The role of therapeutic intervention in the paediatric upper gastrointestinal tract can be divided broadly into (1) emergency and (2) elective procedures.
Emergency procedures
The two most common scenarios faced by the paediatric gastroenterologist is foreign body ingestion in the upper gastrointestinal tract (for example inanimate objects or food bolus and upper gastrointestinal tract bleeding. We discuss this further below.
Foreign body removal (Figures 1 and 2): As the child grows, explores and interacts with their local habitat they inevitably put foreign bodies into their mouths, ingesting a small proportion of them. Of over a 100000 cases of foreign body ingestion in the United States each year, more than 80% occur in children, mainly between the ages of 6 mo and 3 years[19-21]. Fortunately most foreign bodies that enter, pass through the gastrointestinal tract spontaneously, with only about 10%-20% requiring endoscopic removal and less than 1% require surgical removal[19,22]. Deaths are extremely rare but they have been reported[21,23]. The types of objects vary with geography but in the western world, coins are the most frequently encountered foreign body, while in the eastern world, fish bones account for a greater percentage[21,24]. Objects such as batteries or safety pins can add a degree of complexity and risk to foreign body retrieval.
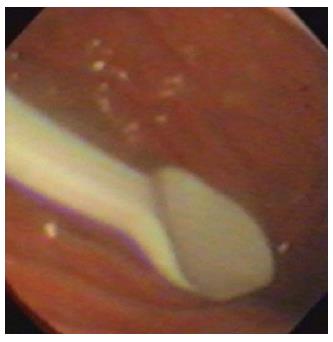
Figure 1 Foreign body (a plastic spoon) in the stomach of a child.
Ingestion of coins and small lithium batteries tend to be much more common.
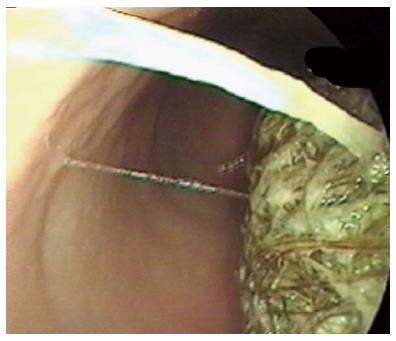
Figure 2 Bezoar seen at endoscopy.
Endoscopic removal wasn’t possible.
After initial workup with a detailed history and biplane X-rays (antero-posterior and lateral), intervention depends on three factors; (1) the object ingested (2) location of the object and (3) the age of the patient. The location is often in areas of physiological narrowing; the upper oesophageal sphincter, the level of the aortic arch, lower oesophageal sphincter or the dependent part of the stomach, usually the gastric fundus[22,25]. It is important to note that the location of the pain or symptom does not always correlate with the associated site of impaction (visceral innervation)[26]. In the very young, due to the compressibility of the trachea, endoscopists need to be aware that even relatively small objects can potentially cause serious tracheal compression leading to respiratory compromise[10].
There are various methods to remove foreign bodies, with the flexible gastroscope being preferred as it allows direct visualisation, manipulation and observation of any potential injury to the adjacent mucosa[27,28]. The endoscopist should have an array of equipment readily available including polyp snares, alligator forceps, rat-tooth forceps, net baskets and overtubes.
Magill forceps, angled forceps commonly used in anaesthesia, are sometimes sufficient to remove a variety of objects in the oropharynx or upper oesophagus providing direct vision is possible. This may require the use of general anaesthesia and a laryngoscope to gently open up the oesophagus[29].
The use of a rubber or plastic dilator (Bougienage) may be used for foreign bodies impacted beyond the reach of forceps in the oesophagus to aid their passage into the stomach. However, careful consideration needs to be taken to assess that the object is judged able to pass along the oesophagus into the stomach without causing significant mucosal injury (e.g., blunt and small objects such as coins) The use of this technique is thus limited and most endoscopists would only advocate this in experienced hands and only in patients where there has been witnessed ingestion within 24 h without existing oesophageal disease[30,31].
An alternative method is extracting the object impacted in the oesophagus with the use of a Foley catheter. This technique involves passing the Foley catheter past the foreign body and inflating the balloon with radio-opaque dye, then with fluoroscopic guidance, gently pulling on the catheter so the object is drawn back into the oral cavity and retrieved[32]. Many endoscopists do not advocate this technique in inexperienced hands as there is the risk of perforation or inadvertent placement of the foreign body into the trachea[33].
Pragmatically, foreign objects beyond the reach of forceps require intubation of the oesophagus with a flexible gastroscope. On entering the oesophagus, occasionally air insufflation or water flush alone may be sufficient to dislodge certain foreign objects to pass the lower oesophageal sphincter into the stomach. Smooth, round objects such as coins or flat batteries can often, more easily, be grasped with alligator jaw forceps. Rubber tipped or specialised alligator forceps are available for the paediatric 2 mm channel.
Special mention needs to be made regarding “button” batteries which are now ever more increasingly being swallowed[34]. Although standard batteries can cause problems due to their size and from the leakage of caustic material, button batteries have the added risk of conducting electricity (as both poles are in direct contact with the mucosa) which can cause significant necrosis and potential perforation[35]. Hence, even if these small batteries are not causing direct impaction, if found anywhere in the upper gastrointestinal tract, they should be removed. The preferable technique is to use a Roth Net® and retracting the basket as far back into the endoscope as possible and removing the endoscope and the foreign body together in one sweep.
Up to 30% of objects ingested are “sharp” such as needles and pins[21]. Unfortunately, the majority of sharp pointed objects are not radio-opaque. Hence, if there is a clinical suspicion of ingestion of these objects, it is of the authors’ opinion that they should all proceed to having an endoscopic assessment and retrieval. Forceps and snares are often suitable as retrieval devices, minimising potential mucosal injury on retraction. This can be achieved by either retrieving the foreign body with the sharp end trailing, using an overtube or even novel devices such as protector hoods on the end of the endoscope[36].
Food bolus: This does not occur as frequently as it does in the adult population (the most common cause of oesophageal foreign body in this group)[37]. The likelihood of there being an underlying oesophageal pathology is higher such as eosinophilic oesophagitis, achalasia or strictures[38].
The indications for intervention is the same as that of other foreign bodies and inability to swallow saliva always requires emergency endoscopy, otherwise there is a risk of aspiration.
The use of medication, for example glucagon, buscopan and proteolytic enzymes, although still being used in current practice lack any evidence and the true likelihood is that the bolus would have passed naturally anyway. Therefore, authors, do not advocate their use considering the associated side effect profile[39]. An overtube may facilitate multiple passes of the endoscope that may be required, but caution with its use needs to be considered, as mentioned earlier.
Methods of removal can be broken into two actions of either “pushing” of the bolus into the stomach or “extraction” of the bolus into the oral cavity. With each method the food may be extracted preferably whole or “piecemeal”. Both methods have been proven to be effective but the former “pushing” method is less preferable considering the unknown potential of pathology distal to the food bolus[40,41]. “Piecemeal” removal can be achieved using alligator forceps, rat-tooth forceps or tripod forceps down the accessory channel facilitating safer “pushing” of contents into the stomach.
Certain food boluses are not easily broken down into smaller pieces, in which case suction can be used with the aid of a cap on the end of an endoscope. If one is not readily available, the friction fit adaptor from an oesophageal band ligation kit can be used, allowing suction to stabilise the food bolus at the distal end more securely before pulling it into the oral cavity[42]. The authors have a preference of using a Roth Net®, with the catheter gently placed alongside the bolus with the net then opened in direct vision carefully in a “to and fro” manner to accommodate the food bolus before angling the net from one wall to the other to then allow the bolus to be caught in the net and retrieved.
Upper gastrointestinal tract bleeding: Life threatening gastrointestinal bleeding in paediatrics is rare but it is important for the endoscopist to recognise when it occurs and act promptly. As this is encountered infrequently in most endoscopy units, much of the evidence for the use of various haemostatic methods in children is inferred from the adult population. It is the common practice for the authors to collaborate with adult gastroenterologists and paediatric surgeons in the case of a serious gastrointestinal bleed.
Bleeding in the upper gastrointestinal tract can arise from peptic ulcers, varices, Mallory-Weiss tears, dieulafoy lesions and angioectasia[43,44]. Unfortunately, there are no large series looking into gastrointestinal bleeding in children overall, with most large prospective studies assessing the incidence in the specialised paediatric intensive care setting[45]. Case series from Asia and developing countries show a higher incidence of variceal bleeding (mainly from extrahepatic portal hypertension) and those in developed countries having a higher incidence of erosive/peptic ulcer bleeding (mainly in the context of a a critically ill state)[45,46].
It is important for the endoscopist to be aware of the different modalities of endoscopic haemostasis available and it is just as important to know when these modalities would be required. Several scoring systems have been created in adults, although not validated in children, that can be used (after certain parameters are adjusted), to ascertain the need for endoscopic intervention. Blatchford and Rockall scores are used worldwide although there has been recent debate on their validity in the prediction of re-bleeding and 30 d mortality[47,48].
For peptic ulcers, the Forrest criteria was created for high-risk bleeding stigmata found during endoscopy. The presence of active bleeding, a non-bleeding visible vessel (re-bleeding rate of 40%-100%) or adherent clot (re-bleeding rate of approximately 25%) are indications for endoscopic treatment. While clean based ulcers do not require endoscopic therapy as the risk of re-bleeding is low (5%)[49,50]. Varices that are not actively bleeding can still be considered at high risk if there are signs of engorged protuberant vessels or a prominent red petechial mark on the vessel (cherry red spot) and therefore therapy should be considered.
The type of therapy used is dependent on the size of child, the type of lesion, the site of bleeding and the judgement and ability of the endoscopist. Three modalities are available to the endoscopist, which can be divided into 3 categories: injection, mechanical haemostasis or thermo-coagulation. Ideally, if the patient size permits, a two channel scope is preferable so that haemostasis can be achieved with concurrent use of flushing of the target area with saline for better visualisation.
(1) Injection therapy: Most injection needles have a small enough diameter to pass through a 2 mm channel in a paediatric gastroscope. Vasoactive agents, sclerosing agents and tissue adhesives can all be delivered by these needles.
Adrenaline is typically available in 1:10000 dilution and its action is via local vasoconstriction, platelet aggregation and mechanical tamponade[51]. In the case of an ulcer, it is important to wash the area, even if it is for a temporary view, in order to visualise the ulcer and identify a possible bleeding vessel. The scope is advanced near to the ulcer and the needle catheter fed through the channel. It is important to have the gastroscope close to the lesion or vessel as the extra length of catheter may predispose it to “kinking”. Ideally, one should aim to inject 1-2 mls aliquots in 4 quadrants around the ulcer or near the vessel (so theoretically to exhibit its 3 effects circumferentially around the bleeding point). Unfortunately, no data exists for exact volumes in children as it does in adults where large volumes of 13-20 mls have been shown to be more efficacious[52].
Sclerosing agents such as sodium tetradecyl sulphate and ethanol act by inducing localised thrombosis over the bleeding vessel. In the past, sclerosing agents had been used for treatment of peptic ulcers and dieulafoy lesions[53]. In the last 2 decades, their role has been more confined to dealing with varices. Although band ligation is more efficacious in the adult population, the benefit of sclerosing agents in children is that they can be used in scenarios where band ligators are too large to pass through the oropharynx of a young child. The exact dose to use is not clear, but recent ASGE (American Society of Gastrointestinal Endoscopy) suggest the use of a quarter to half of what would be used in adults in children under the age of 12 years[54]. Injection can be delivered directly into the varix causing direct thrombosis or para-varix causing tamponade and submucosal fibrosis. Complications can occur including chest pain, mucosal ulceration and stricture formation. The largest case series to date was by Poddar et al[55] who demonstrated the use of alcohol injection in 257 children with varices and showed successful eradication in 95% of patients with a mean of 4.5 sessions (mean volume of 8 mls of absolute alcohol used). In this series 1.4% (n = 3) had perforation and 18% (n = 38) had stricture formation[55].
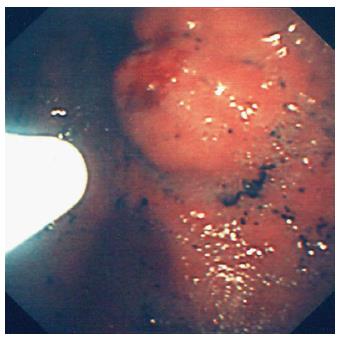
Tissue adhesives such as fibrin glue have emerged as being successful in adult treatment particularly for gastric varices (Figure 3)[56]. There is only one pilot study, to date, in the paediatric population by Rivet et al[57] where 8 infants were treated successfully for varices with fibrin glue. There are technical challenges with this agent, as there is a risk of the needle sticking to the varix or blocking the endoscope channel and causing serious damage. The authors’ preference is to inject between 1-2 mls and flush thoroughly with water and instead of bringing the injection needle back up the channel, to withdraw it together with the endoscope and cut the tip, hence preventing any adhesion to the scope.
Figure 3 Injection of glue into a gastric varix.
(2) Mechanical therapy: Mechanical therapy in the form of clips is ever increasingly being utilised as it has the ability to effectively tamponade areas of bleeding. Its efficacy has been excellent in non variceal bleeding in adults, however published experience in the paediatric setting is lacking. Interestingly, a Japanese series has shown its benefit in prophylaxis. Eighty two children who underwent clipping of their varices, showed a prevention of variceal progression in 90%[58]. One of the limiting factors for its use is that all current brands on the market today need a channel size of 2.8 mm, therefore it is not compatible with paediatric gastroscopes. The jaw length of haemoclips range from 9-11 mm. Each brand has a slightly different clip deployment method, with the option of opening and closing the clips several times as well as clip rotation before deployment.
It is imperative that the endoscopist becomes familiar with the deployment technique. In the authors’ experience, it is often the lack of communication between the endoscopist and assistant that leads to unsuccessful clip deployment. Indications for clip deployment are mainly for a bleeding vessel in an ulcer base, dieulafoy lesion or bleeding from Mallory-Weiss tears. It is the authors’ preference to use a set of commands consisting of: (1) expose (exposing the clip from sheath); (2) open (opening jaws of clip); (3) close (closing of jaws); and (4) deploy (deploying the clip from the shaft). A useful pneumonic to remember is Extreme OCD (expose-open-close-deploy). In a case of severe bleeding that subsequently requires angiography, the radiologist finds the clip a useful aid to identify the site of the bleeding vessel before coil placement.
Band ligation has been the mainstay of treatment for oesophageal variceal haemorrhage for the last 3 decades. It can be utilised for primary haemostasis or for prophylactic measures. The device consists of a cylindrical friction fit adapter cap which has a number of elastic ligating bands fitted around it. The adaptor is placed on the end of an endoscope (minimum tip of 8.5 mm required) and a thread connected to these bands is fed through the channel of the endoscope to a deploying handle positioned on top of the biopsy channel. After the endoscope is placed in the desired location, suction is applied to draw the varix (or other lesion) into the adaptor. The bands are deployed by rotation of the handle, ideally suction should be held for a further 2-3 s to allow the band to fully reach its maximum tension capacity. For varices, this should ideally occur near the GOJ and proceed proximally to avoid obstruction of views by the bands or inadvertent displacement. In contrast to adult studies, randomised control studies are lacking and when they have been undertaken, the sample sizes have been small. As such, there is no consensus on the best modality, although reports suggest fewer complication rates with bands than with sclerotherapy[59].
(3) Thermo-coagulation: Thermo-coagulation devices deliver thermal energy causing coagulation and desiccation which can lead to haemostasis. There are 2 types available, monopolar and bipolar. With monopolar devices, e.g., hot biopsy forceps, an electrical current is passed through the probe tip and conducted through the patient through a grounding pad and back to the diathermy unit. The probe can be applied directly to a vessel until bleeding stops. However, the authors do not use this routinely for haemostasis as the depth of burn is difficult to regulate and a deep thermal injury or perforation is possible[60].
A preferable method is bipolar coagulation. Here, the probe delivers thermal energy by creating an electrical circuit between 2 electrodes on the probe tip. Therefore, the electrical current passes through the affected tissue only, so tissue penetration is less deep. There are 5-French heater probes that can be used with paediatric gastroscopes. Bipolar probes have 6 points through which current can be passed and hence good tissue contact can be made, whether it is used en face or tangentially. As it has less tissue penetration, more pressure is required for deeper penetration and application time is longer. From the authors’ experience when haemostasis is not achieved, it is often when the endoscopist has not taken enough time to place the tip on the bleeding point, which should be a minimum of about 10 s for a bleeding vessel or 3-4 s for angioectasia.
Heater probes have an electrical heated coil inside a Teflon-covered insulated cylinder. Coagulation is performed by directly applying heat through the probe over the bleeding vessel with pressure. There is very little experience of this in paediatrics and currently no probe available for paediatric scopes.
Argon plasma coagulation (APC) is a non-contact form of coagulation in which current is transmitted in an arc of electricity through an ionised gas (argon). It has been shown to be useful in adults for non-variceal bleeding and is commonly used in the treatment of radiation-induced proctitis[61]. The degree of coagulation is dependent on several factors: the power settings, duration of application, distance between tip and tissue and flow rate of the argon gas. Its advantages are that as the tissue coagulates, the conductivity decreases which hence limits the depth of injury and it is available in a 1.5 mm diameter probe for the paediatric gastroscope. There is only one case series of its use in children by Khan et al[62] where 13 children with upper GI lesions (ulcers, haemangiomas and erosions) were successfully treated with APC (flow rate of 0.9 L/min and power at 55 w). Care should be taken to aspirate the argon gas frequently which is potentially combustible in large volumes.
Percutaneous endoscopic gastrostomy
This is now very commonly used since it was first performed by Gauderer et al[63] in 1979. To this day, it is still an effective method of feeding via the stomach where the oral route may not be possible, providing hydration and nutrition[64]. Endoscopic gastrostomy placement compared to surgical placement was developed to avoid surgical intervention. The most common indications for its use in the paediatric setting are neurological impairment or failure to thrive[65,66]. Percutaneous endoscopic gastrostomy (PEG) use is based around the fact that the continuous, suture-less approximation of the stomach to the peritoneum and anterior abdominal wall by a feeding tube leads to the formation of adhesional attachments which subsequently leads to the formation of a tract around the tube[66].
Several modifications of the technique have been introduced since it was first described. The “pull” technique is the most commonly used. This involves performing a gastroscopy to identify the anterior stomach and using sufficient air insufflation to oppose the anterior stomach with the anterior abdominal wall, pushing aside any possible visceral organs that may be inadvertently punctured. The area for insertion of the tube is ascertained by visualisation of trans-illumination of the gastroscope through the abdominal wall and visualisation of a clear finger indentation within the stomach lumen. This area is marked and sterilised before infiltration of local anaesthesia. A skin incision of approximately 0.5 cm is made (only few mm depth required) which can be made horizontally so the scar can be hidden within skin creases for aesthetic purposes. A trocar/angiocath is pushed through this point into the stomach under endoscopic vision. A soft guide wire is then inserted through this so that it just appears within the gastric lumen. This threading wire is then snared through the endoscope and the whole apparatus, scope, snare and thread are withdrawn together. After the guide wire is out, a suitable feeding tube is attached to it and pulled through the mouth and out of the incision. An external bolster/stopper is then placed on the skin to hold this in place. It is at discretion of the endoscopist whether it is necessary to re-intubate the scope to confirm placement of the tube.
The authors would advocate that the distance on the PEG tube is documented, i.e., the distance from the “button/stopper” in the gastric end to that on the skin surface, markings which are available on all feeding tubes. This distance varies according to the size of the child, however, it may be a guide in cases where a larger than expected distance is noted to suggest a possible additional inadvertent visceral attachment. Antibiotics should always be given although the optimal timing, whether pre, post or peri can be left to local microbiology policies.
Oesophageal dilatation: Unlike in adults, where malignancy is the major cause of upper gastrointestinal structuring, in children it is almost always caused by benign disorders. Techniques and equipment used in adult patients can be applied to children, i.e., bougienage, balloon dilatation and self-expanding stents (seldom used). The approach will be determined as with many cases where adult skills are transferred to the paediatric setting by characteristics of the stricture, position, size (both radial and longitudinal), availability of equipment, expertise of the endoscopist and patient size.
The most common cause of oesophageal stricturing worldwide is the ingestion of caustic liquids from around the house, with the other major causes falling into peptic or post-surgical strictures (mainly corrective surgery for oesophageal atresia)[67]. Rarer conditions involve the consequences of prolonged ingestion of certain foreign bodies, strictures associated with eosinophilic oesophagitis, post variceal sclerotherapy and congenital abnormalities.
Dilatation is indicated in patients with symptomatic obstruction. Anastomotic strictures post oesophageal atresia are common, with an incidence of up to 44% in some series[68,69]. Koivusalo et al[70] demonstrated that a watch and wait policy based on symptomatology was superior to routine dilatations as greater than half did not require any subsequent dilatations[70].
The purpose of oesophageal dilatation is to alleviate symptoms, permit free intake of enteral nutrition and reduce complications such as pulmonary aspiration. This must be weighed up against the risk of perforation. This has been reported as 0%-5% after balloon dilatation and 8%-9% after bougienage[71,72].
Bougie dilators come in a range of makes and diameters. However, in the paediatric setting experience is mainly with Savary-Gillard type systems, i.e., a long tapered, radio-opaque, wire-guided and poly-vinyl hollow tubes designed for use in the oesophagus. The bougie system is naturally limited to the oesophagus in the upper GI tract as transmission of the force more distally would be more difficult. Bougie dilators apply axial as well as radial forces[73]. They come in sizes of 5-20 mm diameter and 70-100 cm in length. The technique involves feeding a guide wire through the lumen of the stricture either endoscopically, fluoroscopically or both. When done solely endoscopically, it is worthwhile to note the distance of the stricture from the incisors. After the endoscope is retracted, it is imperative, particularly if fluoroscopy is not used, to maintain the guide wire in a fixed position by an assistant so it does not inadvertently move out of position. The bougie is lubricated well and passed over the guide wire until the maximal diameter has passed over the area of the stricture (as estimated from the previous incisor distance). In adults, it is advocated that only 3 dilators or a maximum increment of 3 mm from initial dilatation occurs in a single session to minimise risk of oesophageal perforation[74]. This data is lacking in children and hence an estimate of the diameter of the adjacent normal calibre oesophagus should be used.
Balloon dilators have the benefit of potentially being used under direct vision and delivering direct radial force across the entire stricture, while controlled manometrically by a hand held device by an assistant. It cannot be passed down the standard 2.0 mm channel of a paediatric scope but in these scenarios, guide wires can be placed via fluoroscopy to enable the balloon catheter to pass over this[75,76]. Balloons are available in 4-40 mm diameter and length varying depending on location used. With this range is mind, in infants, larger length balloons may traverse unnecessarily the entire length of the oesophagus so shorter lengths, pyloric or colonic, should be used in this group. The ideal length of time the balloon is inflated is not known but it is the authors’ experience to leave it inflated for at least 1 min.
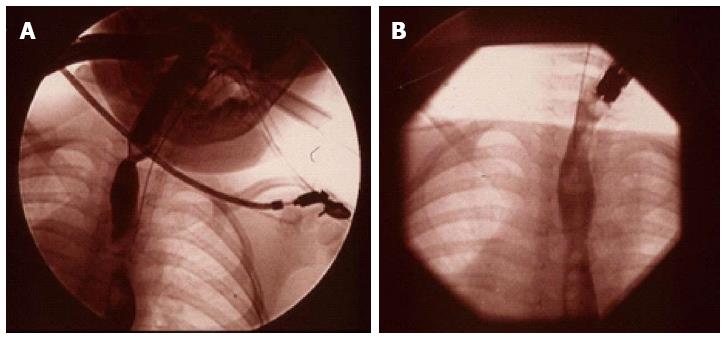
One of the issues with caustic strictures is the frequency of stricture recurrence after dilatation. The authors reported the first use of Mitomycin-C, an antifibrotic agent, for treatment of caustic strictures (Figure 4)[77]. Following the initial report, a case series was reported from 8 paediatric gastroenterology centres around the world about its successful use[78] and it has now been adopted as standard practice in many units[78,79].
Figure 4 (A) Videofluoroscopy image of a proximal and a distal stricture in the oesophagus and (B) resolution of the strictures in the same child 3 mo after treatment with Mitomycin C.
Choice of methods for dilatation is largely down to the experience of the endoscopist and it is not known if one method is better over another for any particular indication. In retrospective data of oesophageal atresia patients, balloons were found to be more effective than bougienage and required fewer dilatations[79]. However, another report showed those with peptic and caustic strictures did better with bougienage[80]. Balloon dilatation does seem to offer a better safety profile and better efficacy[79]. Perforations are a risk although this can be minimised by cautious and gentle dilatation, and avoidance of excessive manipulation that may cause potentially damaging shearing axial forces.
Gastroesophageal reflux disease-novel therapies: The burden of gastro-oesophageal reflux (GORD) is well established in adults with all its associated symptoms including chest discomfort, recurrent cough, chronic respiratory disorders and regurgitation. In the paediatric setting, the additional sequelae of failing to thrive are seen which reduces the threshold for intervention. Those children with frequent symptoms under the age of 2 are more likely to have symptoms later in their childhood[81].
The predominant mechanism causing GORD, as in the adult population, is transient lower oesophageal sphincter (LES) relaxation. This is defined as an abrupt and transient decrease in LES pressure to the level of intra-gastric pressure, unrelated to swallowing and of relatively longer duration than the relaxation triggered by a swallow[82].
The aim of treatment for GORD is to achieve symptom relief whilst preventing complications. Those patients who fail to achieve control with medical therapy or not wishing to be dependent on long term anti-reflux medications may warrant an anti-reflux surgical procedure[83,84].
A variety of endoscopic techniques have been developed for treatment of GORD. These methods can be divided in three broad categories: (1) methods that attempt to create a fundoplication/gastroplication (plicating techniques); (2) methods that create a controlled stricture (radio frequency); and (3) methods that bulk the gastro-oesophageal junction (injecting bulking agents)[85]. There is only experience in the paediatric setting with the first two methods. The ideal procedure should be safe, effective over a long term and should not compromise future surgical options.
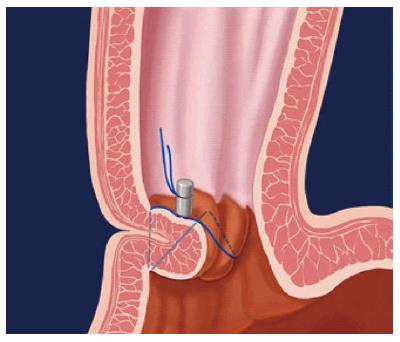
Endoluminal gastroplication (Figures 5-7): Endoluminal plication uses mechanical techniques to decrease reflux by approximation of tissue at or below the Gastro-Oesophageal junction (GOJ). The main plication device be used with the authors’ experience is the EndoCinch® (CR BARD Endoscopic technologies, Massachusetts, United States). This was initially developed by Swain et al[86] in London United Kingdom, in the mid-1980s, and was the first Federal Drug Agency (FDA) approved endoscopic sewing machine method for treating GORD[86].
Figure 5 Endoscopic gastroplication.
This figure the pattern of a zig-zag stich when applied with an Endocinch® sewing maching.
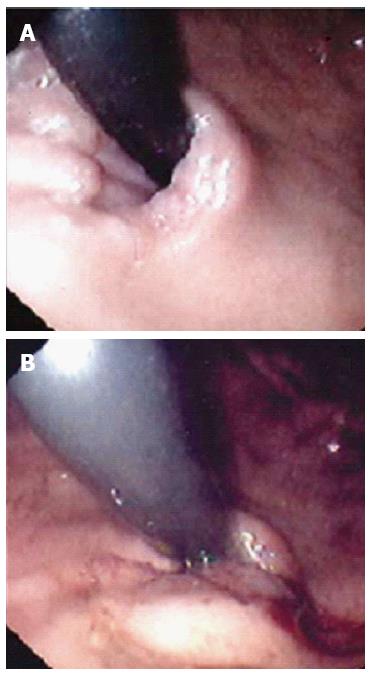
Figure 6 Endoscopic view (J manoeuvre) of a lax Gastro-Oesophageal junction in a child with major reflux before (A) and after (B) application of stitch with the EndoCinch®.
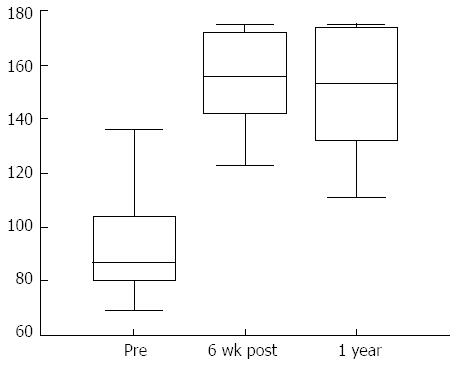
Figure 7 Significant improvement in the total QOLRAD scores (Quality of life in reflux and dyspepsia), 6 wk and 1 year after gastroplication with the Endocinch®.
The method involves placement of an overtube to facilitate repeat intubations that are required for the procedure. An endoscope with a capsule-shaped plication device (with a side hole) mounted at the tip is inserted to the level of the squamo-columnar junction through the overtube, where the side hole is brought into close contact with the wall to draw the mucosa into the capsule with the aid of air suction. A puncture needle with a non-absorbable suture attached (suture tag), is inserted into the biopsy channel and is then passed through. The suction pressure is released and the capsule is carefully rotated away from the stitch side. A suture tag is then set up in the endoscope again and a second set of sutures is placed following the same procedure at a position rotated between 30 and 60 degrees away from the first set. The two sutures form a plication using a knotting device that is inserted into the biopsy channel of a separate endoscope and the process is completed by plicating the tissue in the form of a pouch. The second and third plications are performed in either a linear or circumferential manner, or a combination of the two, depending on the available area within the GOJ and position preference[87].
The procedure can be carried out as a day case, with studies showing it to be relatively quick, non-invasive, effective and safe. Results have been shown it to be comparable to laparoscopic fundoplication in adults[88-90].
The authors have a preference of placing two plication suture lines circumferentially, 1.5 cm below the GOJ and one 0.5 cm below the GOJ, which we believe to be superior to other methods used in adults[88,91]. In a series of 17 children with a median age 13 years, with GORD refractory or dependent on proton pump inhibitors, all patients showed an improvement in symptom severity, frequency and reflux related quality of life scores[92]. Fourteen patients (88%) at 1 year and 9 patients (56%) at 3 years remained without a need for any anti-reflux medication. A sustained improvement in heartburn, regurgitation and vomiting was seen at 3 years. Only one complication of gastric bleeding was observed which resolved spontaneously[93]. The duration of action is conflicting in adults and is under on-going review[94-96]. However, there does appear to be superior efficacy in children and the reasons for this may be due to a relatively deeper suture depth in the thinner paediatric oesophagus[93].
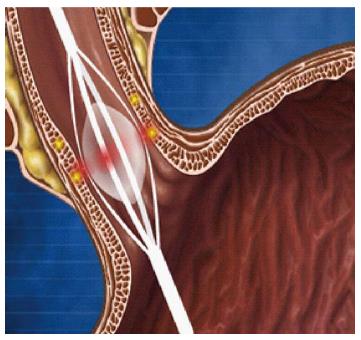
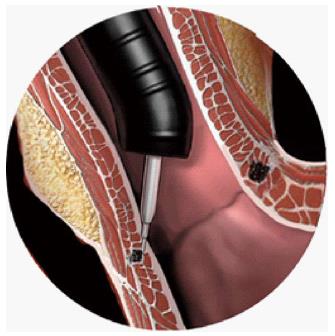
Stricture formation through delivery of radiofrequency energy: Curon Medical designed the STRETTA® system (Figure 8) which gained FDA approval in 2000. The device employs a special balloon on a catheter with four needle electrodes. An upper GI endoscopy is undertaken first to identify the GOJ. A guide wire is then placed into the stomach, the endoscope is then removed and the STRETTA® catheter is then passed over, advancing the balloon to a position at the GOJ. The balloon is inflated and the electrodes are deployed to penetrate into the muscle layer. Radiofrequency energy is delivered through the electrodes to create thermal lesions radially at several levels in the lower oesophageal sphincter and gastric cardia[97]. As the lesions heal, it induces collagen tissue contraction, remodelling and modulation of the triggering threshold for transient LES relaxations[98].
Figure 8 Use of a balloon to deliver radiofrequency energy via needle electrodes to the mucosa.
Evidence for its benefit is promising, as shown in a recent meta-analysis including 1441 patients, although these results need to be interpreted with caution as there was significant heterogeneity between trials[99]. The largest randomised sham-control trial, to date, investigating 64 patients, revealed the radiofrequency group having significant improvement in heartburn symptoms (61% vs 33%) and GORD quality of life score (61% vs 30%) at 6 mo[100]. It is seldom associated with serious complications but there have been reports of delayed gastric emptying in a few[101].
There are only 2 reported case series in the paediatric setting. Islam et al[102] reported its first use in 6 teenagers (mean age 18, range 14-21) in those who had previous surgical reflux surgery. All had an improvement in their GORD symptom score with 5 out of 6 completely asymptomatic at 3 mo[102]. Liu et al[103] reported on 8 children aged 11-16, including 3 children with neurological impairment requiring a concomitant percutaneous gastrostomy feeding tube[103]. The follow-up period was up to 15 mo and 6 of the patients were considered to have a successful outcome, based on improvement of GORD symptoms and tolerability of feeding. Of the two failures, one required continued PPI use and the other a Nissen fundoplication.
Without larger published series in children to date, paediatric gastroenterologists are likely to be reserved in its use, particularly considering that it is unknown what the long-term effects of thermal injury to the GOJ in a child is likely to be.
Another novel endoscopic treatment, the ENTERYX procedure involves injecting a gastro-esophageal biopolymer into the lower oesophageal sphincter (Figure 9). The authors do not recommended its use in paediatric practice though. Besides concerns regarding long-term outcome of the ENTERYX injection, perforation of the oesophagus is a risk during administration of this treatment.
Figure 9 Injection of liquid polymer into the oesophageal mucosa.
The Enteryx® procedure.
Assessment and excision of upper GI polyps: Over the last few years, investigation of number of polyposis syndromes has revealed the presence of upper GI polyps in addition to the more widely documented colorectal polyps. The most common polyposis syndrome, familial adenomatous polyposis (FAP) is an inherited autosomal dominant condition which results from mutations within the gene locus on chromosome 5[104]. In addition to causing the development of numerous colorectal polyps in FAP patients, it has also been found that multiple polyps may occur in the gastric antrum and duodenum[105-108]. Domizio et al[109], investigating a series of patients from St Mark’s Hospital, demonstrated microscopic gastroduodenal pathology in 100/102 asymptomatic FAP patients. This included the presence of duodenal adenomas in 94 patients and gastric fundic gland polyps in 44 patients. Although the significance and natural history of gastric polyps in patients with polyposis syndromes has not been clearly described, it has been shown that patients with FAP have a higher risk of duodenal cancer and various methods of upper GI endoscopic assessment tools have been used including standard endoscopy, endoscopy with a side-viewing scope and double-balloon enteroscopy[110,111].
In addition to FAP, other syndromes are known to predispose to upper GI polyps which can pose management challenges. It is known that children with Peutz-Jeghers (PJ) syndrome have a risk of polyps which can lead to harmful consequences like bleeding and obstruction. Children with PJ may often have to undergo laparotomies to manage these problems but increasingly less invasive, endoscopic management options are being used like balloon enteroscopy which can even be used to remove polyps in the proximal jejunum[112].