Published online Jun 16, 2014. doi: 10.4253/wjge.v6.i6.240
Revised: April 2, 2014
Accepted: May 28, 2014
Published online: June 16, 2014
AIM: To detect the criteria and cause of elevated salivary amylase activity (sAMY) in patients undergoing endoscopic submucosal dissection (ESD) under sedation.
METHODS: A total of 41 patients with early gastric cancer removed via ESD under deep sedation (DS) were enrolled. The perioperative sAMY, which was shown as sympathetic excitements (SE), was measured. The time at which a patient exhibited a relatively increased rate of sAMY compared with the preoperative baseline level (IR, %) ≥ 100% (twice the actual value) was assumed as the moment when the patient received SE. Among the 41 patients, we focused on 14 patients who exhibited an IR ≥ 100% at any time that was associated with sAMY elevation during ESD (H-group) and examined whether any particular endoscopic procedures can cause SE by simultaneously monitoring the sAMY level. If a patient demonstrated an elevated sAMY level above twice the baseline level, the endoscopic procedure was immediately stopped. In the impossible case of discontinuance, analgesic medicines were administered. This study was performed prospectively.
RESULTS: A total of 26 episodes of sAMY eruption were considered moments of SE in the H-group. The baseline level of sAMY significantly increased in association with an IR of > 100% at 5 min, with a significant difference (IR immediately before elevation/IR at elevation of sAMY = 8.72 ± 173/958 ± 1391%, P < 0.001). However, effective intervention decreased the elevated sAMY level immediately within only 5 min, with a significant difference (IR at sAMY elevation/immediately after intervention = 958 ± 1391/476 ± 1031, P < 0.001). The bispectral indices, systolic blood pressure and pulse rates, which were measured at the same time, remained stable throughout the ESD. Forceful endoscopic insertion or over insufflation was performed during 22 of the 26 episodes. Release of the gastric wall tension and/or the administration of analgesic medication resulted in the immediate recovery of the elevated sAMY level, independent of body movement.
CONCLUSION: By detecting twice the actual sAMY based on the preoperative level, the release of the gastric wall tension or the administration of analgesic agents should be considered.
Core tip: The analgesia in patients during endoscopic submucosal dissection (ESD) under deep sedation (DS) has not yet been developed. There was no way of measuring the degree of the pain in those patients. This study revealed that the salivary amylase activity (sAMY) shown as sympathetic excitement (SE) sometimes was elevated during ESD without any change in circulatory dynamics or consciousness. We suggest that sAMY is elevated when patients feel pain during ESD under DS. By detecting twice the actual sAMY based on the preoperative level, the release of gastric wall tension or the administration of analgesic agents should be considered.
- Citation: Uesato M, Nabeya Y, Akai T, Inoue M, Watanabe Y, Horibe D, Kawahira H, Hayashi H, Matsubara H. Monitoring salivary amylase activity is useful for providing timely analgesia under sedation. World J Gastrointest Endosc 2014; 6(6): 240-247
- URL: https://www.wjgnet.com/1948-5190/full/v6/i6/240.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i6.240
Endoscopic submucosal dissection (ESD) is widely used to treat early gastric cancer because the en bloc resection of lesions via ESD provides a detailed pathological assessment and possible radical cure[1-4]. However, technical difficulties and the expanded eligibility criteria for ESD can also result in a prolonged procedure time[1,5,6], and ESD is generally performed under deep sedation (DS)[7,8]. Accordingly, there is an increased risk of anesthesia-related complications that are associated with higher doses of sedative drugs as more opportunities to perform ESD for gastric tumors arise[9]. The effect of analog-sedation for the patients in the intensive care unit has recently attracted attention. Egerod et al[10] recommends an interdisciplinary effort to target patients requiring less because issues of oversedation and inadequate pain management still require additional attention. In addition, the administration of additional analgesics can stabilize the condition of patients under sedation during endoscopic procedures[11]. Consequently, providing timely and adequate analgesia in addition to sedation for the entire duration of ESD is essential. Several methods can be used to determine the state of the consciousness in patients, including the bispectral index monitor designed by Aspect Medical Systems (Norwood, MA, United States) and the Ramsey sedation score. However, a method for measuring analgesic degree has not yet been developed. In practice, endoscopists administer analgesics to patients during ESD without following specific criteria.
The salivary amylase activity (sAMY) is controlled by epinephrine secreted from the adrenal medulla, which is caused by enhanced activity of the sympathetic-nervous-adrenomedullary system[12,13]. Recent studies have demonstrated the efficacy of assessing psychological stress objectively by monitoring sAMY[14,15], and an instrument using this method to assess stress with rapidity and low invasiveness has been marketed for practical use[16,17]. We have already reported that using this instrument, the analgesic level can be monitored easily and accurately according to the sAMY level, which may positively contribute to performing safe and secure ESD under DS[18]. Hence, we first disclosed that monitoring the sAMY level can be used to objectively assess stress in response to pain in patients undergoing ESD[18].
As a next step, two aims of this study are to detect the sAMY level, which can be shown as a significant sympathetic excitement (SE) in patients undergoing ESD for gastric tumors under DS, and to explore which particular endoscopic surgery techniques cause a significant SE.
This study enrolled 41 consecutive patients with early gastric cancer who were treated at the Department of Frontier Surgery or the Department of Endoscopic Diagnostics and Therapeutics, Chiba University Hospital. The patients underwent ESD under properly maintained DS with midazolam (0.04-0.06 mg/kg iv) or propofol (1-2 mg/kg iv) and pentazocine (7.5 mg iv); neither anticholinergic nor vasopressive agents were used. Carbon dioxide was used in the insufflation of the endoscope.
The sAMY levels were determined as previously reported[18]. Briefly, we measured the sAMY level using enzyme analysis equipment, a sAMY Monitor (NIPRO Co., Osaka, Japan), prior to performing ESD in the morning, immediately following the induction of sedation, and every five minutes after the initiation of ESD. sAMY measurement requires only 1 min after saliva sampling under the tongue. We evaluated the intraoperative sAMY value by calculating the relative rate of increase in the sAMY level compared with the control level (IR, %) as follows: (the elevated sAMY level-the baseline level prior to ESD in the morning)/the baseline level × 100. According to the results of our previous study[18], the median (range) of IR was 105.2% (1.7-3050). Taken together, in this study, we assumed the time when a patient exhibited an IR of ≥ 100% (twice the actual value) as the moment when the patient received SE. This study was performed prospectively. In addition, we simultaneously monitored the endoscopic procedures and the perturbation of the sAMY level and examined which techniques were associated with SE during ESD. However, completing ESD as soon as possible was more important than exploring the possible causes of SE. Similar to the case in a previous report[18], intense body movement occurred in a patient after a high sAMY level was overlooked. Therefore, if a patient appeared to a high sAMY level during ESD, the operator attempted to remove the source of the SE immediately and not to overlook it.
Fourteen patients who exhibited an IR of ≥ 100% at any time associated with sAMY elevation during ESD were categorized into the H-group. Nineteen patients who failed to exhibit an IR of ≥ 100% at any time associated with sAMY elevation during ESD were categorized into the L-group. The remaining eight patients exhibited various IR values and were categorized into the M-group. When a patient demonstrated an elevated sAMY level during ESD, the endoscopic procedure was immediately stopped. In the impossible case of discontinuance, analgesic medicines were administered. Therefore, we calculated the recovery rate of sAMY (%) as follows: (the elevated sAMY level-the decreased sAMY level immediately following intervention)/the elevated sAMY level × 100. We defined a forceful endoscopic insertion when the tip of the endoscope was inserted more than 80 cm from the incisor line to stomach and an over insufflation when the gastric fold completely disappeared.
The patient’s blood pressure and pulse rate were also assessed at the time of sAMY measurement. In addition, a bispectral index monitor was used to evaluate the level of consciousness. All patients were interviewed using a questionnaire prior to discharge to determine their subjective consciousness level.
The institutional review board approved the study protocol, and written informed consent was obtained from all patients before enrollment.
Continuous data are presented as the mean ± SD. The Mann-Whitney U test was used to analyze the differences in continuous or ordinal variables between the groups. Fisher’s exact test was used to evaluate the differences in proportions between the groups, and the Kruskal-Wallis test was used in proportion among the three groups. Perioperative changes in the IR values around the moment of sAMY elevation were compared using the Wilcoxon signed rank-sum test. All statistical analyses were conducted using the SPSS 15.0 software package (SPSS Inc., Chicago, IL, United States). P values of less than 0.05 were considered to be statistically significant.
The patient characteristics are shown in Table 1. No significant differences were observed among the three groups in terms of clinical characteristics, including the procedure time. The H-group demonstrated 26 episodes of sAMY elevation (with an IR of ≥ 100%). The M-group exhibited 30 episodes of sAMY elevation (11 episodes of an IR of ≥ 100% and 19 episodes of an IR of < 100%), and the L-group exhibited 16 episodes of sAMY elevation (with an IR of < 100%). The number of episodes of an elevated sAMY level associated with body movement was higher in the H-group than it was in the L-group (P = 0.078) (Table 2). However, even in the H-group, nine (34.6%) of the 26 episodes of an elevated sAMY (with an IR of ≥ 100%) were not accompanied by simultaneous body movement. The method of sedation failed to affect the sAMY level immediately after the induction of sedation (midazolam/propofol = 39.70 ± 49.18/29.26 ± 44.62 KU/L, P = 0.926). All 41 patients responded with “I did not wake up at all” on the post-ESD questionnaire.
| H-group | M-group | L-group | P value | |
| No. of patients | 14 | 8 | 19 | |
| Gender (male/female) | 9/5 | 6/2 | 16/3 | 0.429 |
| Age (yr) | 71.5 ± 11.7 | 71.6 ± 8.9 | 69.9 ± 7.0 | 0.569 |
| (range) | (40-84) | (58-86) | (58-81) | |
| Body weight (kg) | 57.3 ± 10.6 | 62.4 ± 10.0 | 58.8 ± 8.7 | 0.443 |
| (range) | (43.1-82) | (49-80.3) | (44-76) | |
| No. of tumors | 14 | 8 | 19 | |
| Location U/M/L | 1/5/8 | 0/2/6 | 3/6/10 | 0.464 |
| Less, post/great, ant | 9/5 | 3/5 | 10/9 | 0.485 |
| Resected tumor size (mm) | 29.0 ± 10.0 | 29.3 ± 12.7 | 30.2 ± 11.4 | 0.827 |
| (range) | (15-49) | (17-58) | (12-50) | |
| Procedure time (min) | 78.0 ± 54.1 | 92.5 ± 55.9 | 73.7 ± 46.8 | 0.717 |
| (range) | (35-240) | (35-200) | (20-205) |
| H-group | M-group | L-group | P value | |
| No. of patients | 14 | 8 | 19 | |
| No. of elevated sAMY (times) | 26 | 30 | 16 | |
| ≥ twice the actual value | 26 | 11 | 0 | |
| < twice the actual value | 0 | 19 | 16 | |
| with body movement | 17 | 16 | 6 | 0.215 (10.078) |
| without body movement | 9 | 14 | 10 |
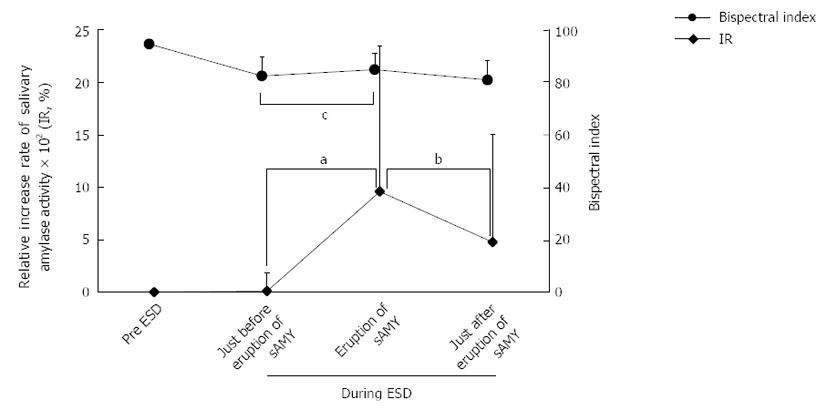
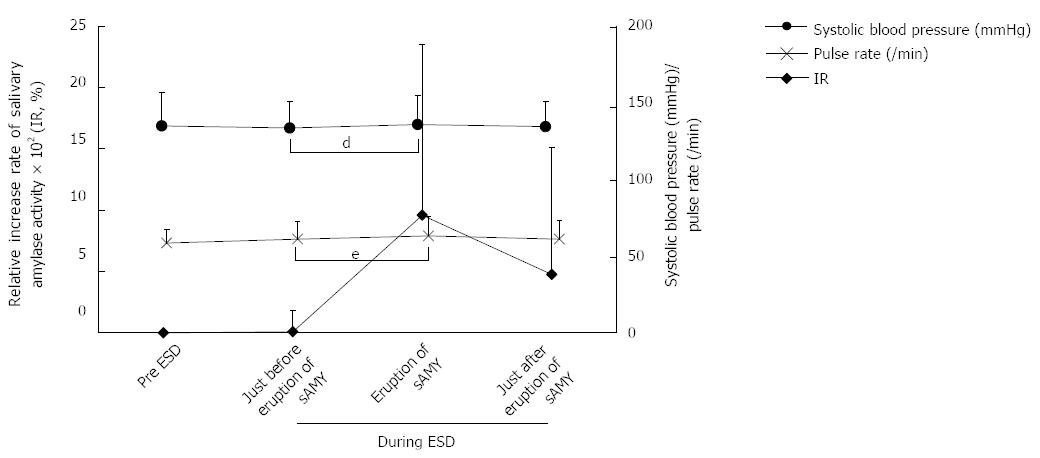
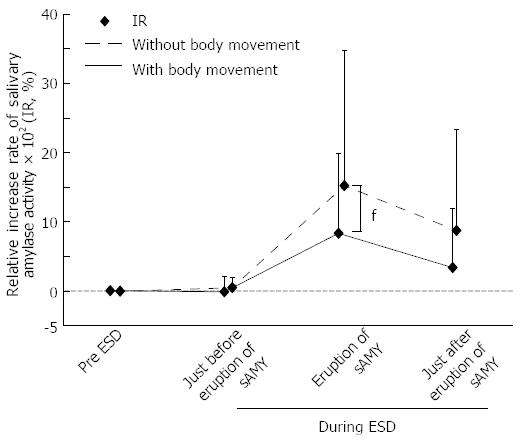
In this study, because we aimed to explore the relationships among the sAMY elevation associated with SE, the patients’ condition, and the endoscopic procedure, we focused on the patients in the H-group, who were considered to experience the potential pain at any time of sAMY elevation during ESD compared with the patients in the “painless” L-group. Figure 1 shows the variation in the IR and bispectral index associated with the 26 episodes of sAMY elevation in the H-group. The baseline level of sAMY significantly increased in association with an IR of > 100% at 5 min, with a significant difference (IR immediately before elevation/IR at sAMY elevation = 8.72 ± 173/958 ± 1391%, P < 0.001). However, an effective intervention decreased the elevated sAMY level immediately within only 5 min, with a significant difference (IR at sAMY elevation/immediately after intervention = 958 ± 1391/476 ± 1031, P < 0.001). The bispectral indices in the patients undergoing ESD proved to be stable throughout the procedures, even when the sAMY level was elevated in association with an IR of > 100%, i.e., when the patient received SE (Figure 1). Figure 2 shows the variations in systolic blood pressure and pulse rate that were associated with perturbation in the IR in the H-group. The systolic blood pressure values and pulse rates were also stable throughout ESD. The status of simultaneous body movement did not significantly affect the IR in the H-group, while the IR values that were not associated with body movement (nine episodes) were relatively higher than those associated with body movement (17 episodes) (Figure 3, P = 0.236).
The technical status at the moment of sAMY elevation was compared between the H- and L-groups (Table 3). In both groups, the most frequent operative technique was “Dissection” (H-group/L-group; 11/26 = 42.3%/10/16=62.5%), and no significant differences were found in the frequency of this technique (P = 0.430). “Inversion” was the most frequent direction (H-group/L-group; 14/26 = 53.8%/10/16, 62.5%) in both groups, without significant intergroup differences in the frequency of this direction (P = 0.582). Forceful endoscopic insertion or over insufflation were performed during 22 of the 26 episodes (84.6%) of sAMY elevation in the H-group; the frequency of these procedures was significantly higher in the H-group than it was in the L-group (56.3%, P = 0.042). The interventions used to treat sAMY elevation, which indicated SE, in the H-group are shown in Table 4. To relieve SE immediately, either release of gastric wall tension or pentazocine injection were performed during the 14 episodes of sAMY elevation associated with body movement. In two cases, both technical and medical interventions (i.e., release of gastric wall tension and medication administration) were concomitantly performed. The recovery rate of a sAMY elevation that was not associated with body movement did not significantly differ from that of a sAMY elevation that was associated with body movement. Midazolam or propofol were administered in only two patients with high bispectral indices and were very effective in both cases.
| H-group | L-group | P value | |
| No. of elevated sAMY (times) | 26 | 16 | |
| Operative techniques | |||
| Incision | 9 | 4 | 0.430 |
| Dissection | 11 | 10 | |
| Hemostasis | 6 | 2 | |
| Endoscopic direction | |||
| Straight | 12 | 6 | 0.582 |
| Inversion | 14 | 10 | |
| Forceful endoscopic insertion or over insufflation | |||
| Presence | 22 | 9 | 0.042 |
| Absence | 4 | 7 | |
| Body movement | Presence | Absence |
| Number (times) | 17 | 9 |
| Release of gastric wall tension only | 2/86.2 | 5/66.1 |
| Medication (pentazocine injection) only | 12/94.7 | 3/95.9 |
| Release and medication (pentazocine) | 2/119.6 | 0/- |
| Medication (midazolam or propofol injection) only | 1/124.2 | 1/85.6 |
The results of this study first demonstrated that the gastric wall tension caused by forceful endoscopic insertion or over insufflation is a major cause of SE in patients undergoing ESD for gastric tumors under DS. A link between SE and the status of the endoscopic procedure was clearly shown by monitoring the sAMY level, which objectively reflects the analgesic level in unconscious gastric ESD patients. The management of the sAMY might prevent the unanticipated body movement in patients during ESD.
Kiriyama et al[19] reported that local lidocaine injections into the submucosal layer are effective for local pain control both immediately after and during ESD, because local pain can be caused by the formation of artificial gastric ulcers. In their study, the level of pain and the effects of lidocaine during surgery were evaluated indirectly based on the reduced total dose of pentazocine[19]. However, our current study demonstrated that an IR of sAMY ≥ 100%, which indicates intraoperative SE, was not always observed, although every patient suffered from artificial ulcers induced by ESD. Moreover, there were no significant differences between the H- and L-groups in terms of the size of the resected tumors. Therefore, the degree of SE demonstrated by the sAMY level may not necessarily depend on ulcer formation, and the size of an artificial ulcer may not be crucial for SE, at least in patients undergoing gastric ESD. This idea is supported by our experience, as most patients who are conscious do not feel pain when they are treated with gastric or colonic endoscopic mucosal resection. We therefore hypothesized that the operative time or some particular technique of the operative procedure, which varies among individuals, is associated with the development of SE in patients undergoing gastric ESD.
Our data suggest that the development of SE during gastric ESD is not related to a long operative time (Table 1). However, we found that the status of forceful endoscopic insertion or over insufflation significantly differed between the H- and L-groups (Table 3). Regarding the sudden production of sAMY, sympathetic fibers directly trigger the salivary gland, which secretes amylase before the gland responds to norepinephrine from the adrenal medulla[20]. In the current study, the systolic blood pressure values and pulse rates remained stable, even when the sAMY level suddenly changed during gastric ESD. Most likely, an increased sAMY level reflects sympathetic nerve excitement before circulatory dynamics become unstable. If the endoscopic procedures were to be subsequently continued, the sympathetic nerves would be further excited, and the blood pressure and pulse rate would become unstable. In this study, we successfully demonstrated this relationship by monitoring the sAMY level, which reflects the degree of potential pain during gastric ESD under DS and proper interventions.
Sensory receptors (mechanoreceptors) that are present in the mucosa, musculature (bowel wall), serosal surface, and mesentery[21-23] primarily respond to mechanical events, such as distension, torsion, contraction, and compression or stretching of the gut[23]. According to basic science experiments, gastric and/or colorectal distention induces acute visceral pain[24,25]. In particular, colorectal distension in rats stimulates cardiovascular and visceromotor responses[25]. Moreover, both morphine and clonidine produce a dose-dependent inhibition of cardiovascular and visceromotor responses to colorectal distension[25]. Clinically, the degree of discomfort a patient feels during a colonoscopic examination varies considerably and is related to the force imparted on the colon by the colonoscopy instruments, stretching the colonic wall, and mesenteric attachments, causing excessive gaseous insufflation[26,27]. These previously reported findings are consistent with the results of our gastroscopy study. However, there have been no such reports on the link between the objective evaluation of pain, i.e., measurement of the sAMY level, and the technical status during gastric ESD. If ESD is performed under steady pressure automatically controlled endoscopy[28], we might reveal more clinical details of the relationship between the pain and the over insufflation.
While assessing and measuring pain are very important considerations for both patients and physicians, as previously described[19], pain tolerance varies greatly among individuals. Accordingly, the results of our study are significant with respect to the individualized, safe management of patients who undergo ESD for gastric tumors under DS. First, the operator should avoid causing gastric wall tension to relieve intraoperative pain. However, if releasing gastric wall tension cannot be achieved due to necessary technical steps or if it is not effective at reducing the patient’s pain, analgesic drugs, such as pentazocine, should be administered immediately. These results support the findings of a previous report showing that morphine produces a dose-dependent inhibition of visceromotor responses to colorectal distension in rats[25]. In addition, in our study, analgesic evasion resulted in a significant decrease in the sAMY level within only 5 min.
Until recently, ESD operators have typically used body movement to indicate the moment that a patient feels pain during ESD performed under DS. However, it is important to note that 34.6% of the patients in the H-group exhibited no body movement in our study. This result suggests that, when sAMY elevation indicating pain is observed, analgesic drugs should be administered immediately to decrease the pain, even in patients without body movement. If the sAMY elevation is overlooked, significant variations in systolic blood pressure, pulse rate, and body movement will occur. Therefore, an elevation of the sAMY level is a timely, practical, and objective indicator of intraoperative pain during gastric ESD, even when the patient fails to move simultaneously. The incidence of complications, such as bleeding or perforation, increases if the patient moves during ESD. It is therefore clinically important to address pain before movement occurs. In this study, we focused on the patients in the H-group, who were considered to experience potential pain at any time of sAMY elevation during ESD, compared with the patients in the “painless” L-group. However, the degree of sAMY elevation varied among the patients. Therefore, to safely complete gastric ESD, continuously monitoring the sAMY level throughout the ESD procedure is advisable to accurately assess the real-time degree of pain in individual patients and to determine when to release endoscopic stretching or appropriately administer analgesics after detecting twice the actual sAMY based on the preoperative value.
In this study, even when an elevated sAMY level was observed in the patients undergoing ESD, the average bispectral index was stable (Figure 1). Furthermore, all patients responded with “I did not wake up at all” on the post-ESD questionnaire. Midazolam and/or propofol injection was effective in two patients with both high bispectral indices and high sAMY elevation levels (one case without body movement) in the H-group. High levels of both the bispectral index and sAMY suggest that a patient may be in a waking state. Accordingly, monitoring the sAMY level simultaneously with the bispectral index enables physicians to differentially understand the levels of pain and consciousness in patients undergoing gastric ESD under DS and is of great clinical significance.
In conclusion, pain, as represented by twice the actual sAMY based on the preoperative level, in unconscious patients undergoing ESD under DS for gastric tumors may be caused by the gastric wall tension, which can elevate the sAMY level quickly, even without body movement, before a change in cardiovascular response. Therefore, continuously monitoring the changes in the sAMY level and either modifying the endoscopic technique or administering analgesics can be used to treat pain in a timely manner, and patients undergoing ESD for gastric tumors under DS can be managed more securely.
Endoscopic submucosal dissection (ESD) is widely used to treat early gastric cancer under deep sedation (DS) and analgesia. Accordingly, there is an increased risk of anesthesia-related complications associated with higher doses of sedative and analgesic drugs as the opportunities to perform ESD for gastric tumors arise. There are several methods to know the state of the consciousness in patients. However, a method to measure analgesic degree has not yet been established.
Recent studies have demonstrated the efficacy of assessing psychological stress objectively by monitoring salivary amylase activity (sAMY), and an instrument using this method to assess stress with rapidity and low invasiveness has been marketed for practical use.
Until recently, ESD operators have usually judged body movement to indicate the moment that a patient feels discomfort during ESD performed under DS and given the analgesics to patients without criteria. The authors aimed to detect the criteria of sAMY level shown as a significant sympathetic excitement in patients undergoing ESD of gastric tumors under DS and to explore which particular techniques of endoscopic surgery cause the sAMY elevation.
The study results suggest that by detecting twice the actual sAMY based on the preoperative level, the release of gastric wall tension or the administration of analgesic agents should be considered.
sAMY: salivary amylase activity is controlled by epinephrine secreted from the adrenal medulla, caused by enhanced activity of the sympathetic nervous-adrenomedullary system.
In this manuscript, Uesato et al provided a novel way to measure the depth of analgesia by a quantitative marker. This manuscript is interesting.
P- Reviewers: Albuquerque A, He SB, Pierzchalski P, Uen YH S- Editor: Song XX L- Editor: A E- Editor: Zhang DN
| 1. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [Cited in This Article: ] |
| 2. | Miyazaki S, Gunji Y, Aoki T, Nakajima K, Nabeya Y, Hayashi H, Shimada H, Uesato M, Hirayama N, Karube T. High en bloc resection rate achieved by endoscopic mucosal resection with IT knife for early gastric cancer. Hepatogastroenterology. 2005;52:954-958. [Cited in This Article: ] |
| 3. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [Cited in This Article: ] |
| 4. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [Cited in This Article: ] |
| 5. | Naruse M, Inatsuchi S. Risk management in endoscopic submucosal dissection in upper gastrointestinal endoscopy: Risk management for sedation in endoscopic submucosal dissection. Dig Endosc. 2007;19:S2-S4. [Cited in This Article: ] |
| 6. | Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, Bae JM. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc. 2005;62:860-865. [Cited in This Article: ] |
| 7. | Chun SY, Kim KO, Park DS, Kim SY, Park JW, Baek IH, Kim JH, Park CK. Safety and efficacy of deep sedation with propofol alone or combined with midazolam administrated by nonanesthesiologist for gastric endoscopic submucosal dissection. Gut Liver. 2012;6:464-470. [Cited in This Article: ] |
| 8. | Kang KJ, Min BH, Lee MJ, Lim HS, Kim JY, Lee JH, Chang DK, Kim YH, Rhee PL, Rhee JC. Efficacy of Bispectral Index Monitoring for Midazolam and Meperidine Induced Sedation during Endoscopic Submucosal Dissection: A Prospective, Randomized Controlled Study. Gut Liver. 2011;5:160-164. [Cited in This Article: ] |
| 9. | Hata K, Andoh A, Hayafuji K, Ogawa A, Nakahara T, Tsujikawa T, Fujiyama Y, Saito Y. Usefulness of bispectral monitoring of conscious sedation during endoscopic mucosal dissection. World J Gastroenterol. 2009;15:595-598. [Cited in This Article: ] |
| 10. | Egerod I, Jensen MB, Herling SF, Welling KL. Effect of an analgo-sedation protocol for neurointensive patients: a two-phase interventional non-randomized pilot study. Crit Care. 2010;14:R71. [Cited in This Article: ] |
| 11. | Terui T, Inomata M. Administration of additional analgesics can decrease the incidence of paradoxical reactions in patients under benzodiazepine-induced sedation during endoscopic transpapillary procedures: prospective randomized controlled trial. Dig Endosc. 2013;25:53-59. [Cited in This Article: ] |
| 12. | Chatterton RT, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16:433-448. [Cited in This Article: ] |
| 13. | Speirs RL, Herring J, Cooper WD, Hardy CC, Hind CR. The influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Arch Oral Biol. 1974;19:747-752. [Cited in This Article: ] |
| 14. | Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol. 2004;49:963-968. [Cited in This Article: ] |
| 15. | Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg. 2005;101:1873-1876. [Cited in This Article: ] |
| 16. | Yamaguchi M, Kanemori T, Kanemaru M, Takai N, Mizuno Y, Yoshida H. Performance evaluation of salivary amylase activity monitor. Biosens Bioelectron. 2004;20:491-497. [Cited in This Article: ] |
| 17. | Yamaguchi M, Deguchi M, Wakasugi J, Ono S, Takai N, Higashi T, Mizuno Y. Hand-held monitor of sympathetic nervous system using salivary amylase activity and its validation by driver fatigue assessment. Biosens Bioelectron. 2006;21:1007-1014. [Cited in This Article: ] |
| 18. | Uesato M, Nabeya Y, Akai T, Inoue M, Watanabe Y, Kawahira H, Mamiya T, Ohta Y, Motojima R, Kagaya A. Salivary amylase activity is useful for assessing perioperative stress in response to pain in patients undergoing endoscopic submucosal dissection of gastric tumors under deep sedation. Gastric Cancer. 2010;13:84-89. [Cited in This Article: ] |
| 19. | Kiriyama S, Oda I, Nishimoto F, Mashimo Y, Ikehara H, Gotoda T. Pilot study to assess the safety of local lidocaine injections during endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2009;12:142-147. [Cited in This Article: ] |
| 20. | Skosnik PD, Chatterton RT, Swisher T, Park S. Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. Int J Psychophysiol. 2000;36:59-68. [Cited in This Article: ] |
| 21. | Cervero F. Neurophysiology of gastrointestinal pain. Baillieres Clin Gastroenterol. 1988;2:183-199. [Cited in This Article: ] |
| 22. | Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271-293. [Cited in This Article: ] |
| 23. | Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut. 1999;45 Suppl 2:II6-II16. [Cited in This Article: ] |
| 24. | Sakurai J, Obata K, Ozaki N, Tokunaga A, Kobayashi K, Yamanaka H, Dai Y, Kondo T, Miyoshi K, Sugiura Y. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology. 2008;134:1094-1103. [Cited in This Article: ] |
| 25. | Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153-169. [Cited in This Article: ] |
| 26. | Shah SG, Brooker JC, Thapar C, Williams CB, Saunders BP. Patient pain during colonoscopy: an analysis using real-time magnetic endoscope imaging. Endoscopy. 2002;34:435-440. [Cited in This Article: ] |
| 27. | Appleyard MN, Mosse CA, Mills TN, Bell GD, Castillo FD, Swain CP. The measurement of forces exerted during colonoscopy. Gastrointest Endosc. 2000;52:237-240. [Cited in This Article: ] |