zmora O, Koller M, Kundel Y, Fidder H, Bar-Meir S, Avidan B. Endoscopic ultrasound staging of rectal cancer: diagnostic value before and following chemoradiation. J Gastroenterol Hepatol. 2006;21:454-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Mezzi G, Arcidiacono PG, Carrara S, Perri F, Petrone MC, De Cobelli F, Gusmini S, Staudacher C, Del Maschio A, Testoni PA. Endoscopic ultrasound and magnetic resonance imaging for re-staging rectal cancer after radiotherapy. World J Gastroenterol. 2009;15:5563-5567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Radovanovic Z, Breberina M, Petrovic T, Golubovic A, Radovanovic D. Accuracy of endorectal ultrasonography in staging locally advanced rectal cancer after preoperative chemoradiation. Surg Endosc. 2008;22:2412-2415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Löhnert MS, Doniec JM, Henne-Bruns D. Effectiveness of endoluminal sonography in the identification of occult local rectal cancer recurrences. Dis Colon Rectum. 2000;43:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Hünerbein M, Totkas S, Moesta KT, Ulmer C, Handke T, Schlag PM. The role of transrectal ultrasound-guided biopsy in the postoperative follow-up of patients with rectal cancer. Surgery. 2001;129:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Marone P, De Bellis M, Rossi GB, Tempesta AM. Effectiveness of endoscopic ultrasonography in the follow up of patients operated on for rectal cancer. Digestive and Liver Diseases. 2001;33:S143. [Cited in This Article: ] |
| 98. | Săftoiu A. State-of-the-art imaging techniques in endoscopic ultrasound. World J Gastroenterol. 2011;17:691-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Saftoiu A, Gheonea DI. Tridimensional (3D) endoscopic ultrasound - a pictorial review. J Gastrointestin Liver Dis. 2009;18:501-505. [PubMed] [Cited in This Article: ] |
| 100. | Giovannini M, Bories E, Pesenti C, Moutardier V, Lelong B, Delpéro JR. Three-dimensional endorectal ultrasound using a new freehand software program: results in 35 patients with rectal cancer. Endoscopy. 2006;38:339-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Miyata T, Kitano M, Sakamoto H, Imai H, Kamata K, Kadosaka K, Omoto S, Kudo M. Role of Contrast-Enhanced Harmonic EUS in Differentiating Malignant From Benign Lymphadenopathy. Gastrointestinal Endoscopy. 2013;77:AB142. [Cited in This Article: ] |
| 102. | Giovannini M. Contrast-enhanced endoscopic ultrasound and elastosonoendoscopy. Best Pract Res Clin Gastroenterol. 2009;23:767-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |