Published online Jun 10, 2015. doi: 10.4253/wjge.v7.i6.575
Peer-review started: January 28, 2015
First decision: March 6, 2015
Revised: April 1, 2015
Accepted: April 16, 2015
Article in press: April 20, 2015
Published online: June 10, 2015
Fecal incontinence has a profound impact in a patient’s life, impairing quality of life and carrying a substantial economic burden due to health costs. It is an underdiagnosed condition because many affected patients are reluctant to report it and also clinicians are usually not alert to it. Patient evaluation with a detailed clinical history and examination is very important to indicate the type of injury that is present. Endoanal ultrasonography is currently the gold standard for sphincter evaluation in fecal incontinence and is a simple, well-tolerated and non-expensive technique. Most studies revealed 100% sensitivity in identifying sphincter defect. It is better than endoanal magnetic resonance imaging for internal anal sphincter defects, equivalent for the diagnosis of external anal sphincter defects, but with a lower capacity for assessment of atrophy of this sphincter. The most common cause of fecal incontinence is anal sphincter injury related to obstetric trauma. Only a small percentage of women are diagnosed with sphincter tears immediately after vaginal delivery, but endoanal ultrasonography shows that one third of these women have occult sphincter defects. Furthermore, in patients submitted to primary repair of these tears, ultrasound revealed a high frequency of persistent sphincter defects after surgery. Three-dimensional endoanal ultrasonography is currently largely used and accepted for sphincter evaluation in fecal incontinence, improving diagnostic accuracy and our knowledge of physiologic and pathological sphincters alterations. Conversely, there is currently no evidence to support the use of elastography in fecal incontinence evaluation.
Core tip: Clinicians need to be more alert to fecal incontinence, which is a serious under-reported problem. Endoanal ultrasonography is currently the gold standard for sphincter evaluation in these patients. The most important cause of fecal incontinence is obstetric injury and the most relevant questions and controversies are related to this. The diagnosed of sphincter injury after delivery and after complete primary repair is much lower to that found by ultrasonography, and many of these women developed fecal incontinence. The clinical evaluation, technical aspects, advantages and limitations and the current role of three dimensional ultrasonography and real-time elastography will also be discussed.
- Citation: Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc 2015; 7(6): 575-581
- URL: https://www.wjgnet.com/1948-5190/full/v7/i6/575.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i6.575
Fecal incontinence (FI) has a profound impact in a patient’s personal life, impairing social interaction, professional and sexual activity and carries a substantial economic burden due to health costs.
The prevalence varies from 2.2% to 25 % in the community[1] and up to 50% of the nursing home residents[2]. Although a relevant problem, it is an underdiagnosed condition, since many affected patients are reluctant and embarrassed to report it. In a study by Sultan et al[3], none of the women that developed FI after vaginal delivery spontaneously reported their symptoms or sought medical attention. So, it is essential that health professionals, mainly those who look after women ask about symptoms of FI, especially in the postpartum period.
Loss of continence can result from several mechanisms, dysfunction of the anal sphincters, abnormal rectal compliance, decreased rectal sensation, altered stool consistency, or a combination of any of these abnormalities. FI is often multifactorial condition, may be the consequence of local, anatomical or systemic disorders, non-traumatic or traumatic lesions. Not every patient with sphincter injury develops incontinence, and, in addition, patients can have incontinence without sphincter injury. There are several women that only develop FI several years (20 or 30 decades) after delivery.
Patient evaluation should always include a detailed clinical history, inspection of the perianal area and a digital rectal examination. The type of incontinence (urge or passive), obstetric history (vaginal deliveries, use of forceps, perineal laceration), previous anorectal surgery, coexisting comorbidities, anal resting tone and squeeze pressure are fundamental to understand the mechanism behind the impairment and this information should never be neglected. Patients with urge incontinence often have weakness of the external anal sphincter (EAS) and reduced squeeze pressures or reduced rectal capacity with rectal hypersensitivity. Patients with passive FI, often have weakness of the internal anal sphincter (IAS) and lower resting pressure[4]. Taking all this information into consideration before endoanal ultrasonography (EAUS) is performed, can indicate the type of injury found.
There are several clinical scores that can be used to access severity, like the American Medical System, Pescatori score, Vaizey scale, Rockwood score or the Cleveland Clinic (Wexner) Incontinence Score[5]. These scores allow a more objective and reproducible assess of FI severity and a comparison of patients and treatments, namely the outcomes of both conservative and surgical treatments.
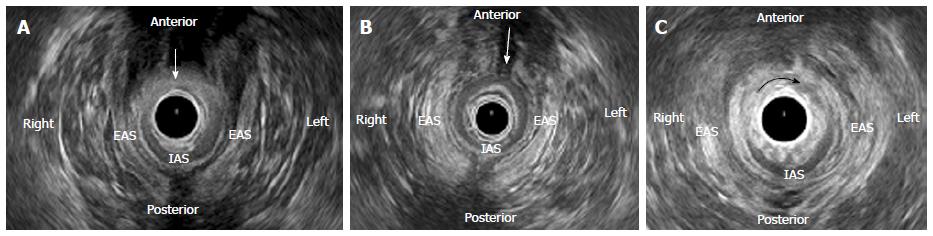
EAUS is currently the gold standard technique for sphincter evaluation in FI[6]. The first studies in EAUS were performed by Law et al[7,8], in the early 1990s, comparing EAUS with electromyography, EAUS proved to be better tolerated and a useful technique for assessing defects of the anal sphincters. Most studies revealed 100% sensitivity in identifying sphincter defect. It is important to search for sphincter discontinuity, sphincter thinning and perform perineal body measurement. Discontinuity of the sphincter indicates a tear, and scarring is characterized by loss of the normal texture that usually has low reflectiveness. IAS tears appear normally as hyperechoic breaks and EAS tears appear as relatively hypoechoic areas (Figure 1). IAS thickness measurement in adults is abnormal if less than 2 mm (suggestive of degeneration) and generalized EAS atrophy is difficult to evaluate in EAUS. Perineal body measurement improves visualization of anterior sphincter lesions in females. A perineal body thickness of 10 mm or less is considered abnormal, whereas 10 mm to 12 mm is associated with sphincter defect in one-third of patients and those with 12 mm or more are unlikely to harbour a defect unless they previously have undergone reconstructive perineal surgery[9-11].
During the exam, the number, the circumferential extent (radial angle in degrees or in hours of the clock) and longitudinal extent (proximal, distal or full length) of the defect should be reported.
There are several possible pitfalls during EAUS that can simulate sphincter tears. A correct diagnose is important for FI assessment and for choosing the best therapeutic approach; a proper training in EAUS is fundamental. In many cases, it is not the endoscopic ultrasound practitioner that is performing the EAUS. These are two different techniques and specific training is needed for endoscopic ultrasound practitioners enrolled in EAUS.
Anal sphincteroplasty should be considered in patients with FI who do not respond to conservative therapy and who have an anatomic sphincter defect. Short-term outcomes suggest good-to-excellent results, but the benefits tend to deteriorate with long-term follow-up[4].
EAUS and endoanal magnetic resonance imaging (MRI) are comparable for the diagnosis of EAS defects, but IAS defects are less well assessed on MRI[12]. EAUS is simple, well-tolerated and non expensive. Endoanal MRI is expensive, not generally available, unsuitable in claustrophobic patients and those with metal implants. Endoanal MRI is superior to two-dimensional (2D) EAUS for identifying EAS atrophy. EAUS cannot distinguish fatty infiltration from normal muscle tissue and the boundaries of the EAS are harder to determine. Comparison between endoanal MRI and three-dimensional (3D) EAUS capacity for EAS atrophy evaluation revealed conflicting results. Cazemier et al[13] showed that both techniques are comparable in detecting EAS atrophy, although there is a substantial difference in grading. West et al[14] demonstrated that no 3D EAUS measurements are suitable parameters for assessing EAS atrophy. It is important to recognize atrophy because it is associated with a poor clinical outcome of sphincter repair.
EAUS and anorectal manometry are complementary investigations. EAUS allows anal sphincter morphology assessment and manometry anal sphincter function evaluation. Studies comparing both techniques show good correlation between them in partial or complete defects of the anal sphincter[15].
Most studies show poor agreement between digital anorectal examination and EAUS. In a study by Sultan et al[16] the clinical examination was only 50% accurate at predicting anal sphincter defects and Jeppson et al[17] show a specificity of 32% for digital anorectal examination in detecting anal sphincter defects seen on EAUS; however, Dobben et al[18] reported increased correlation between digital examination and EAUS based on size of the sphincter defect. Notwithstanding, performing digital anorectal examination is important in the evaluation of a patient with FI, helping to differentiate other potential causes such as tumor or fecal impaction.
It is important to ask patients about the presence of FI directly rather than relying on spontaneous reporting[4] and initial patient evaluation should include a detailed clinical history, inspection of the perianal area and a digital rectal examination. Manometry is important for anal sphincters function evaluation, anal sphincter resting and squeeze pressures are the key parameters[4]. EAUS is the gold standard for diagnosing anal sphincters tear and IAS degeneration. If EAS atrophy is suspected, endoanal MRI should be performed. Needle electromyography of the anal sphincter should be considered in patients with clinically suspected neurogenic sphincter weakness, particularly if there are features suggestive of proximal (i.e., sacral root) involvement[4].
The most common cause of FI is anal sphincter injury related to vaginal delivery in female, due to direct anal sphincter laceration or indirect damage to sphincter innervation.
Two EAUS-based scoring systems have been proposed to define the severity of anal sphincter damage, both of them in women with obstetric anal sphincter injuries (OASIS). Starck et al[19] introduced a specific score, with 0 indicating no defect and 16 corresponding to a defect > 180° involving the whole length and depth of both sphincters. Norderval et al[20] reported a simplified system, including fewer categories and not recording partial defects of the IAS. The maximal score of 7 denotes defects in both the EAS and the IAS exceeding 90° in the axial plane and involving more than half of the length of each sphincter. Both scoring systems have demonstrated a good correlation between the extent of sphincter defects and the degree of FI. Scoring systems may help the clinician in choosing the appropriate treatment for patients with FI, but studies are needed.
Obstetric tears are divided into several subclasses, initially described by Sultan[21], and then adopted by the Royal College of Obstetricians and Gynaecologists (RCOG): injury to the perineal skin grade 1; injury to the perineum involving the perineal muscles grade 2; involving the anal sphincter < 50% EAS grade 3a; > 50% EAS grade 3b; involvement of the IAS grade 3c; involvement of the anal sphincter as well as the anorectal epithelium grade 4[19]. OASIS encompasses both third- and fourth-degree perineal tears. They are identified in 0.6%-9.0% of vaginal deliveries where mediolateral episiotomy is performed, but the detection in EAUS is much higher[22].
A landmark study by Sultan et al[3] in 1993, using EAUS reported occult anal sphincter injury in 35% of women, six weeks after their first vaginal delivery. The incidence of de novo defects in multiparous females was 4.2%. The incidence of occult sphincter damage after vaginal delivery was unknown, previously to this study. Only 3% of primiparous women had an injury during delivery that was apparent in clinical examination. Results also suggested that the structural injury to the sphincters was permanent, since they were also present at 6 mo. Notably, only one third of women with sphincter defects in EAUS had FI.
In 2003, Oberwalder et al[23] published a meta-analysis of 717 vaginal deliveries (including the study by Sultan[21]) and found an incidence of occult sphincter damage of 26.9% in primiparous women and 8.5% of new defects in multiparous women. In one third of these women, postpartum sphincter damage was symptomatic.
Perhaps women with occult sphincter defect, but without FI can have sufficient residual sphincter function[21] or, since several mechanisms contribute to continence, they may compensate for this injury. The peak of incidence of FI is in the fifth and sixth decades of life in women, so the cumulative effect of deliveries, aging, menopause, progression of neuropathy may contribute for sphincter weakness in the long term and FI developing several years (20 or 30 decades) after delivery.
The clinical relevance of screening for occult anal sphincter laceration is controversial, mainly in asymptomatic defects. In a prospective cohort study by Frudinger et al[24], including primiparas with occult anal sphincter lacerations, at 10-year follow-up, only women who were symptomatic in the immediate postpartum period had deterioration over time of FI. Conversely, a randomized control trial by Faltin et al[25] showed that EAUS after childbirth improves the diagnosis of anal sphincter tears, and their immediate repair decreases the risk of severe FI. In this study, 752 primiparas with no clinically recognized anal sphincter laceration (occult) were assigned to undergo or not an EAUS immediately after delivery and diagnosed lacerations were repaired. In the EAUS group significantly fewer women reported severe FI at 3 and 12 mo compared to those who did not undergo EAUS. Using these data, it was estimated that 29 women would have to undergo EAUS to prevent one case of severe FI.
The current guidelines of the RCOG from 2007[22] state that “As there are clear difficulties with availability, access to staff trained in EAUS on the labour ward, image quality and patient acceptability, the use of EAUS in detecting anal sphincter injury immediately after delivery should be viewed as a research tool at present”. There is no recommendation about screening women later after vaginal delivery for occult sphincter defects. Thus, data are controversial for asymptomatic patients. There are no cost-benefit studies of EAUS in this setting, or whether asymptomatic patients could benefit from it. Currently, the major investment should be in improving the identification of OASIS immediately after delivery. It is unclear, if occult sphincter defects are missed tears or true “occult” defects; probably the vast majority are not diagnosed clinically at time of delivery.
If an OASIS is identified immediately after vaginal delivery, it should be repaired. The RCOG[22] recommend that for repair of the external anal sphincter, either an overlapping or end-to-end (approximation) method can be used; if the IAS is identified, it is advisable to repair separately with interrupted sutures. Repair should be conducted in an operating theatre, under regional or general anaesthesia, by appropriately trained practitioners. Although primary reconstruction of the sphincters, more than 50% of women experience some change in continence (mainly to flatus) and the effect deteriorates with time[26]. Having a persistent sonographic defect after primary repair of OASIS has been shown to be associated with ongoing incontinence symptoms[27,28]. Studies show a high frequency of endosonographic sphincter defects after primary repairs, between 54% and 93% of women[29-32]. In a study using EAUS performed 2-7 d after delivery in women who had undergone a primary repair of an OASIS, 90% had endosonographic sphincter defects. In this study the extent of the endosonographic defects were mainly determined by the surgical experience of the doctor performing the repair, and not by the clinical degree of the tear[19].
The current guidelines of the RCOG[22] also do not make recommendations about using EAUS for confirming a complete primary repair. According to these guidelines “If a woman is experiencing incontinence or pain at follow-up, referral to EAUS and anorectal manometry should be considered”. Considering the very high rate of sphincter defects detected by EAUS after primary repair, the high percentage of women that have some continence alteration and the difficulty in assessing the complete reparation of defects immediately after delivery, is EAUS confined to symptomatic women enough? In 2006, Starck et al[32] conducted a prospective study that included women who had suffered an OASIS at delivery and underwent EAUS at 1 wk, 3 mo and 1 year after primary suture. There was a positive correlation between the endosonographic sphincter defect score at 1 wk, 3 mo and 1 year and the Wexner incontinence score at 1 and 4 years. Endosonographic sphincter defect score at 1 wk was the variable that was most predictive of the Wexner score at 4 year. There are no systematic reviews or randomised controlled trials to suggest the best method of follow-up after obstetric anal sphincter repair[22].
EAUS can also be important to aid decision for future delivery. According to the RCOG guidelines[22], “all women who have sustained an OASIS in a previous pregnancy and who are symptomatic or have abnormal EAUS and/or manometry should have the option of elective caesarean birth. Between 17% and 24% of these women with previous third-degree tear developed worsening fecal symptoms after a second vaginal delivery”.
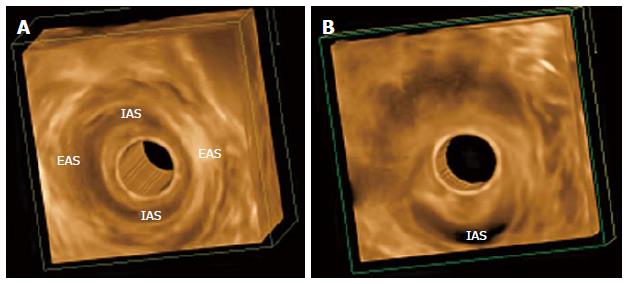
Three-dimensional EAUS has been used in the evaluation of the anal canal since the late 1990s[33,34]. Before 3D, imaging of the anal canal was mainly limited to the axial plane, impairing accurate longitudinal measurement, which is important for complete surgical repair. Three-dimensional EAUS produces a digital volume that can be seen from any plane, allowing length, thickness, area, and volume measurement (Figure 2).
Christensen et al[35] conducted a study to investigate the differences of 3D and 2D EAUS in visualizing damage to the anal sphincter complex. The agreement between the two observers that evaluated the images was better when using 3D (98.2% using 3D and 87.9% using 2D), so 3D improved diagnostic confidence.
The studies involving 3D EAUS also allowed for a better understanding of sex differences in sphincter configuration and between parous and non-parous females, continent and incontinent patients[36]. FI was not associated with loss of sphincter volume, but anterior sphincter length and EAS thickness is smaller[36]. Williams et al[37] assessed changes to anal canal morphology after delivery, in the absence of sphincter trauma, and there was a decrease in the length of the anterior portion of the EAS following childbirth.
Real-time elastography (RTE) has been evaluated previously in tumours and inflammatory tissues, and has proven to provide valuable additional information.
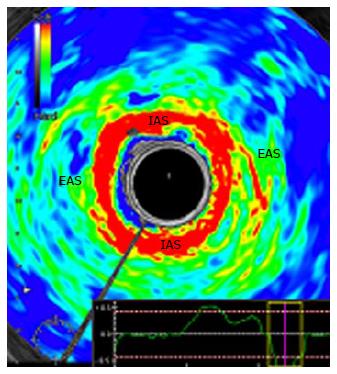
In 2010, Allgayer et al[38] performed the first study to access RTE in FI, 50 patients were included. The IAS, a smooth muscle, consisted of softer areas (red) than the EAS and, conversely, the EAS, a striated type of muscle, contained harder elements (blue) than the IAS (Figure 3). There was an absence of a correlation of elastogram color distributions of the IAS and EAS with major clinical, functional and gray-scale B-mode parameters, so RTE did not seem to provide additional information in the diagnostic workup of FI. However, there was a non-significant increase in the percentage of blue (hard) areas in the IAS in patients neoadjuvantly irradiated for rectal or cervical cancer compared to non-irradiated patients. To confirm this data, the authors performed a larger study[39], but RTE with quantitation of sphincter elastic properties yielded no further diagnostic and prognostic information compared to conventional EAUS in irradiated and non-irradiated patients and, therefore, cannot be regarded as a new tool in the assessment of those patients.
Hence, currently there is not evidence to support the use of RTE in FI evaluation.
FI is a serious clinical and social problem, frequently under-reported, and clinicians need to be more alert to it in the routine clinical practice. EAUS is a fundamental tool when assessing these patients.
The most important cause of FI is obstetric injury and the more relevant questions and controversies in EAUS are related to this aetiology. The diagnosed of sphincter injury after delivery and after complete primary repair is much lower to that found by EAUS, and many of these women developed FI, later in life.
While three-dimensional EAUS is currently accepted for sphincter evaluation in FI, there is presently no evidence to support the use of elastography.
P- Reviewer: Amornyotin S, Jonaitis L, Tham T, Yan SL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Norton C, Whitehead WE, Bliss DZ, Harari D, Lang J. Management of fecal incontinence in adults. Neurourol Urodyn. 2010;29:199-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Nelson R, Furner S, Jesudason V. Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum. 1998;41:1226-1229. [PubMed] [Cited in This Article: ] |
| 3. | Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329:1905-1911. [PubMed] [Cited in This Article: ] |
| 4. | Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157; (Quiz) 1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 5. | Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77-97. [PubMed] [Cited in This Article: ] |
| 6. | Tjandra JJ, Dykes SL, Kumar RR, Ellis CN, Gregorcyk SG, Hyman NH, Buie WD. Practice parameters for the treatment of fecal incontinence. Dis Colon Rectum. 2007;50:1497-1507. [PubMed] [Cited in This Article: ] |
| 7. | Law PJ, Kamm MA, Bartram CI. A comparison between electromyography and anal endosonography in mapping external anal sphincter defects. Dis Colon Rectum. 1990;33:370-373. [PubMed] [Cited in This Article: ] |
| 8. | Law PJ, Kamm MA, Bartram CI. Anal endosonography in the investigation of faecal incontinence. Br J Surg. 1991;78:312-314. [PubMed] [Cited in This Article: ] |
| 9. | Saranovic D, Barisic G, Krivokapic Z, Masulovic D, Djuric-Stefanovic A. Endoanal ultrasound evaluation of anorectal diseases and disorders: technique, indications, results and limitations. Eur J Radiol. 2007;61:480-489. [PubMed] [Cited in This Article: ] |
| 10. | Santoro GA, Wieczorek AP, Dietz HP, Mellgren A, Sultan AH, Shobeiri SA, Stankiewicz A, Bartram C. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol. 2011;37:381-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Zetterström JP, Mellgren A, Madoff RD, Kim DG, Wong WD. Perineal body measurement improves evaluation of anterior sphincter lesions during endoanal ultrasonography. Dis Colon Rectum. 1998;41:705-713. [PubMed] [Cited in This Article: ] |
| 12. | Malouf AJ, Williams AB, Halligan S, Bartram CI, Dhillon S, Kamm MA. Prospective assessment of accuracy of endoanal MR imaging and endosonography in patients with fecal incontinence. AJR Am J Roentgenol. 2000;175:741-745. [PubMed] [Cited in This Article: ] |
| 13. | Cazemier M, Terra MP, Stoker J, de Lange-de Klerk ES, Boeckxstaens GE, Mulder CJ, Felt-Bersma RJ. Atrophy and defects detection of the external anal sphincter: comparison between three-dimensional anal endosonography and endoanal magnetic resonance imaging. Dis Colon Rectum. 2006;49:20-27. [PubMed] [Cited in This Article: ] |
| 14. | West RL, Dwarkasing S, Briel JW, Hansen BE, Hussain SM, Schouten WR, Kuipers EJ. Can three-dimensional endoanal ultrasonography detect external anal sphincter atrophy? A comparison with endoanal magnetic resonance imaging. Int J Colorectal Dis. 2005;20:328-333. [PubMed] [Cited in This Article: ] |
| 15. | Reddymasu SC, Singh S, Waheed S, Oropeza-Vail M, McCallum RW, Olyaee M. Comparison of anorectal manometry to endoanal ultrasound in the evaluation of fecal incontinence. Am J Med Sci. 2009;337:336-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Sultan AH, Kamm MA, Talbot IC, Nicholls RJ, Bartram CI. Anal endosonography for identifying external sphincter defects confirmed histologically. Br J Surg. 1994;81:463-465. [PubMed] [Cited in This Article: ] |
| 17. | Jeppson PC, Paraiso MF, Jelovsek JE, Barber MD. Accuracy of the digital anal examination in women with fecal incontinence. Int Urogynecol J. 2012;23:765-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Dobben AC, Terra MP, Deutekom M, Gerhards MF, Bijnen AB, Felt-Bersma RJ, Janssen LW, Bossuyt PM, Stoker J. Anal inspection and digital rectal examination compared to anorectal physiology tests and endoanal ultrasonography in evaluating fecal incontinence. Int J Colorectal Dis. 2007;22:783-790. [PubMed] [Cited in This Article: ] |
| 19. | Starck M, Bohe M, Valentin L. Results of endosonographic imaging of the anal sphincter 2-7 days after primary repair of third- or fourth-degree obstetric sphincter tears. Ultrasound Obstet Gynecol. 2003;22:609-615. [PubMed] [Cited in This Article: ] |
| 20. | Norderval S, Markskog A, Røssaak K, Vonen B. Correlation between anal sphincter defects and anal incontinence following obstetric sphincter tears: assessment using scoring systems for sonographic classification of defects. Ultrasound Obstet Gynecol. 2008;31:78-84. [PubMed] [Cited in This Article: ] |
| 21. | Sultan AH. Editorial: Obstetric perineal injury and anal incontinence. Clin Risk. 1999;5:193-196. [Cited in This Article: ] |
| 22. | Royal College of Obstetricians and Gynaecologists (RCOG). The Management of Third- and Fourth- Degree Perineal Tears. RCOG Guideline. 2007;1-11. [Cited in This Article: ] |
| 23. | Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333-1337. [PubMed] [Cited in This Article: ] |
| 24. | Frudinger A, Ballon M, Taylor SA, Halligan S. The natural history of clinically unrecognized anal sphincter tears over 10 years after first vaginal delivery. Obstet Gynecol. 2008;111:1058-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6-13. [PubMed] [Cited in This Article: ] |
| 26. | Soerensen MM, Pedersen BG, Santoro GA, Buntzen S, Bek K, Laurberg S. Long-term function and morphology of the anal sphincters and the pelvic floor after primary repair of obstetric anal sphincter injury. Colorectal Dis. 2014;16:O347-O355. [PubMed] [Cited in This Article: ] |
| 27. | Reid AJ, Beggs AD, Sultan AH, Roos AM, Thakar R. Outcome of repair of obstetric anal sphincter injuries after three years. Int J Gynaecol Obstet. 2014;127:47-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Zetterström J, López A, Holmström B, Nilsson BY, Tisell A, Anzén B, Mellgren A. Obstetric sphincter tears and anal incontinence: an observational follow-up study. Acta Obstet Gynecol Scand. 2003;82:921-928. [PubMed] [Cited in This Article: ] |
| 29. | Sultan AH, Kamm MA, Hudson CN, Bartram CI. Third degree obstetric anal sphincter tears: risk factors and outcome of primary repair. BMJ. 1994;308:887-891. [PubMed] [Cited in This Article: ] |
| 30. | Poen AC, Felt-Bersma RJ, Strijers RL, Dekker GA, Cuesta MA, Meuwissen SG. Third-degree obstetric perineal tear: long-term clinical and functional results after primary repair. Br J Surg. 1998;85:1433-1438. [PubMed] [Cited in This Article: ] |
| 31. | Nielsen MB, Hauge C, Rasmussen OO, Pedersen JF, Christiansen J. Anal endosonographic findings in the follow-up of primarily sutured sphincteric ruptures. Br J Surg. 1992;79:104-106. [PubMed] [Cited in This Article: ] |
| 32. | Starck M, Bohe M, Valentin L. The extent of endosonographic anal sphincter defects after primary repair of obstetric sphincter tears increases over time and is related to anal incontinence. Ultrasound Obstet Gynecol. 2006;27:188-197. [PubMed] [Cited in This Article: ] |
| 33. | Gold DM, Bartram CI, Halligan S, Humphries KN, Kamm MA, Kmiot WA. Three-dimensional endoanal sonography in assessing anal canal injury. Br J Surg. 1999;86:365-370. [PubMed] [Cited in This Article: ] |
| 34. | Wisser J, Schär G, Kurmanavicius J, Huch R, Huch A. Use of 3D ultrasound as a new approach to assess obstetrical trauma to the pelvic floor. Ultraschall Med. 1999;20:15-18. [PubMed] [Cited in This Article: ] |
| 35. | Christensen AF, Nyhuus B, Nielsen MB, Christensen H. Three-dimensional anal endosonography may improve diagnostic confidence of detecting damage to the anal sphincter complex. Br J Radiol. 2005;78:308-311. [PubMed] [Cited in This Article: ] |
| 36. | West RL, Felt-Bersma RJ, Hansen BE, Schouten WR, Kuipers EJ. Volume measurements of the anal sphincter complex in healthy controls and fecal-incontinent patients with a three-dimensional reconstruction of endoanal ultrasonography images. Dis Colon Rectum. 2005;48:540-548. [PubMed] [Cited in This Article: ] |
| 37. | Williams AB, Bartram CI, Halligan S, Marshall MM, Spencer JA, Nicholls RJ, Kmiot WA. Alteration of anal sphincter morphology following vaginal delivery revealed by multiplanar anal endosonography. BJOG. 2002;109:942-946. [PubMed] [Cited in This Article: ] |
| 38. | Allgayer H, Ignee A, Dietrich CF. Endosonographic elastography of the anal sphincter in patients with fecal incontinence. Scand J Gastroenterol. 2010;45:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Allgayer H, Ignee A, Zipse S, Crispin A, Dietrich CF. Endorectal ultrasound and real-time elastography in patients with fecal incontinence following anorectal surgery: a prospective comparison evaluating short- and long-term outcomes in irradiated and non-irradiated patients. Z Gastroenterol. 2012;50:1281-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |