Published online Mar 16, 2015. doi: 10.4253/wjge.v7.i3.206
Peer-review started: September 2, 2014
First decision: November 19, 2014
Revised: December 5, 2014
Accepted: December 18, 2014
Article in press: December 19, 2014
Published online: March 16, 2015
Zenker’s diverticulum (ZD) is an abnormal hypopharyngeal pouch often presenting with dysphagia. Treatment is often sought with invasive surgical management of the diverticulum being the only mode of definitive therapy. Primarily done by an open transcervical approach in the past, nowadays treatment is usually provided by otolaryngologists using a less invasive trans-oral technique with a rigid endoscope. When first described, this method grew into acceptance quickly due to its similar efficacy and vastly improved safety profile compared to the open transcervical approach. However, the main limitation with this approach is that it may not be suitable for all patients. Nonetheless, progress in the field of natural orifice endoscopic surgery over the last 10-20 years has led to the increase in utilization of the flexible endoscope in the treatment of ZD. Primarily performed by interventional gastroenterologists, this approach overcomes the prior limitation of its surgical counterpart and allows adequate visualization of the diverticulum independent of the patient’s body habitus. Additionally, it may be performed without the use of general anesthesia and in an outpatient setting, thus further increasing the utility of this modality, especially in elderly patients with other comorbidities. Today, results in more than 600 patients have been described in various published case series using different techniques and devices demonstrating a high percentage of clinical symptom resolution with low rates of adverse events. In this article, we present our experience with flexible endoscopic therapy of Zenker’s diverticulum and highlight the endoscopic technique, outcomes and adverse events related to this minimally invasive modality.
Core tip: Definitive therapy for Zenker’s diverticulum (ZD) typically includes either diverticulectomy or myotomy/septotomy of the cricopharyngeus muscle. Previously done as an open transcervical approach by surgeons, treatment has now evolved to include a minimally invasive trans-oral approach with flexible endoscopy performed by gastroenterologists. In this article we highlight our experience with flexible endoscopic therapy of ZD at our institution, describe commonly used flexible endoscopic techniques and devices, and assess efficacy and safety data related to this minimally invasive modality.
- Citation: Perbtani Y, Suarez A, Wagh MS. Techniques and efficacy of flexible endoscopic therapy of Zenker’s diverticulum. World J Gastrointest Endosc 2015; 7(3): 206-212
- URL: https://www.wjgnet.com/1948-5190/full/v7/i3/206.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i3.206
Zenker’s diverticulum (ZD) is a pharyngeal outpouching caused by increased intraluminal pressure, in conjunction with an area of inherent weakness in the hypopharynx known as the Killian triangle. This area of vulnerability is formed in between two pharyngo-esophageal muscles, the inferior pharyngeal constrictor and the cricopharyngeus. Since the original description of the condition by Ludlow[1] and then Zenker von Zeimssen[2]; the pathophysiology that leads to the ZD has been poorly understood. Currently, the mechanism that leads to increase in luminal pressures causing the ZD is thought to be due to poor upper esophageal sphincter (UES) compliance[3,4].
Characteristic symptoms for ZD include dysphagia, which is the presenting symptom in 80%-90% of patients. Additionally, patients may present with cough, dysphonia, malnutrition and weight loss. Occurrence is not usual in patients under the age of 40 years with incidence most prevalent in males in the seventh or eighth decade of life[5]. The diagnosis of ZD is based on clinical and radiographic findings, with dynamic barium esophagram being the confirmatory study[6]. Surgical intervention involving disruption of the cricopharyngeus by myotomy and/or diverticulectomy is the mainstay of treatment. The open trans-cervical approach that was originally described by Wheeler[7] has now evolved after a sentinel paper published by Dohlman and Mattson[8] to a less invasive trans-oral approach using a rigid endoscope. This technique currently performed by otolaryngologists, is the method of choice due to similar efficacy, reduced patient morbidity and overall shorter hospital stay compared to traditional open transcervical surgery[9].
Indeed, all patients who are diagnosed with ZD would ideally undergo endoscopic therapy as the benefits mentioned previously make this a more favorable choice for patients and clinicians. However, as with all surgical procedures there are several pre-interventional considerations. At the crux of these issues are the needs to visualize the diverticulum trans-orally. Several patient indicators including high body mass index and poor neck flexibility predispose the patient to higher risk of adverse events and procedural failure. As such, an open approach is still used in 15% to 68% of cases[5]. Nevertheless, within the past 20 years the trans-oral approach has progressed with the advent use of a flexible endoscope. Currently performed by interventional gastroenterologists/endoscopists, this method helps overcome the prior concerns of visual limitations while still providing a minimal invasive approach to this complex surgical condition. Several variations to this procedure have been explored and published in recent times, though, lacking comprehensive long-term analysis and comparative effectiveness of these various techniques. In this article we highlight our experience with flexible endoscopic therapy of ZD at our institution, describe commonly used flexible endoscopic techniques and devices, and assess efficacy and safety data related to this minimally invasive modality.
The aim of this study was to assess the efficacy and safety of patients undergoing flexible endoscopic treatment of ZD. Efficacy was defined by: (1) technical success of endoscopic therapy; and (2) improvement in dysphagia score. Safety was characterized by the lack of development of intra-procedural or post-procedural adverse events (AE).
Technical success: Procedural technical success was defined as the ability to successfully perform flexible endoscopic cricopharyngeus myotomy.
Dysphagia score: A score range (0-4) was used to quantify dysphagia prior to and after endoscopic treatment[10].
Adverse events: Endoscopic adverse events were assessed based on previously established criteria by the American Society of Gastrointestinal Endoscopy (ASGE)[11].
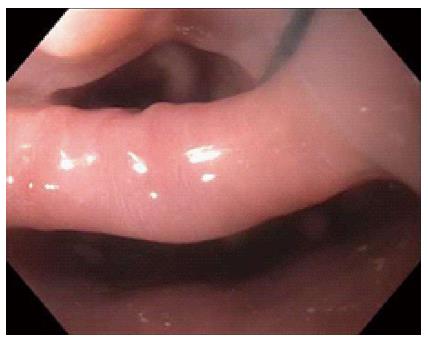
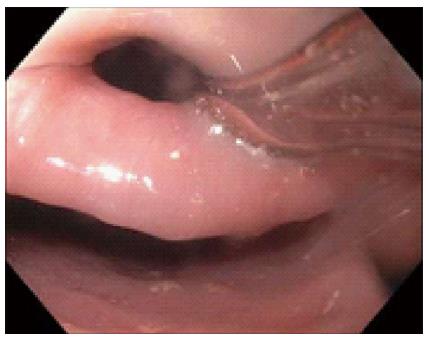
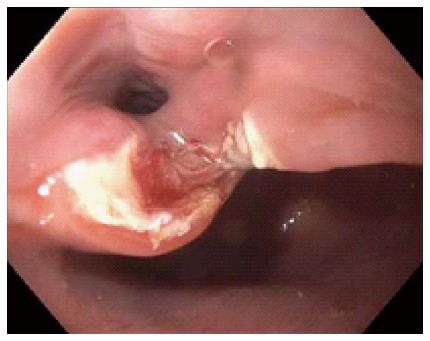
This study was approved by the University of Florida Institutional Review Board (IRB). Our electronic endoscopy database was queried from January 2006 through June 2014 for patients who were referred to a single interventional endoscopist for flexible endoscopic treatment of ZD. Diagnosis of ZD was made with either barium esophagram, computed tomography or direct endoscopic visualization. General anesthesia was used per anesthesiologist recommendations and prophylactic antibiotics were typically not given. The procedure was begun after general endoscopic evaluation of the ZD (Figure 1), stomach and duodenum. A nasogastric or orogastric tube (Figure 2) was then placed over a guidewire for improving visualization of the diverticulum and protection of the anterior esophageal wall during myotomy. A needle knife was then used to perform the myotomy (Figure 3) exposing the transverse fibers of the cricopharyngeus. Following the procedure, all patients were then admitted overnight for observation and gradual advance of their diet.
A total of 8 patients [50% male, mean age 72.4 years (range 58-88)] underwent technically successful flexible endoscopic myotomy of their ZD. One endoscopic treatment session was performed per patient and all patients noted improvement in their dysphagia symptoms after therapy. The mean pre-procedure dysphagia score was 2.6 (range 2-4) and post-procedure dysphagia score was 0.4 (range 0-2). There were no AE and mean follow-up time was 5.8 mo (range 0-17). Two patients with mild residual dysphagia did not wish to undergo a repeat procedure or other interventions.
Since landmark studies by Mulder[12] and Ishioka[13], the use of flexible endoscopy to treat ZD has come into many centers as an additional minimally invasive modality for management. Described by radiographic and manometric studies by Cook[3], pathogenesis of this abnormality seems to be related to poor UES compliance leading to increase swallowing pressures and in turn a Killian’s dehiscence. As such, the mainstay of treatment has been ablation or division of the cricopharyngeus “septum” (cricopharyngeus myotomy or septotomy) and there have been many variations in the way this myotomy is performed. Additionally, most institutions have employed tools aiding to secure and expose the septum such as the diverticuloscope and clear cap assisted devices. Currently, 19 case series/analyses[10,12-29] have been published describing flexible endoscopic therapy in over 600 patients with ZD (Table 1).
| Ref. | Total patients (n = 678) | Age (range) | Device for myotomy | Assist device | Pre-procedure dysphagia score | Post-procedure dysphagia score | Clinical symptom resolution rate | Adverse events | Recurrences | Followup (range) |
| Mulder et al[12] | 20 | Mean 82 (41-100) | FC | None | NA | NA | 85% | 0% | 0 | Mean 7 (1-18) |
| Ishioka et al[13] | 42 | Mean 68 (46-102) | NK | None | NA | NA | 93% | 5% | 7 | Mean 38 (12-96) |
| Hashiba et al[14] | 47 | (58-81) | NK | None | NA | 0 or 1 | 96% | 14.9% | 0 | (0-12) |
| Mulder et al[15] | 125 | Median 77 (41-100) | APC | None | NA | NA | 100% | 20% | NA | NA |
| Sakai et al[16] | 10 | (67-87) | NK | Cap | 1.8 | 0 | 100% | 0% | 0 | (2-12) |
| Evrard et al[17] | 31 | Median 78 | NK | DS | 93% | 13% | 9 | Median 12.5 | ||
| Costamagna et al[18] | 28 | Median 66 (47-86) | NK | Cap | NA | NA | 43% | 32% | 4 | Median 36(9-60) |
| Costamagna et al[18] | 11 | Median 70 (63-84) | NK | DS | NA | NA | 91% | 0% | 1 | Median 6.5(3-15) |
| Rabenstein et al[19] | 41 | Mean 73 | APC | Cap | NA | NA | 95% | 19.5% | 5 | Mean 16 |
| Christiaens et al[10] | 21 | Median 77.5 (52-89) | FC | Cap | 1.5 | 0 | 100% | 3% | 2 | Median 22.4 |
| Volgelsang et al[20] | 31 | Median 69 (52-92) | NK | Cap | NA | NA | 100% | 23% | 10 | Mean 26 |
| Manner et al[21] | 8 | Mean 66 | APC | Cap | NA | NA | NA | 37.5% | NA | NA |
| Tang et al[22] | 6 | Mean 71 (48-91) | NK | Endo Clips | NA | NA | 100% | 0% | 0 | NA |
| Al-Kadi et al[23] | 18 | Mean 80 (68-91) | NK | None | (2-4) | NA | 87.5% | 5.5% | NA | Mean 27.5 |
| Case et al[24] | 22 | Median 85 | NK | None | NA | NA | 100% | 32% | 4 | Mean 12.7 |
| Repici et al[25] | 32 | Mean 74.8 (58-92) | HK | Cap | 2.96 | 0.62 | NA | 6.25% | 3 | Mean 23.9(12-48) |
| Hondo et al[26] | 5 | Median 69.6 (59-83) | HS | DS | 2 | 0.20 | NA | 0% | 0 | Mean 1 |
| Huberty et al[27] | 150 | Median 73 (42-94) | NK | DS | 1.88 | 0.34 | 90.3% | 2.2% | 31 | Median 43(13-121) |
| Ramchandani et al[28] | 3 | Mean 79 | SB-K | DS | NA | NA | 100% | 0% | 0 | NA |
| Manno et al[29] | 19 | Median 74 (46-84) | IT-K | DS | NA | NA | 100% | 0% | 2 | Median 27 |
| Perbtani(current study) | 8 | 72.4 | NK | None | 2.6 | 0.4 | 100% | 0% | 2 | Mean 5.8(1-17) |
Typically, symptomatic patients undergo barium esophagram and index upper endoscopy for diagnosis of ZD. One of the advantages of flexible endoscopic therapy for ZD is the ability to perform the procedure without general anesthesia in many cases. This allows patients who are not ideal candidates for endotracheal intubation to undergo either moderate sedation (conscious sedation/CS) or deep sedation/monitored anesthesia care (MAC). In published studies (Table 2) where mode of anesthesia was mentioned, greater than half of all patients underwent endoscopic procedures with either CS or MAC, without mention of intraoperative adverse events related to airway compromise. Nevertheless, some authors[26] still insist in using general anesthesia to protect the airway in case of bleeding at the UES and since the improved muscle relaxation provides greater safety assurance when manipulating the endoscope.
| Sedation type | Patients (n = 678) |
| Conscious sedation | 352 (52) |
| Monitored anesthesia care | 60 (9) |
| General anesthesia | 77 (11) |
| Not reported | 189 (28) |
Prior to performing the procedure various steps are essential to ensure safety in performing the myotomy: (1) Placement of a nasogastric (NG) or orogastric (OG) tube is a common practice that has been introduced with two potential benefits: First, it allows enhanced visualization of the esophageal lumen and diverticulum, and secondly it protects the anterior esophageal wall from injury from instruments used during myotomy; (2) Accessories to improve visualization: Sakai et al[16] originally described the use of an assist or accessory device during ZD therapy to stabilize and visualize the septum. A transparent oblique-end hood was used at the tip of the endoscope that extended distally. This in turn served to prevent closure of the upper esophageal sphincter thus allowing for better visualization of the tissue bridge between the esophagus and the diverticulum. Similarly, clear mucosectomy caps[19] have been used with similar intentions. In 2003 Evrard et al[17] described the use of a soft diverticuloscope as an adjunct that served a similar function as the clear cap device. The diverticuloscope is placed as an overtube on the endoscope and contains two distal flaps that serve to straddle the septum and safeguard the anterior esophageal and posterior diverticular walls. At this point the instrument used to divide the septum is introduced either alongside or through a channel within the endoscope. In review of the published cases, only one study looked at outcomes between accessories. Costamagna et al[18] documented lower complication rates and procedural time with the diverticuloscope vs using a clear cap. However, it is worth noting that the diverticuloscope is only commercially available in Canada and Europe.
In performing the cricopharyngeus myotomy the optimal instrument for ablation remains debatable. Moreover, due to the lack of prospective comparative trials the device chosen is often dependent on prior training and preference of the endoscopist. The most commonly used device is the needle knife as is our practice, followed by argon plasma coagulation (APC) and forceps coagulation (Table 3). This is notably different amongst otolaryngologists, where either carbon dioxide laser or a stapler-assisted device is most frequently employed[30]. When using the needle knife the tip of the instrument is placed at the center of the septum where coagulation, blended or alternating current can be used[16,18,20]. The division through the septum occurs in craniocaudal motion, which exposes the transverse fibers of the cricopharyngeal muscle. The incision should not extend past the inferior portion of the diverticulum, as risk for perforation significantly increases. Length of the myotomy has been described safely up to 5-10 mm from the bottom of the ZD[16,26,27] with endoclips placed by some endoscopists[18] distally for prophylaxis against microperforations.
| Device for myotomy | Patients (n = 678) |
| Needle knife | 404 (59.6) |
| APC | 174 (25.7) |
| Forceps coagulation | 41 (6) |
| Hook knife | 32 (4.7) |
| Insulated tip knife | 19 (2.9) |
| Harmonic scalpel | 5 (0.7) |
| Stag beetle knife | 3 (0.4) |
Emerging techniques: Recent advances in natural orifice translumenal endoscopic surgery (NOTES) have given rise to novel myotomy techniques including per oral endoscopic myotomy (POEM)[31]. Similarly, endoscopic submucosal dissection (ESD) techniques have recently been reported[32] to extend its application to aid in cricopharyngeal myotomies. In this small case series, a modified clear cap overtube was created to secure the diverticular wall while indigo carmine solution was injected into the septum. A submucosal bleb or lift was then created which served to increase exposure of the cricopharyngeal muscle fibers and theoretically enabling a safer and more complete myotomy. Although this variation of flexible endoscopic treatment of ZD is in it’s infancy, this study highlights the continual innovative modifications being made to optimize clinical outcome, reduce recurrence rate and sidestep technical hurdles faced by its predecessor.
In the post-operative period patients have been discharged as outpatients after as short as 6 h as long as there were no apparent adverse events[20]. However, in most cases, patients are hospitalized for 24-48 h with gradual progression of their diet 12 h post-operatively. Post-procedural radiologic studies remain institution dependent. The development of perforations is a concern but there is a low sensitivity for detection of microperforations using this method. Additionally, little correlation has been seen between radiographic findings and patient symptoms[6,33]. We endorse imaging only if there is a clinical suspicion for perforation.
Multiple centers have reported their results of the flexible endoscopic approach to ZD therapy since it’s original description in 1995. There have been 19 reported case series that have been published consisting of 670 patients (Table 1). However, due to the subjective manner in which procedural outcome is determined in these series, it is difficult to gauge true objective clinical success. Often, success is based on patient symptoms and not on objective data. Moreover, there are no guidelines or studies that suggest if endoscopic or radiologic surveillance would be beneficial. To improve upon this aspect some centers have instituted using a dysphagia score[10]. The scale ranges from 0-4 as follows: 0: no dysphagia; 1: dysphagia to solids; 2: dysphagia to semi solids; 3: dysphagia to liquids; 4: patient cannot swallow saliva. A score of this manner provides an objective measurement of outcome as is routinely used at our center and in this study as well. In studies where dysphagia score was used, the average pre- and post- treatment dysphagia score was 2.1 (range 1.8-4) and 0.26 (range 0-0.6) respectively. More routinely reported is the clinical resolution rate (CRR). This is commonly described as a symptom improvement either immediately or 2-4 wk post-procedurally. Of the available studies the reported CRR was over 90% (Table 1) and patients with persistent symptoms typically underwent either a repeat procedure or were referred to otolaryngologists for surgical management. Recurrence rate (RR) for symptoms was near 15% from the available data with an average follow-up time of 20 mo. However, follow-up period was not mentioned in nearly a quarter of the reports and is commonly seen as a shortcoming when reporting outcomes for this procedure.
Adverse events (AE) for the flexible endoscopic therapy of ZD have been well reported since first being described. However, due to the lack of standardization there remains heterogeneity of how accounted complications are reported and designated. In the 678 patients that have been reported to undergo the flexible endoscopic procedures, including our current study, 80 patients (11.8%) were reported to have AE (Table 4), the most common being micro-perforations, which encompasses greater than half of reported complications. These are described as the patient developing either: cervical, subcutaneous or mediastinal emphysema. Most of these documented by various radiographic studies, had inconsequential medical outcomes and were treated conservatively with or without antibiotics[34]. Macroscopic perforations, the more morbid AE, were only reported in 4 cases. These perforations were seen either during endoscopic visualization or by oral contrast extravasation and were typically managed with endoscopic clipping without any long-term sequelae being reported. Bleeding occurred in 6 of the reported cases, mostly intra-procedural and treated with epinephrine injection, endoclips or electrocautery. Prolonged post-procedural bleeding has only been recorded in 1 case[14] with hemostasis achieved with endoscopic injection of epinephrine. Fever was reported as the most common presentation of infections reported in published cases. Patients were usually treated with antibiotics if specific organ involvement was apparent or for fever lasting more than 24 h. If fever persisted and a focus of infection was not found, then appropriate testing to rule out perforation or mediastinitis is essential[35]. Patient mortality is infrequent, with only one case being reported[15] due to pulmonary embolism. At our institution, similar to previously published series from other centers, we did not encounter any procedural adverse events. However, as mentioned earlier, accurate reporting of AE is likely best achieved in prospective studies using objective predetermined criteria as suggested by the ASGE[11].
| Adverse events | 80/678 (11.8%) |
| Micro perforations | 52/678 (7.7) |
| Cervical emphysema | 1 |
| Mediastinal emphysema | 5 |
| Subcutaneous emphysema | 25 |
| Unspecified | 21 |
| Macro perforations | 4/678 (0.6) |
| Bleeding | 9/678 (1.3) |
| Infection | 12/678 (1.8) |
| Fever | 10 |
| Pneumonia | 1 |
| Neck abscess | 1 |
| Death | 1/678 (0.1) |
| Adverse events, not otherwise specified | 2/678 (0.3) |
Flexible endoscopy therapy appears to be a minimally invasive option for the treatment of ZD with several studies showing favorable clinical outcomes and an adequate safety profile. Future efforts should include prospective trials with further standardization of technical aspects, comparison of endoscopic devices and accessories, and report of long-term clinical outcomes with this technique.
P- Reviewer: Muguruma N, Takayama S S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Ludlow A. A case of obstructed deglutition from a preternatural dilatation of and bag formed in the pharynx. Medical Observations and Inquiries. 1764;3:85-101. [Cited in This Article: ] |
| 2. | Zenker FA, von Ziemssen H. Cyclopaedia of the Practice of Medicine, vol 3. London: Low, Marston, Searle, Rivington Pub 1878; 46-68. [Cited in This Article: ] |
| 3. | Cook IJ. Clinical disorders of the upper esophageal sphincter. GI Motility Online. 2006. Available from: http://www.nature.com/gimo/contents/pt1/full/gimo37.html. [Cited in This Article: ] |
| 4. | Cook IJ, Gabb M, Panagopoulos V, Jamieson GG, Dodds WJ, Dent J, Shearman DJ. Pharyngeal (Zenker’s) diverticulum is a disorder of upper esophageal sphincter opening. Gastroenterology. 1992;103:1229-1235. [Cited in This Article: ] |
| 5. | Law R, Katzka DA, Baron TH. Zenker’s Diverticulum. Clin Gastroenterol Hepatol. 2014;12:1773-1782; quiz 1773-1782. [Cited in This Article: ] |
| 6. | Mantsopoulos K, Psychogios G, Karatzanis A, Künzel J, Lell M, Zenk J, Koch M. Clinical relevance and prognostic value of radiographic findings in Zenker’s diverticulum. Eur Arch Otorhinolaryngol. 2014;271:583-588. [Cited in This Article: ] |
| 7. | Wheeler WI. Pharyngocoele and dilation of the pharynx, with existing diverticulum at lower portion of pharynx lying posterior to the oesophagus, cured by pharyngotomy, being the first of the kind recorded. Dublin J Med Sci. 1886;82:349-356. [Cited in This Article: ] |
| 8. | Dohlman G, Mattsson O. The endoscopic operation for hypopharyngeal diverticula: a roentgencinematographic study. AMA Arch Otolaryngol. 1960;71:744-752. [Cited in This Article: ] |
| 9. | Aly A, Devitt PG, Jamieson GG. Evolution of surgical treatment for pharyngeal pouch. Br J Surg. 2004;91:657-664. [Cited in This Article: ] |
| 10. | Christiaens P, De Roock W, Van Olmen A, Moons V, D’Haens G. Treatment of Zenker’s diverticulum through a flexible endoscope with a transparent oblique-end hood attached to the tip and a monopolar forceps. Endoscopy. 2007;39:137-140. [Cited in This Article: ] |
| 11. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [Cited in This Article: ] |
| 12. | Mulder CJ, den Hartog G, Robijn RJ, Thies JE. Flexible endoscopic treatment of Zenker’s diverticulum: a new approach. Endoscopy. 1995;27:438-442. [Cited in This Article: ] |
| 13. | Ishioka S, Sakai P, Maluf Filho F, Melo JM. Endoscopic incision of Zenker’s diverticula. Endoscopy. 1995;27:433-437. [Cited in This Article: ] |
| 14. | Hashiba K, de Paula AL, da Silva JG, Cappellanes CA, Moribe D, Castillo CF, Brasil HA. Endoscopic treatment of Zenker’s diverticulum. Gastrointest Endosc. 1999;49:93-97. [Cited in This Article: ] |
| 15. | Mulder CJ. Zapping Zenker’s diverticulum: gastroscopic treatment. Can J Gastroenterol. 1999;13:405-407. [Cited in This Article: ] |
| 16. | Sakai P, Ishioka S, Maluf-Filho F, Chaves D, Moura EG. Endoscopic treatment of Zenker’s diverticulum with an oblique-end hood attached to the endoscope. Gastrointest Endosc. 2001;54:760-763. [Cited in This Article: ] |
| 17. | Evrard S, Le Moine O, Hassid S, Devière J. Zenker’s diverticulum: a new endoscopic treatment with a soft diverticuloscope. Gastrointest Endosc. 2003;58:116-120. [Cited in This Article: ] |
| 18. | Costamagna G, Iacopini F, Tringali A, Marchese M, Spada C, Familiari P, Mutignani M, Bella A. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs. diverticuloscope-assisted technique. Endoscopy. 2007;39:146-152. [Cited in This Article: ] |
| 19. | Rabenstein T, May A, Michel J, Manner H, Pech O, Gossner L, Ell C. Argon plasma coagulation for flexible endoscopic Zenker’s diverticulotomy. Endoscopy. 2007;39:141-145. [Cited in This Article: ] |
| 20. | Vogelsang A, Preiss C, Neuhaus H, Schumacher B. Endotherapy of Zenker’s diverticulum using the needle-knife technique: long-term follow-up. Endoscopy. 2007;39:131-136. [Cited in This Article: ] |
| 21. | Manner H, May A, Rabenstein T, Pech O, Nachbar L, Enderle MD, Gossner L, Ell C. Prospective evaluation of a new high-power argon plasma coagulation system (hp-APC) in therapeutic gastrointestinal endoscopy. Scand J Gastroenterol. 2007;42:397-405. [Cited in This Article: ] |
| 22. | Tang SJ, Jazrawi SF, Chen E, Tang L, Myers LL. Flexible endoscopic clip-assisted Zenker’s diverticulotomy: the first case series (with videos). Laryngoscope. 2008;118:1199-1205. [Cited in This Article: ] |
| 23. | Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg. 2010;211:239-243. [Cited in This Article: ] |
| 24. | Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc. 2010;85:719-722. [Cited in This Article: ] |
| 25. | Repici A, Pagano N, Romeo F, Danese S, Arosio M, Rando G, Strangio G, Carlino A, Malesci A. Endoscopic flexible treatment of Zenker’s diverticulum: a modification of the needle-knife technique. Endoscopy. 2010;42:532-535. [Cited in This Article: ] |
| 26. | Hondo FY, Maluf-Filho F, Giordano-Nappi JH, Neves CZ, Cecconello I, Sakai P. Endoscopic treatment of Zenker’s diverticulum by harmonic scalpel. Gastrointest Endosc. 2011;74:666-671. [Cited in This Article: ] |
| 27. | Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc. 2013;77:701-707. [Cited in This Article: ] |
| 28. | Ramchandani M, Nageshwar Reddy D. New endoscopic “scissors” to treat Zenker’s diverticulum (with video). Gastrointest Endosc. 2013;78:645-648. [Cited in This Article: ] |
| 29. | Manno M, Manta R, Caruso A, Bertani H, Mirante VG, Osja E, Bassotti G, Conigliaro R. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc. 2014;79:168-170. [Cited in This Article: ] |
| 30. | Parker NP, Misono S. Carbon dioxide laser versus stapler-assisted endoscopic Zenker’s diverticulotomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2014;150:750-753. [Cited in This Article: ] |
| 31. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [Cited in This Article: ] |
| 32. | Kedia P, Fukami N, Kumta NA, Kahaleh M, Sharaiha RZ. A novel method to perform endoscopic myotomy for Zenker’s diverticulum using submucosal dissection techniques. Endoscopy. 2014;46:1119-1121. [Cited in This Article: ] |
| 33. | Witterick IJ, Gullane PJ, Yeung E. Outcome analysis of Zenker’s diverticulectomy and cricopharyngeal myotomy. Head Neck. 1995;17:382-388. [Cited in This Article: ] |
| 34. | Dzeletovic I, Ekbom DC, Baron TH. Flexible endoscopic and surgical management of Zenker’s diverticulum. Expert Rev Gastroenterol Hepatol. 2012;6:449-465; quiz 466. [Cited in This Article: ] |
| 35. | Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus. 2008;21:1-8. [Cited in This Article: ] |