Published online Mar 16, 2015. doi: 10.4253/wjge.v7.i3.224
Peer-review started: August 23, 2014
First decision: December 4, 2014
Revised: December 12, 2014
Accepted: December 18, 2014
Article in press: December 19, 2014
Published online: March 16, 2015
Processing time: 209 Days and 17.2 Hours
Advanced endoscopic imaging is revolutionizing our way on how to diagnose and treat colorectal lesions. Within recent years a variety of modern endoscopic imaging techniques was introduced to improve adenoma detection rates. Those include high-definition imaging, dye-less chromoendoscopy techniques and novel, highly flexible endoscopes, some of them equipped with balloons or multiple lenses in order to improve adenoma detection rates. In this review we will focus on the newest developments in the field of colonoscopic imaging to improve adenoma detection rates. Described techniques include high-definition imaging, optical chromoendoscopy techniques, virtual chromoendoscopy techniques, the Third Eye Retroscope and other retroviewing devices, the G-EYE endoscope and the Full Spectrum Endoscopy-system.
Core tip: Here we focus on the newest developments in the field of colonoscopic imaging to improve adenoma detection rates. Described techniques include high-definition imaging, optical chromoendoscopy techniques, virtual chromoendoscopy techniques, the Third Eye Retroscope and other retroviewing devices, the G-EYE endoscope and the Full Spectrum Endoscopy-system.
- Citation: Neumann H, Nägel A, Buda A. Advanced endoscopic imaging to improve adenoma detection. World J Gastrointest Endosc 2015; 7(3): 224-229
- URL: https://www.wjgnet.com/1948-5190/full/v7/i3/224.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i3.224
Colorectal cancer is the second most common cause for cancer related death in developed countries. The age-adjusted incidence of colorectal cancer is estimated 61.2 cases and 44.8 cases per 100000 populations among men and women, respectively[1]. Colonoscopy is considered the golden standard for screening of colorectal cancer and its precursor lesions, the colorectal adenomas. The main advantage of colonoscopy in comparison to non-endoscope based screening tests is that it also allows therapy or at least tissue acquisition of colorectal lesions to guide subsequent therapy.
Recently, Nishihara and coworkers examined the association of the use of colonoscopy with colorectal-cancer incidence and colorectal-cancer mortality among participants in the Nurses’ Health Study and the Health Professionals Follow-up Study[2]. Overall, more than 88000 participants were followed over a period of 22 years. Within this time, 1815 incident colorectal cancers and 474 deaths from colorectal cancer were documented. Multivariate hazard ratios for colorectal cancer were 0.57 after polypectomy, 0.60 after negative sigmoidoscopy, and 0.44 after negative colonoscopy. In addition, negative colonoscopy was associated with a reduced incidence of proximal colon cancer. Moreover, a reduced mortality from proximal colon cancer was observed after screening colonoscopy but not after sigmoidoscopy. Accordingly, this long-term study confirmed the efficacy of screening colonoscopy to reduce colorectal cancer.
Very recently, Corley and coworkers evaluated the association between the adenoma detection rate and patients’ risk of subsequent colorectal cancer (i.e., interval cancer) and death[3]. Over 314000 colonoscopies performed by 136 endoscopists were included. The adenoma detection rates ranged from 7.4% to 52.5%. During the follow-up period, 712 interval cancers were diagnosed. The adenoma detection rate was inversely associated with the risks of interval colorectal cancer, advanced-stage interval cancer, and fatal interval cancer. Importantly, each 1% increase in the adenoma detection rate was associated with a 3% decrease in the risk of colorectal cancer.
Therefore, the above mentioned studies highlighted the importance of a precise colonoscopic examination to reduce colorectal cancer incidence. Within recent years, various new endoscopic imaging techniques have been introduced to assist endoscopists in performing accurate endoscopic examinations. In this review we will focus on the newest developments in the field of colonoscopic imaging including high-definition imaging, optical chromoendoscopy techniques, virtual chromoendoscopy techniques, the Third Eye Retroscope and other retroviewing devices, the G-EYE endoscope and the Full Spectrum Endoscopy (FUSE)-system.
Multiple studies have addressed the specific issue whether high-definition white-light imaging is superior to standard white-light endoscopy for diagnosis of colorectal adenomas. Results of those studies are sometimes conflicting. In addition, interpretation is often difficult as new endoscopes are not only equipped with newer chip technology allowing high-definition imaging, but also with wide-field optics and closer focus modes. Therefore, it is not possible to determine which of these individual factors led to altered adenoma detection. One recent meta-analysis compared the diagnostic yield of colonic polyps between high-definition colonoscopy and standard video endoscopy[4]. Five studies involving 4422 patients were included. The incremental yield of high definition colonoscopy for the detection of any polyp was 3.8% with a number needed to treat of 26. For the detection of adenomatous polyps the incremental yield was 3.5% with a number needed to treat of 28. There were no significant differences between high-definition and standard video endoscopy in the detection of high-risk adenomas. Nonetheless, the pooled weighted mean difference in small adenoma detection was significantly higher with high-definition colonoscopy. In a retrospective study including 2430 consecutive patients the adenoma detection rate was significantly higher among patients who underwent high-definition white-light endoscopy compared with standard white-light colonoscopies[5]. These data are in contrast to one recent trial including 426 individuals who underwent high-definition white-light endoscopy and 426 individuals who underwent conventional colonoscopy[6]. In this study, high-definition endoscopy did not increase the detection of individuals with polyps, adenomas, or high-risk adenoma features. High-definition did also not increase the detection of individuals with clinically insignificant colonic lesions.
Importantly, one recent study aimed to investigate whether detection rates of individual endoscopists increase within 1 year before and 1 year after the switch from standard to high-definition endoscopy[7]. In this study, the adenoma detection rates of endoscopists with a low detection rate (< 20%) increased significantly after switch from standard to high-definition endoscopy (P = 0.0076) while this effect was not measurable for high-adenoma detectors (≥ 20%).
Optical chromoendoscopy uses optical filters within the light source of the endoscope to narrow the bandwidth of the light. The normal bandwidth consists of a red-green-blue image. The narrow band imaging (NBI; Olympus, Tokyo, Japan) narrows the red light. The resulting green-blue image improves imaging of the mucosal vascular and surface pattern morphology[8].
To date, four meta-analyses evaluated the impact of NBI for colon polyp detection as compared to white-light endoscopy. None of these could find convincing evidence that NBI is significantly better than white-light endoscopy for detection of colorectal polyps[9-12]. The most recent meta-analysis included 7 studies with a total of 2936 patients[12]. No statistically significant difference in the overall polyp or adenoma detection rate with the use of NBI or white-light endoscopy was detected. In addition, when the number of adenomas and polyps per patient was analyzed, no significant difference was found between NBI and white-light endoscopy.
One main disadvantage of the NBI system is the relatively dark image according to its principle of light filtering. While NBI has proven its efficacy for characterization of lesions in multiple studies its value for detection of lesions seems to be limited as the darker NBI image does mostly not allow a detailed view of the colonic structures.
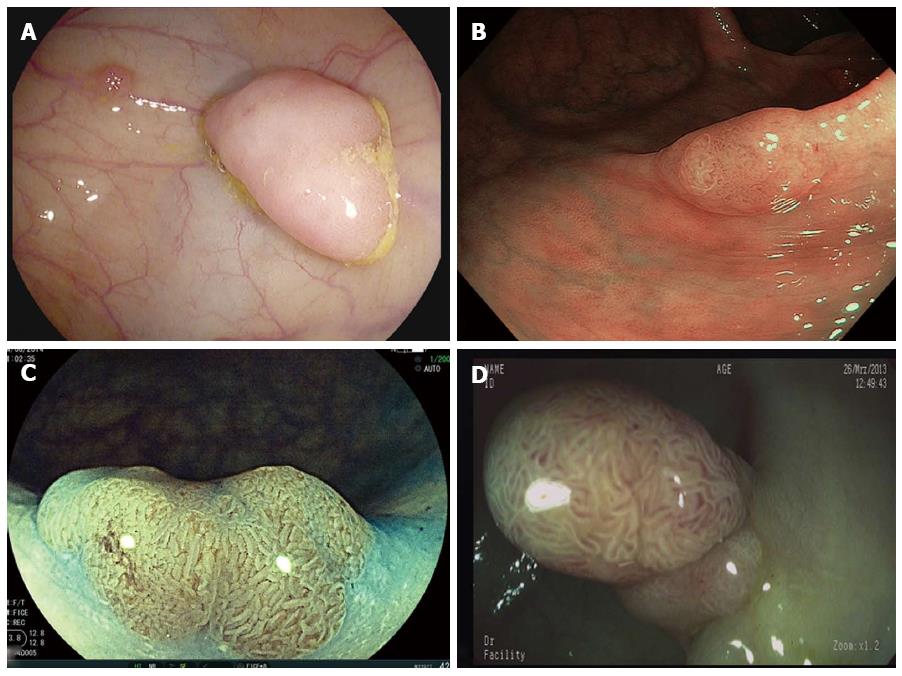
Very recently, a new NBI system was launched (Olympus, Tokyo, Japan), now allowing an up to 4-times brighter image (Figure 1). The new system was already evaluated in a trial by Leung et al[13] which included 360 patients. Patients were randomized to undergo either NBI or high-definition white-light endoscopy. In this well designed study, both the adenoma and polyp detection rates were significantly higher in the NBI group as compared with the high-definition white-light group. No significant differences were observed in the adenoma miss rates between the two groups. Therefore, these early results suggest that the new NBI system is superior to conventional white-light endoscopy. The final results of multicenter studies addressing this issue are therefore highly anticipated.
Virtual chromoendoscopy techniques rely on the principle of digital postprocessing and include Fujinon Intelligent Color Enhancement (FICE, Fujifilm, Tokyo, Japan), i-scan (Pentax, Tokyo, Japan) and the recently introduced SPIES system (Storz, Tuttlingen, Germany) (Figure 2). The technical details of the systems have been reviewed in detail elsewhere[14,15].
Similar to optical chromoendoscopy, results on the efficacy of virtual chromoendoscopy for improved adenoma detection are contrary with studies reporting on improved adenoma detection rates and others not. One early study by Arthur Hoffman included 220 patients which were randomized in a 1:1 ratio to undergo high-definition white-light endoscopy or i-scan[16]. Colonoscopy performed with i-scan detected significantly more patients with colorectal neoplasia (38%) as compared to standard white-light endoscopy (13%). These data were confirmed in a retrospective study by Testoni et al[17] reporting significantly more detected lesions with i-scan as compared to standard white-light endoscopy. Contrary, Hong et al[18] performed a prospective, randomized trial using a back-to-back colonoscopy design. Overall, 389 patients were randomized. The adenoma detection rates during the first withdrawal of high-definition white-light endoscopy and i-scan and the adenoma miss rates of each group were not statistically different between the different groups. Based on the multivariate analysis, the application of i-scan was not associated with an improvement in adenoma detection and the prevention of missed polyps. While there are currently no data on the newly introduced SPIES-system, even the results of studies evaluating the FICE system produced inconsistent data. In this context, one study enrolled 359 patients and randomly assigned those to the white-light group followed by the FICE group and the FICE group followed by the white-light endoscopy group. There was no significant difference between FICE and white-light endoscopy in the adenoma detection rate[19]. Another study examined 135 consecutive patients by total colonoscopy and 128 patients were randomized to compare white-light colonoscopy and FICE[20]. Colonoscopy with FICE identified significantly more patients with small colorectal adenomas than conventional white-light colonoscopy.
In 2007, the Third Eye Retroscope (Avantis Medical Systems, Sunnyvale, United Ststes) was introduced[21]. The device consists of a fiber optic which is introduced through the working channel of a standard colonoscope until it extends beyond its other end. Afterwards, the Third Eye Retroscope turns around 180 degrees. The endoscopist has now two images on one monitor. One image is showing the standard colonoscopic view and one image is providing the retrograde view. Main advantage of the system is that it allows to visualize lesions located proximal (i.e., behind) the colonic folds. Various studies have evaluated the Third Eye Retroscope. One multicenter study included eight different centers and a total of 249 patients[22]. 257 polyps were identified with the colonoscope alone while the Third Eye Retroscope detected significantly more additional polyps and adenomas. For lesions 6mm or larger and 10 mm or larger, the additional detection rates with the Third Eye Retroscope for adenomas was 25% and 33%, respectively. Every polyp that was detected with the Third Eye Retroscope was subsequently located with the colonoscope and removed. Another, open-labeled, prospective, multicenter study at nine sites evaluated the impact of the Third Eye Retroscope on adenoma detection rates during colonoscopy[23]. Overall, a 16% increase in the adenoma detection rate by using the Third Eye Retroscope was detected. For lesions 6mm or larger and 10 mm or larger, the overall additional detection rates with the Third Eye Retroscope for all adenomas were 24% and 19%, respectively. Meanwhile, the data have also been confirmed by other investigators demonstrating an improved adenoma detection rate with the Third Eye Retroscope by visualizing areas located proximal (i.e., behind) colonic folds[24,25].
However, despite its efficacy, one potential limitation of the Third Eye Retroscope might be that the working channel is blocked. Accordingly, in order to perform endoscopic therapy of detected lesions, one have to withdraw the device first before advancing additional equipment necessary for polyp removal. In the attempt to offer a hybrid of a therapeutic scope which also allows relatively easy visualization of areas located behind the colonic folds, new “RetroView” devices were recently introduced. These devices (3490TFi, Pentax, Tokyo, Japan and 580RD, Fujifilm, Tokyo, Japan) are slim colonoscopes allowing retroflexion of the distal tip at 210 degrees (Figure 3) In addition, the endoscopes are equipped with latest virtual chromoendoscopy techniques (i.e., i-scan; FICE) and working channels of 3.2mm thereby allowing characterization, demarcation and endoscopic therapy at once. Currently, no scientific evidence regarding the new retroviewing devices is available but multiple groups are already evaluating the potential beneficial effect of the technology.
FUSE (EndoChoice, GA, United States) was recently introduced as a new platform (Figure 3). The FUSE-colonoscope consists of three imagers integrated into the distal tip of the endoscope and at the lateral sides thereby enabling a 330° angle of view of the colon. Images are displayed on three contiguous monitors. Very recently, Ian Gralnek et al[26] presented the results of a large international multicenter study comparing standard forward viewing endoscopy with the FUSE system. Patients underwent same-day, back-to-back tandem colonoscopy with a standard forward-viewing colonoscope and the full-spectrum colonoscope after a 1:1 randomization. Overall, 185 patients were included and randomly assigned to both groups. By per-lesion analysis, the adenoma miss rate was significantly lower in patients receiving FUSE than in those in the standard forward-viewing group (7% vs 41%). Therefore, the FUSE platform represents a new and promising technology to improve the efficacy of colorectal cancer screening and surveillance.
Very recently, the G-EYE endoscope (Smart Medical, Ra’anana Israel) was launched. The G-EYE relies on a standard endoscope in which a permanently integrated balloon was incorporated at its distal bending section (Figure 4). The balloon is inflated in the cecum and the endoscope is withdrawn with the balloon inflated until the rectum is reached. The inflated balloon stabilizes the endoscope during the withdrawal phase and interventions and provides additional folds straightening in order to improve adenoma detection rates. Early data provided by Kiesslich and coworkers suggest that the adenoma detection rate with the G-EYE endoscope could be increased by at least 48% (personal communication). Final results of the ongoing multicenter studies are expected by the end of the year.
In the attempt to improve adenoma detection rates various advanced endoscopic imaging techniques have been introduced within the past 5 years. Scientific evidence is still missing for some of the new technologies. It is still not fully known whether pure high-definition white-light endoscopy improves adenoma detection rates. Therefore, prospective, randomized, multicenter studies addressing this issue are highly warranted. While there was no beneficial effect of the first NBI system, recent evidence suggests that the new NBI system is superior to conventional white-light endoscopy and could improve adenoma detection rates. Again, results of multicenter studies addressing this issue are highly anticipated. Study results on the potential of virtual chromoendoscopy techniques using digital postprocessing for improved adenoma detection in the colorectum are still inconsistent. Multiple, large and multicenter studies are currently addressing this issue and the results of those studies are anticipated latest within the next two years. New endoscope platforms now allow for a more detailed view of the luminal gastrointestinal tract. Early data demonstrate the impressive potential of those new platforms to improve early diagnosis of colorectal lesions without detriment to procedure time or procedure complications. Therefore, new endoscopic imaging techniques will assist the endoscopists to improve adenoma detection rates for better diagnosis and early therapy of colorectal lesions.
P- Reviewer: Agresta F, Aytac E, Lee CL S- Editor: Tian YL L- Editor: A E- Editor: Zhang DN
| 1. | Lieberman DA. Clinical practice. Screening for colorectal cancer. N Engl J Med. 2009;361:1179-1187. |
| 2. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. |
| 3. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. |
| 4. | Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499-505. |
| 5. | Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M, Gross SA. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364-370. |
| 6. | Burke CA, Choure AG, Sanaka MR, Lopez R. A comparison of high-definition versus conventional colonoscopes for polyp detection. Dig Dis Sci. 2010;55:1716-1720. |
| 7. | Waldmann E, Britto-Arias M, Gessl I, Heinze G, Salzl P, Sallinger D, Trauner M, Weiss W, Ferlitsch A, Ferlitsch M. Endoscopists with low adenoma detection rates benefit from high-definition endoscopy. Surg Endosc. 2015;29:466-473. |
| 8. | Mönkemüller K, Fry LC, Zimmermann L, Mania A, Zabielski M, Jovanovic I. Advanced endoscopic imaging methods for colon neoplasia. Dig Dis. 2010;28:629-640. |
| 9. | Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;1:CD008361. |
| 10. | Sabbagh LC, Reveiz L, Aponte D, de Aguiar S. Narrow-band imaging does not improve detection of colorectal polyps when compared to conventional colonoscopy: a randomized controlled trial and meta-analysis of published studies. BMC Gastroenterol. 2011;11:100. |
| 11. | Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363-70; quiz 371. |
| 12. | Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc. 2012;75:604-611. |
| 13. | Leung WK, Lo OS, Liu KS, Tong T, But DY, Lam FY, Hsu AS, Wong SY, Seto WK, Hung IF. Detection of colorectal adenoma by narrow band imaging (HQ190) vs. high-definition white light colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2014;109:855-863. |
| 14. | Neumann H, Fujishiro M, Wilcox CM, Mönkemüller K. Present and future perspectives of virtual chromoendoscopy with i-scan and optical enhancement technology. Dig Endosc. 2014;26 Suppl 1:43-51. |
| 15. | Tontini GE, Vecchi M, Neurath MF, Neumann H. Review article: newer optical and digital chromoendoscopy techniques vs. dye-based chromoendoscopy for diagnosis and surveillance in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1198-1208. |
| 16. | Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827-833. |
| 17. | Testoni PA, Notaristefano C, Vailati C, Di Leo M, Viale E. High-definition colonoscopy with i-Scan: better diagnosis for small polyps and flat adenomas. World J Gastroenterol. 2012;18:5231-5239. |
| 18. | Hong SN, Choe WH, Lee JH, Kim SI, Kim JH, Lee TY, Kim JH, Lee SY, Cheon YK, Sung IK. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest Endosc. 2012;75:1011-1021.e2. |
| 19. | Chung SJ, Kim D, Song JH, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc. 2010;72:136-142. |
| 20. | Cha JM, Lee JI, Joo KR, Jung SW, Shin HP. A prospective randomized study on computed virtual chromoendoscopy versus conventional colonoscopy for the detection of small colorectal adenomas. Dig Dis Sci. 2010;55:2357-2364. |
| 21. | Triadafilopoulos G, Watts HD, Higgins J, Van Dam J. A novel retrograde-viewing auxiliary imaging device (Third Eye Retroscope) improves the detection of simulated polyps in anatomic models of the colon. Gastrointest Endosc. 2007;65:139-144. |
| 22. | Waye JD, Heigh RI, Fleischer DE, Leighton JA, Gurudu S, Aldrich LB, Li J, Ramrakhiani S, Edmundowicz SA, Early DS. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos). Gastrointest Endosc. 2010;71:551-556. |
| 23. | DeMarco DC, Odstrcil E, Lara LF, Bass D, Herdman C, Kinney T, Gupta K, Wolf L, Dewar T, Deas TM. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc. 2010;71:542-550. |
| 24. | Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480-489. |
| 25. | Siersema PD, Rastogi A, Leufkens AM, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI. Retrograde-viewing device improves adenoma detection rate in colonoscopies for surveillance and diagnostic workup. World J Gastroenterol. 2012;18:3400-3408. |
| 26. | Gralnek IM, Siersema PD, Halpern Z, Segol O, Melhem A, Suissa A, Santo E, Sloyer A, Fenster J, Moons LM. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol. 2014;15:353-360. |