INTRODUCTION
Pancreatic neoplasms have a wide range of pathology, from pancreatic adenocarcinoma to cystic mucinous neoplasms. Endoscopic ultrasound (EUS) with or without fine needle aspiration (FNA) is a helpful diagnostic tool in the work-up of pancreatic neoplasms. Its utility in pancreatic malignancy is well known. Over the last two decades it has become the procedure of choice for tissue diagnosis and staging of pancreatic adenocarcinoma. In this review the utility of EUS in the diagnosis of pancreatic adenocarcinoma and technical aspects of the procedure that can increase rates of pathology diagnosis will be discussed. Examples of rare and atypical lesions and the role of EUS-FNA will be highlighted. Also reviewed are the advances in differentiation and diagnosis of pancreatic cysts, including new tests (DNA analysis, k-ras measurement) that may play a role in the future discriminating cystic lesions. The current evidence, limitations, and complications of EUS-FNA in the evaluation of both solid and cystic pancreatic neoplasms will be reviewed.
PANCREATIC ADENOCARCINOMA
Pancreatic adenocarcinoma remains a rising and leading cause of cancer death in the United States. The five year survival is less than 5%[1,2], which stems from the fact that more than 80% of pancreatic adenocarcinomas present as advanced disease at time of diagnosis[2]. Often the diagnosis and stage can be clearly established with cross sectional imaging and patients can be taken for definitive surgical management. However, when there is lack of clarity in the diagnosis or stage of the disease, EUS-FNA can play an important role. Additionally, it is useful when neoadjuvant therapy is planning to be used and tissue diagnosis is needed. EUS alone is a valuable tool for staging pancreatic lesions. Figure 1 demonstrates an endoscopic ultrasound image (Figure 1A) and typical cytology of a pancreatic adenocarcinoma (Figure 1B and C). EUS has been shown to be superior to other imaging [computed tomography (CT) or abdominal US] in pancreatic tumor detection, specifically in tumors < 3 cm[3]. Earlier studies showed that EUS may be superior to CT in staging and determining surgical resectability. However with the advances in CT imaging, whether EUS still holds advantage in this setting appears to be less clear[4]. It is likely that these two modalities are complimentary in the staging of pancreatic adenocarcinoma.
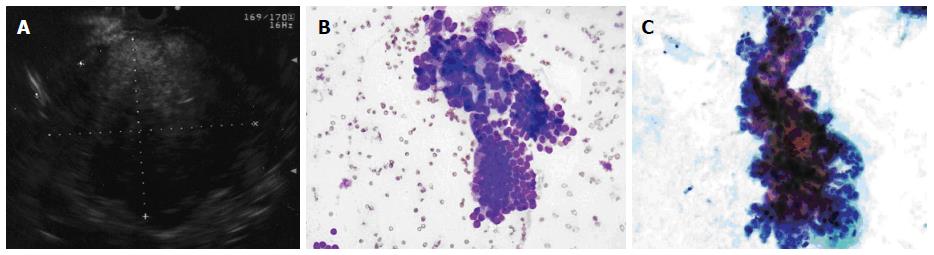
Figure 1 Pancreatic adenocarcinoma.
A: Endoscopic ultrasound image demonstrating a large pancreatic adenocarcinoma; B: Pancreatic adenocarcinoma. A crowded group of large, pleomorphic ductal cells with irregular hyperchromatic nuclei and prominent anisocytosis. These contrast well with an orderly sheet of benign ductal epithelial cells with round, uniform nuclei (bottom) (Diff-QuikTM stain, × 100); C: Similar in appearance malignant cells in a Papanicolaou-stained preparation (× 400).
EUS-FNA was first described in the early 1990’s and since then it has become the standard of care in diagnosis of pancreatic masses[5]. Much of the data regarding EUS-FNA is in regards to diagnosing pancreatic adenocarcinoma. EUS-FNA is highly sensitive and specific for solid lesions, with sensitivities as high as 80%-95% for pancreatic masses and specificity as high as 75%-100%[6-8]. More recently a meta-analysis of 41 studies of EUS-FNA found a pooled sensitivity of 87%[9]; additionally, a recent systemic review of ten high-quality studies showed a pooled sensitivity and specificity of 94% and 95%, respectively[10]. When compared to CT-guided biopsy and endoscopic retrogrograde cholangiopancreatography (ERCP) with brush cytology, EUS-FNA has a distinct advantage. ERCP brush cytology sensitivity is quite low ranging from 30% to 85%[11]. CT-guided biopsy is a more invasive procedure than EUS-FNA and has a lower diagnostic yield. CT guided biopsy also carries the risk of peritoneal seeding, with one retrospective study showing rates as high as 16.3% compared to 2.2% with EUS-FNA[12]. Currently more centers are performing EUS-FNA so there may be a wide range of diagnostic yield in pancreatic masses, but the general trend over the last 10 years is towards higher sensitivity and specificity for pancreatic masses[9].
OPTIMIZING EUS-FNA OF PANCREATIC MASSES
Much of the research in EUS-FNA has focused on optimizing diagnostic yield for pancreatic masses. Multiple aspects of the procedure have been studied including cytopathologist involvement, needle size, providing suctioning and experience of endoscopist. The current data regarding optimization of EUS-FNA results will be reviewed below.
Studies have shown that the total number of EUS-FNA performed within a facility have been linked to higher diagnostic yield. Additionally, the availability of rapid on-site cytopathology evaluation (ROSE) evaluation also significantly increased diagnostic yield of EUS-FNA[13,14]. ROSE has become much more common in practice. All studies to date have shown that ROSE improves diagnostic yield for EUS-FNA and reduces the need for more passes and duration of the procedure[15-17]. An EUS-FNA study of 182 patients showed that with ROSE there was a significantly lower number of inadequate samples (1% vs 12.6%) and a much higher diagnostic sensitivity (96.2% vs 78.2%)[18].
Cytopathologist availability may be difficult and costly; many institutions do not have a cytopathologist readily available to come to endoscopy suites. Two studies have shown that having cytopathologist available via telepathology for rapid review is as effective as when they are present in the room[19,20]. Further studies are looking at the impact of individual cytopathologists and cytology technicians on diagnostic yield. Recently it was shown that providing specific training to cytology technicians can dramatically impact their personal ability to confirm accuracy and diagnosis[21].
The use of optimal equipment for EUS-FNA, including optimal needle size, has been studied extensively. Most commonly 22 or 25 gauge needles are used in EUS-FNA of pancreatic masses. There have been three randomized control studies looking at 22 gauge vs 25 gauge needles. The overall trend of these studies was a slightly more favorable yield with the 25 gauge needle, however none showed a statistically significant difference[22-24].
Beyond choosing the appropriate needle size, different aspects of obtaining cytology including suctioning and stylet use have been studied. The role of suctioning in EUS-FNA has been studied with two randomized control trials showing no difference in diagnostic yield. One study did show higher cellularity with suctioning, however this did not lead to an increase in diagnostic accuracy[25,26]. Most experts agree that suction does not increase diagnostic yield, and in fact likely increases the amount of blood in specimens[27]. Use of stylet has also shown no benefit in improving diagnostic yield, with studies showing that it also increases the amount of blood thus leading to poorer samples[28,29].
There is a definite learning curve in performing EUS-FNA for pancreatic masses. As endoscopists perform more EUS-FNA, sensitivity rises[30]. The current ASGE guidelines recommend 25 supervised EUS-FNA for the diagnosis of pancreatic adenocarcinoma, however literature supports more experience. It has been shown that rates of complications and number of passes needed also decrease with more experience. This study looked specifically at the performance of one endoscopist over the course of the first 300 EUS-FNAs, showing improved performance when comparing the last 100 procedures performed to the first 100[31].
NON-ADENOCARCINOMA MASSES
Pancreatic adenocarcinoma is the most common pancreatic mass lesion, however approximately 10%-15% masses are due to other lesions including cystic neoplasms and neuroendocrine tumors[32]. Thus, getting an accurate diagnosis is important to devise an appropriate management plan. Recently, primary non-adenocarcinoma of the pancreas was found in 25% of EUS-FNA of pancreatic masses[33]. Neuroendocrine tumors comprised 37.5% of the primary non-adenocarcinomas of the pancreas while mucinous neoplasms with mixed cystic/solid components made up 25%. In this study, masses in the tail of the pancreas were more commonly primary non-adenocarcinoma of the pancreas, and these masses were less likely to have vascular invasion or malignant lymphadenopathy when compared to adenocarcinoma[33]. Primary non-adenocarcinoma of the pancreas is often difficult to differentiate from adenocarcinoma with EUS alone. Cytopathology becomes more useful in these cases. The differential diagnosis for pancreatic masses should include not only adenocarcinoma but also neuroendocrine tumors, lymphoma, and metastatic disease.
NEUROENDOCRINE TUMORS
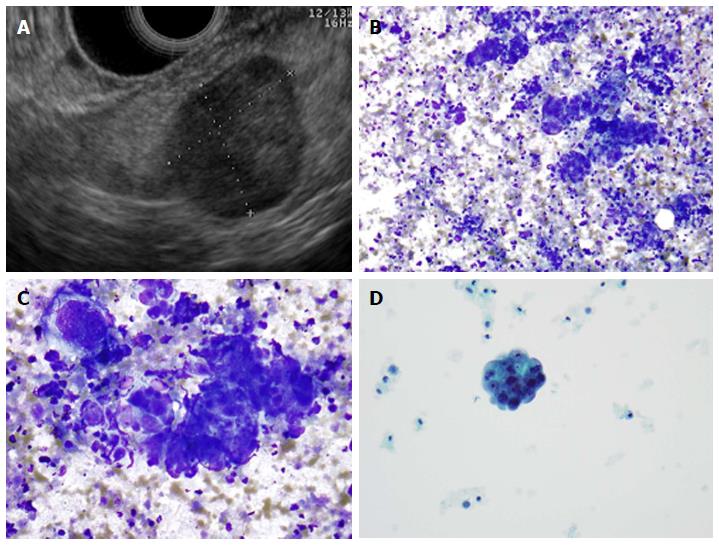
Neuroendocrine tumors of the pancreas are most commonly sporadic but some arise in context of inherited genetic syndromes, including multiple endocrine neoplasia type 1 and 2. Pancreatic neuroendocrine tumors are non-functional 40%-91% of time; the most common functioning tumors are insulinomas followed by glucagonomas, gastrinomas (Zollinger-Ellison syndrome) and somatostatinomas[34]. Some studies have shown that EUS-FNA is effective for obtaining preoperative determination of Ki-67 expression, which is an important prognostic factor for grading pancreatic endocrine tumors[35]. EUS-FNA is highly accurate for neuroendocrine tumors with sensitivity above 90%; thus it is helpful for making a diagnosis[35,36]. Typical EUS imaging of a neuroendocrine tumor and cytologic appearance of the tumor cells are presented in Figure 2.
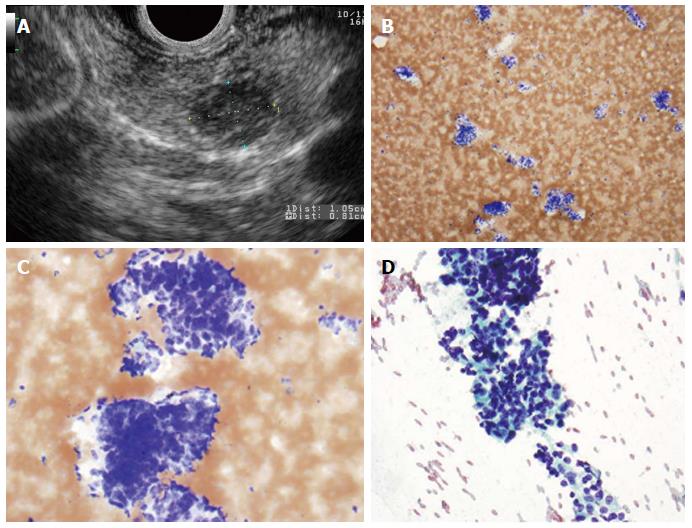
Figure 2 Pancreatic neuroendocrine neoplasm.
A: Endoscopic ultrasound image showing a 9 mm × 10 mm neuroendocrine tumor (insulinoma); B: Low-power view shows a cellular aspirate composed of clusters of uniform cells (Diff-QuikTM stain, × 100); C: High power view shows uniform cells with high N:C ratios and coarse chromatin (Diff-QuikTM stain, × 400); D: Papanicolaou stain highlights coarse, evenly distributed chromatin (× 400).
LYMPHOMA
Primary pancreatic lymphoma is rare, comprising only 0.5% of all pancreatic masses[37]. In one study of EUS-FNA, lymphoma made up to 8% of the non-adenocarcinoma masses[33]. Most pancreatic lymphomas are non-Hodgkin lymphomas. Making an accurate diagnosis of lymphoma is important as treatment is generally chemotherapy and/or radiation as opposed to adenocarcinoma which is most often managed by surgery[37]. EUS-FNA has become more commonly used in the diagnosis of pancreatic lymphoma. Pancreatic lymphomas are less likely to present with jaundice. The addition of flow cytometry has greatly improved lymphoma diagnosis compared to cytology alone[38]. Figure 3 represents cytology from pancreatic follicular lymphoma showing a cellular aspirate composed of relatively monotonous in appearance lymphocytes with mild atypia.
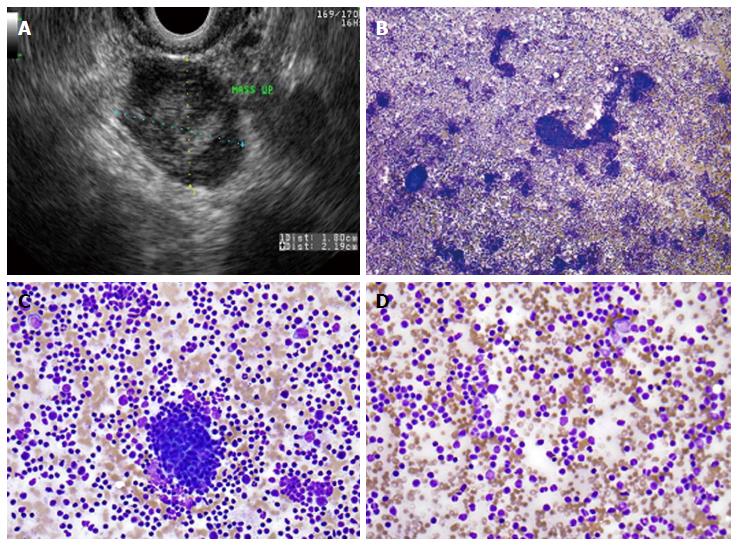
Figure 3 Primary pancreatic lymphoma.
A: Endoscopic ultrasound demonstrating a 1.8 cm × 2.2 cm lymphoma in the uncinate process of the pancreas; B: Low-power view showing a very cellular aspirate composed of discohesive lymphoid cells (Diff-QuikTM stain, × 100); C: High-power view showing an admixture of mature lymphocytes of various sizes with no more than a minimal atypia; lymphoid aggregates resembling a germinal center are also present (bottom); D: Small mature lymphocytes with cleaved and irregular nuclei raising suspicion for a mature B-cell lymhoma. (Diff-QuikTM stain, × 400).
PANCREATIC GASTROINTESTINAL STROMAL TUMOR
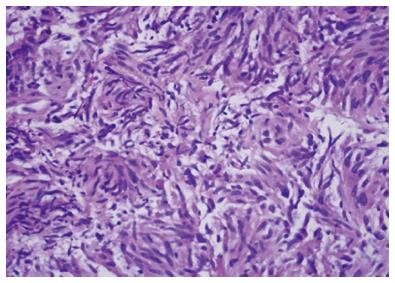
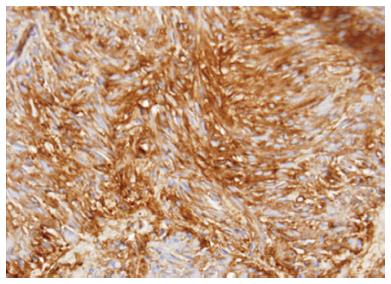
Primary extra-gastrointestinal stromal tumor arising in the pancreas is exceedingly rare. There have been 21 cases reported in the English literature in the last 10 years. The diagnosis of gastrointestinal stromal tumor (GIST) is based on histological, immunohistochemical, and molecular features. Microscopically the tumor usually consists of spindle and/or epithelioid cells typically arranged in fascicles or nests. GIST can often have the appearance of neuroendocrine tumors on EUS (Figure 4) thus an addition of EUS-FNA is highly valuable for differentiating these tumor types[39]. Figure 5 represents cytology from a primary pancreatic GIST tumor. Immunoistochemical positivity of CD117 confirms the diagnosis of GIST (Figure 6).
Figure 4 Endoscopic ultrasound image of large, 3.
5 cm × 4.4 cm, round, hypoechoic, heterogenous mass lesion arising from the tail of the pancreas.
Figure 5 Cytology from a primary pancreatic gastrointestinal stromal tumor.
Figure 6 Pancreatic gastrointestinal stromal tumor, cytology demonstrates a spindle cell neoplasm with moderate nuclear pleomorphism which stains strongly positive for CD117 and negative for desmin, consistent with a gastrointestinal stromal tumor arising from the pancreas.
(Courtesy of Rashmi Agni, University of Wisconsin Department of Pathology and Laboratory Medicine).
METASTATIC DISEASE
Metastatic disease to the pancreas is uncommon. The most common metastatic disease found with EUS-FNA includes renal cell carcinoma, melanoma and small cell lung cancer with renal cell carcinoma being the most common[33,40,41]. Other tumors metastatic to pancreas include papillary serous carcinoma (Figure 7), breast cancer, and rarely, sarcoma. EUS-FNA may be helpful in making these rare diagnoses.
Figure 7 Metastatic high-grade serous carcinoma of the ovary.
A: Endoscopic ultrasound image of a metastatic high-grade serous carcinoma of the ovary; B: Low-power view showing a cellular aspirate with a necrotic background (Diff-QuikTM stain, × 100); C: High-power view showing groups of malignant cells with large nuclei and prominent nucleoli. These cells are difficult to distinguish from a primary pancreatic ductal adenocarcinoma; however, necrotic background is not common in a primary tumor (Diff-QuikTM stain, × 400); D: Papanicolaou stain showing a cannon ball shaped group of malignant cells with large, round nuclei and prominent nucleoli, characteristic of serous ovarian carcinoma (× 400).
NON-DIAGNOSTIC SAMPLES
Despite pancreatic adenocarcinoma being the most common mass of the pancreas, the above examples highlight the broad differential that exists with a pancreatic mass. It also highlights the importance of tissue diagnosis especially when diagnosis is not clear. While EUS-FNA remains the procedure of choice for obtaining tissue from pancreas lesions, non-diagnostic samples are not uncommon. Determining what to do when FNA is non-diagnostic is difficult. Multiple studies have shown the benefit of repeat EUS-FNA with high diagnostic yield rates of 61% to 84%[42-44]. Given this data, many authors recommend repeat EUS-FNA when providers are faced with a non-diagnostic sample.
PANCREATIC NEOPLASMS-CYSTIC LESIONS
EUS-FNA plays a vital role in the examination of pancreatic cystic lesions. Pancreatic cysts are quite common with incidental cysts being reported in range of 2.6%-13.6% depending on imaging modality used[45,46]. In one autopsy study cysts occurred in 24.3% of patients[47]. The true incidence of neoplastic pancreatic cysts is difficult to determine. Deciding which pancreatic cysts require EUS-FNA for evaluation is one of the first steps in management. With advances and ease of EUS-FNA, it would be tempting for endoscopists to perform FNA on all lesions referred to them; however there are certain attributes on imaging which may help to avoid FNA altogether. Magnetic resonance imaging (MRI) and CT are valuable in assessing cystic size and determining if cystic lesions have worrisome findings such as connection with the pancreatic main duct. MRI has a distinct advantage over CT in visualizing fluid, particularly in T2 weighted series[46]. EUS alone has a particular advantage over other imaging modalities for evaluation of cysts due to the close proximity of lesions. EUS is particularly good at examining cyst morphology including size location, internal structural features, wall thickness, the presence of calcifications and ductal communication.
Generally cystic lesions are divided into two categories: neoplastic cystic tumors and non-neoplastic cystic tumors. Neoplastic cystic tumors include mucinous cystic neoplasm (MCN), intraductal papillary-mucinous neoplasm (IPMN), and serous cystic neoplasm (SCN). Morphologic features are different for each cyst type.
SCNs, often called microcystic adenoma or glycogen-rich cystadenoma, are generally considered benign lesions as they have been associated with only a few cases of malignant conversion. On imaging, SCNs often have a honeycomb appearance. A central stellate scar is pathognomonic for SCN. There tend to be thin internal septa that are hypervascular on Doppler. Around 10% of SCNs are unilocular without an obvious microcystic component[48,49].
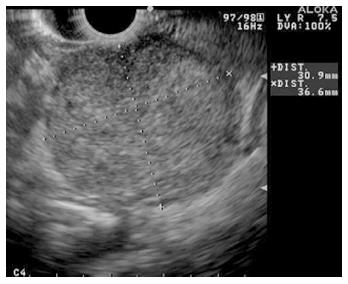
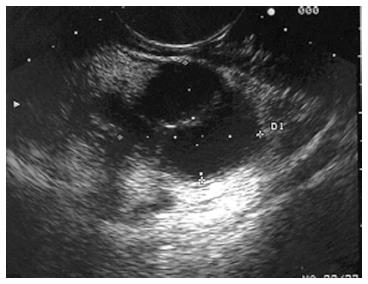
MCNs are found almost exclusively in the distal pancreas. They tend to occur in middle-aged women and generally considered to have a low malignant potential[50]. MCNs are characterized by two distinct histologic components: an inner epithelial layer composed of tall mucin-secreting cells, and a densely cellular ovarian-type stroma[50]. On imaging, MCNs are multiloculated cysts with a visible cystic wall. Peripheral calcification (egg shell calcification) can be seen in 10%-25% of MCNs and help to differentiate them from SCN. It is not always possible to determine lesions to be MCN on imaging alone thus FNA can be helpful. Due to malignant potential, most MCNs are removed surgically. Lesions less than 4 cm have low malignant potential, and in elderly patients who are not strong surgical candidates, these lesions can be observed[50]. Differentiating MCN from mucinous cystadenocarcinoma (Figure 8) by imaging alone is difficult; cytology and fluid analysis are both helpful in differentiating the two.
Figure 8 Endoscopic ultrasound image demonstrating a cystadenocarcinoma.
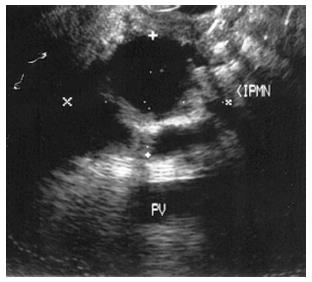
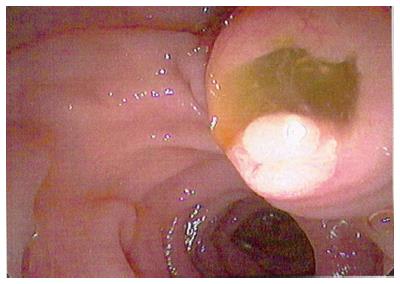
IPMNs were first recognized in 1982 and since then these cysts are commonly seen incidentally on cross sectional imaging. IPMN can appear as a cyst or a cluster of cysts in the uncinate process (Figure 9). IPMNs are generally defined as intraductal epithelial neoplasms of mucin-producing cells of the main duct or side branches[51]. Main duct IPMNs can cause dilation of the pancreatic duct up often > 5 cm; and have higher malignant potential thus are generally managed surgically[52]. Main duct IPMNs can create the classic “fish mouth papilla” due to the presence of mucin within the main duct (Figure 10).
Figure 9 Endoscopic ultrasound image demonstrating an intraductal papillary-mucinous neoplasm.
Figure 10 Endoscopic view of “fish mouth papilla” due to the presence of mucin within the main duct.
Despite the advances of EUS in visualizing cystic lesions, EUS alone is often not enough in determining if malignancy is present. The addition of cystic fluid analysis further helps differentiate cysts. Currently, measurement of amylase and carcino-embryonic antigen (CEA) are the most routinely used in clinical practice. Amylase is often used in differentiating cystic neoplasms from pseudocysts, with amylase < 250 U/L being highly specific for SCN and MCNS (98%). In a review of 12 studies, the median values of amylase in pseudocysts, SCN, MCN and mucinous cystadenocarcinoma were 11000, 250, 8000 and 150 IU/L, respectively[53].
Multiple tumor markers have been studied to help differentiate mucinous neoplasms from non-mucinous neoplasms. These markers include CEA, CA 19-9, CA 72-4 and CA-125; ultimately CEA was determined to be the most useful in this setting[53]. A cut off of 192 ng/mL for CEA was first demonstrated by Brugge et al[54] as providing the greatest area under the curve (0.79) for differentiating mucinous vs nonmucinous cystic lesions. Additionally, a CEA > 800 ng/mL has been shown to be 79% accurate for mucinous lesions (MCN or mucinous cystadenocarcinoma)[53]. Higher CEA levels are more often associated with malignant lesions. Cyst fluid cytology can also be helpful in determining if there is an underlying mucinous cystadenocarcinoma although sensitivity is not high (sensitivity of 48% for malignant cystic lesions)[53]. Brugge et al[54] showed the sensitivity of cytology for MCN to be as low as 34.5% with a specificity of 83%. Figure 11A and B represents cytology from a mucinous neoplasm; mucinous cystic neoplasm and intraductal papillary mucinous neoplasm are indistinguishable cytologically. Most centers combine amylase and CEA measurements and fluid cytology to establish the diagnosis of mucinous cystic neoplasm.
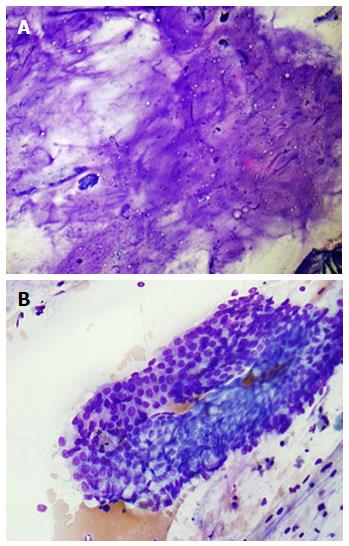
Figure 11 Pancreatic mucinous neoplasm.
A: Low-power view of pancreatic mucinous neoplasm showing copious thick, colloid-like mucin (Diff-QuikTM stain, × 100); B: High-power view of pancreatic mucinous neoplasm showing sheets of only mildly atypical columnar cells containing intracytoplasmic mucin; these cells are very difficult to distinguish from benign gastric or duodenal epithelium (Diff-QuikTM stain, × 400).
Recently DNA analysis and k-ras mutation have also been shown to be useful to determine pancreatic cyst type and the presence of malignancy. In the PANDA study, using the criteria of a high amplitude k-ras mutation followed by allelic loss showed a maximum specificity of 96% for malignancy. Additionally, this study was able to demonstrate that all malignant cysts that were negative by conventional cytologic evaluation could be diagnosed as malignant by using DNA analysis[55]. Recently two studies have used microRNAs (miRNA) with good success differentiating pancreatic cysts[56,57] with one study showing a panel of miRNA being able to distinguish MCN from SCN, branch duct-IPMN, main duct-IPMN, and adenocarcinoma with a sensitivity and specificity of 100%.
COMPLICATIONS
One of the biggest concerns when considering aspiration of a cystic lesion is the introduction of infection. Although rare, multiple aspirations increase this risk. The current guidelines recommend one aspiration for cysts to minimize this risk, followed by 48 h of antibiotic therapy[58]. Another complication when aspirating cysts is intracystic hemorrhage, also rare, endoscopists should be aware of this complication. Most patients with intracystic hemorrhage can still be managed on an outpatient basis with antibiotics[59].
The overall rate of complication with EUS alone or EUS-FNA is quite low. Complications other than infection and bleeding include perforation and the unique risk of pancreatitis. Perforation with EUS is rather rare. In a survey study, cervical esophageal perforation occurred in only 16 of 43852 reported upper EUS procedures at a frequency of 0.03%. Most of these patients were elderly, and most of the EUS procedures were done by trainees or personal with limited experience (less than 1 year)[60]. Experts agree that EUS is associated with a similar rate of perforations compared with standard endoscopy[58].
Pancreatitis is a unique complication associated with EUS-FNA for aspiration of both masses and cysts. Reported rates of pancreatitis associated with pancreatic EUS-FNA range from 0% to 2%[58]. In one study where two cases of pancreatitis were reported, both were mild and both patients had a recent history of pancreatitis. Authors concluded a history of recent pancreatitis could be potential risk factor for procedure- induced pancreatitis[61].
CONCLUSIONS
EUS-FNA is a safe and effective procedure for the evaluation of solid and cystic lesions of the pancreas. Ways to optimize diagnostic yield for pancreatic masses continue to be investigated; overall the availability of ROSE seems to have the biggest impact on results. Optimal needle size appears to be 22 or 25 gauge, while suctioning and stylet do not have a positive impact on performance. EUS-FNA is helpful in differentiating adenocarcinoma from other more rare lesions including neuroendocrine tumors, lymphoma and metastatic lesions, and whenever diagnostic uncertainty exists; EUS-FNA should be pursued. In the evaluation of cystic lesions, EUS-FNA is a safe and effective way of classifying lesions. Measurement of cystic fluid CEA, amylase and cytology remain valuable in routine aspiration. Studies on DNA markers show promise in optimizing the detection of cystic malignancies, although currently routine use of DNA markers is not recommended. Whether it is evaluating a solid or cystic pancreatic lesion, EUS-FNA plays a pivotal role, as technology improves this role will continue to grow.
P- Reviewer: Barreto S, Neri V, Shi XY S- Editor: Gong XM L- Editor: A E- Editor: Wu HL