Published online Nov 16, 2013. doi: 10.4253/wjge.v5.i11.551
Revised: July 1, 2013
Accepted: August 4, 2013
Published online: November 16, 2013
AIM: To investigate the clinical impact of capsule endoscopy (CE) after an obscure gastrointestinal bleeding (OGIB) episode, focusing on diagnostic work-up, follow-up and predictive factors of rebleeding.
METHODS: Patients who were referred to Hospital del Mar (Barcelona, Spain) between 2007 and 2009 for OGIB who underwent a CE were retrospectively analyzed. Demographic data, current treatment with non-steroid anti-inflammtory drugs or anticoagulant drugs, hemoglobin levels, transfusion requirements, previous diagnostic tests for the bleeding episode, as well as CE findings (significant or non-significant), work-up and patient outcomes were analyzed from electronic charts. Variables were compared by χ2 analysis and Student t test. Risk factors of rebleeding were assessed by Log-rank test, Kaplan-Meier curves and Cox regression model.
RESULTS: There were 105 patients [45.7% women, median age of 72 years old (interquartile range 56-79)] and a median follow-up of 326 d (interquartile range 123-641) included in this study. The overall diagnostic yield of CE was 58.1% (55.2% and 63.2%, for patients with occult OGIB and overt OGIB, respectively). In 73 patients (69.5%), OGIB was resolved. Multivariate analysis showed that hemoglobin levels lower than 8 g/dL at diagnosis [hazard ratios (HR) = 2.7, 95%CI: 1.9-6.3], patients aged 70 years and above (HR = 2.1, 95%CI: 1.2-6.1) and significant findings in CE (HR = 2.4, 95%CI: 1.1-5.8) were independent predictors of rebleeding.
CONCLUSION: One third of the patients presented with rebleeding after CE; risk factors were hemoglobin levels < 8 g/dL, age ≥ 70 years or the presence of significant lesions.
Core tip: This paper describes a large cohort of patients with obscure gastrointestinal bleeding who underwent a capsule endoscopy. The diagnostic yield was analyzed with further exploration motivated by the capsule findings, as well as the outcome during follow-up. Risk factors of rebleeding were also analyzed. Interestingly, old age, a lower hemoglobin level at diagnosis and significant lesions in capsule endoscopy were found to be predictors of rebleeding in this cohort.
- Citation: Cañas-Ventura A, Márquez L, Bessa X, Dedeu JM, Puigvehí M, Delgado-Aros S, Ibáñez IA, Seoane A, Barranco L, Bory F, Andreu M, González-Suárez B. Outcome in obscure gastrointestinal bleeding after capsule endoscopy. World J Gastrointest Endosc 2013; 5(11): 551-558
- URL: https://www.wjgnet.com/1948-5190/full/v5/i11/551.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i11.551
Since 2001, video capsule endoscopy (CE) has become an important tool for the diagnosis of small bowel diseases. Its most important indication is in the study of obscure gastrointestinal bleeding (OGIB), defined as persistent or recurrent bleeding from the gastrointestinal (GI) tract after a non-conclusive conventional endoscopic examination [upper-GI endoscopy (GIE)] and colonoscopy. Two different presentations of OGIB can be distinguished: obscure-occult GI bleeding [persistent or recurrent iron deficiency anemia and/or positive fecal occult blood test (occult-OGIB)] and obscure-overt GI bleeding [recurrent visible blood loss: melena or hematochezia (overt-OGIB)][1]. CE provides a non-invasive examination of the small intestine that is not accessible through conventional endoscopy or push or balloon enteroscopy. Several studies have shown good specificity and sensitivity of CE in the setting of OGIB and better diagnostic yield than other techniques (radiological or endoscopic procedures)[2-5]. CE is well-tolerated and its rate of complications is very low. The disadvantages of CE are the impossibility to treat and the impossibility to obtain tissue biopsies. Although the diagnostic yield of CE is high, the impact on the outcome of the patients with OGIB after a CE is still unclear.
A single center retrospective study of a long follow-up cohort of 105 patients with OGIB who underwent a CE study is presented. The aim of this study was to evaluate the usefulness of CE, focusing on the subsequent treatment and the outcome of the OGIB episode. Secondary objectives were to define predictive factors of rebleeding.
All patients with OGIB referred to the endoscopy unit (Hospital del Mar, Barcelona, Spain) between January 2007 and June 2009 were analyzed retrospectively. On all these patients, at least one upper-GIE and one colonoscopy were run. Those endoscopies were considered normal or the findings were insufficient to explain the patient’s symptoms. By using the electronic charts of all patients, we collected data on the complete episode of OGIB; including previous procedures to CE and follow-up data. The collected variables were: demographic data, history of intake of non-steroidal anti-inflammatory drugs (NSAIDs), anticoagulant and antiplatelet therapy (aspirin and clopidogrel), hemoglobin levels (Hb) at diagnosis, transfusion requirements, time from overt bleeding to CE procedure, and results of previous diagnostic tests [upper-GIE, colonoscopy, computed tomography (CT) scan, radiographic series of small bowel, angiography, Tc99 red cell scan and Meckel’s scan].
The procedure was performed in ambulatory and in-hospital patients, using PillCam SB2© (Given Imaging, Yoqneam, Israel). Bowel preparation consisted of an oral purge (two liters of polyethylene glycol-based solution) ingested the night before the procedure. CE was swallowed in the morning and the data recorder was removed 8-9 h later. Patients were allowed to drink fluids 2 h after the administration of CE and to eat 4 h after the ingestion. Patients were asked to verify the excretion of the capsule in the stool and to alert the endoscopy unit if it was not excreted.
In order to test small bowel patency, a previous exam was performed with Agile capsule© (Given Imaging, Yoqneam, Israel) on patients with a history of sub-occlusive intestinal episodes, chronic NSAID intake, i.e., longer than 6 mo, established or suspected inflammatory bowel disease, previous abdominal surgery of small bowel or bowel strictures demonstrated by radiological techniques. Capsule retention was defined as the presence of the capsule in the GI tract for at least 2 wk after ingestion. Two gastroenterologists with extensive experience in small bowel endoscopy (González-Suárez B and Dedeu JM) evaluated the images recorded by CE.
The CE findings were classified as significant and non-significant. Significant findings were those that explained the clinical situation (i.e., ulcers, active bleeding, tumors and angiodysplasias). Non-significant findings were those where the mucosa was normal or with minimal changes with an uncertain relationship to the bleeding (i.e., small erosions, small and isolated angiodysplasia).
Therapeutic strategy was classified into two different groups: (1) specific treatment focused on the main cause of bleeding: invasive therapies (i.e., endoscopic treatment or surgery) and medical treatment (i.e., proton-pump inhibitors (PPIs), NSAIDs or anticoagulant drugs withdrawal); and (2) non-specific treatment (i.e., iron supplementation, blood transfusions, watchful waiting and NSAID withdrawal if CE findings were not significant or different to ulcer/erosion). Therapeutic strategy was chosen based on the patient’s overall condition and the nature of the disease.
Complete follow-up information was obtained from electronic charts: hemoglobin levels, transfusion requirement after treatment, recurrence of OGIB and CE complications. Follow-up time was defined as the time between the CE and the date of rebleeding or the last follow-up visit. Anemia was defined as Hb level < 13 g/dL in men and < 12 g/dL in women. A patient’s outcome was considered favorable or resolved if no overt bleeding was present and the anemia was resolved completely after treatment. Rebleeding was defined as overt bleeding or reappearance of anemia.
Continuous data were expressed as median and percentiles [interquartile range (IQR) 25th-75th percentile] and were compared using the Student t test or the U test. Categorical data were expressed by percentages with a 95%CI and compared by the χ2 test or the F test.
Independent predictors for rebleeding were first analyzed by univariate analysis using the Log-rank test in the Kaplan-Maier model (setting the rebleeding variable as “event”). All variables from the univariate analysis with a P < 0.05 were included in a Cox proportional hazards regression using the stepwise selection method. Results were reported as hazard ratios (HR) with 95%CI. All P values were two-sided and P < 0.05 was considered to indicate a statistically significant difference.
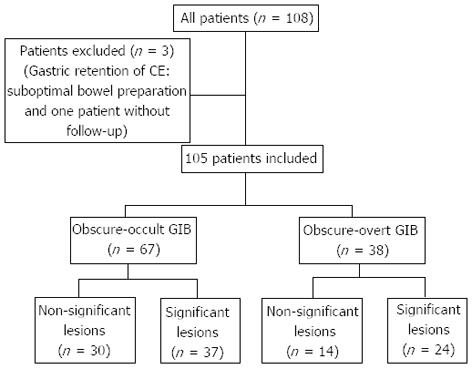
There were 108 patients included in the study. In two patients, the CE failed to achieve complete small bowel visualization: in one patient CE was retained for eight hours in the stomach and in the other one, bowel preparation was not optimal for image evaluation. In one patient, follow-up was not available because she moved back to her country the day after CE. Hence, 105 patients were available for data analysis (Figure 1).
According to the definition of OGIB, 67 patients (64.2%) with occult-OGIB and 38 patients (35.8%) with an overt-OGIB were identified. The baseline characteristics of patients included in the cohort are summarized in Table 1. Follow-up time and hemoglobin at diagnosis were similar in both groups. Mean of transfusion units was higher in the overt-OGIB group than in the occult-OGIB group (2.5 vs 1.7 units, P = 0.037).
| Characteristics | Overall | Occult-OGIB | Overt-OGIB | P value |
| Patients | 100.00% | 63.80% | 36.20% | - |
| Age [yr, median (IQR)] | 72 (5-79) | 71 (5-78) | 73 (49-82) | 0.800 |
| Gender (female) | 45.70% | 55.20% | 28.90% | 0.014 |
| Bleeding-related drugs | 41.90% | 37.30% | 50% | 0.200 |
| NSAIDs | 30.50% | 26.90% | 36.80% | 0.300 |
| Hemoglobin level [g/dL, median (IQR)] | 7.5 (6.4-9.3) | 7.4 (6.0-9.1) | 7.6 (6.5-9.4) | 0.600 |
| Transfusion requirements | 61% | 55.20% | 71.10% | 0.080 |
| Transfusion requirements (blood units, mean ± SD) | 2.01 | 1.7 ± 1.7 | 2.5 ± 2.3 | 0.037 |
| Follow-up [d, median (IQR)] | 326 (123-641) | 330 (154-691) | 217 (84-476) | 0.500 |
There were 44 patients (41.9%) that had been taken a bleeding-related drug before the OGIB episode: clopidogrel (n = 8); warfarin (n = 9) or NSAIDs (including acetylsalicylic acid) (n = 26), without statistical significant differences comparing the two groups (P = 0.2).
All patients were previously submitted to at least one upper-GIE and colonoscopy that were considered normal or whose findings were insufficient to explain the bleeding episode. Other procedures were performed before CE in 12 patients: four CT-scans focused on small bowel, two mesenteric angiographies, three Tc99 red cell scans, three Meckel’s scans, and all of them were negative for the diagnosis of cause of bleeding.
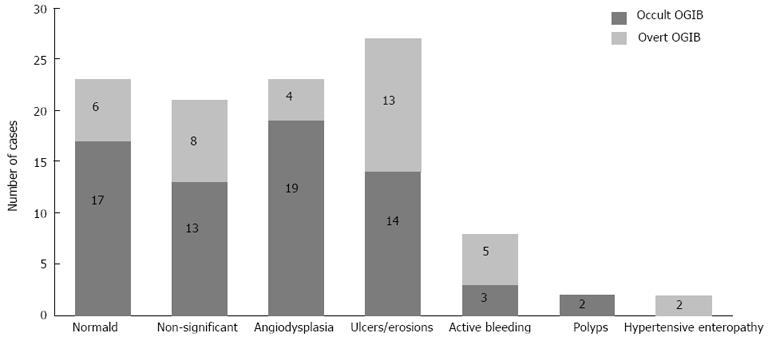
CE findings were considered significant, according to previous definition, in 37 patients of the occult-OGIB group and in 24 patients of the overt-OGIB group, which represent a diagnostic yield of 55.2% and 63.2%, respectively (P = 0.5). The overall diagnostic yield of CE in our cohort was 58.1%. Intestinal angiodysplasia (21%) and small bowel ulcers (27%) were the most frequent lesions (Figure 2). A total of seven lesions (6.6%) were found in the upper GI tract of these patients: five ulcers and two erythematous duodenitis, classified as non-significant lesions.
In two patients, CE was retained in the small bowel. In one patient, retention was due to a pelvic relapse of a previous colorectal cancer that involved the ileum; the patient remained asymptomatic until surgery and the device was removed. Another patient was diagnosed with an intestinal T-cell lymphoma and CE was removed during an oral balloon enteroscopy performed to take biopsies.
All patients with overt-OGIB were submitted to a CE within the first three weeks after the bleeding episode. There were no differences between patients with significant and non-significant lesions regarding the time interval between bleeding and CE [8.5 d (95%CI: 11.4-5.6) vs 6.9 d (95%CI: 8.5-5.3), P = 0.06, respectively].
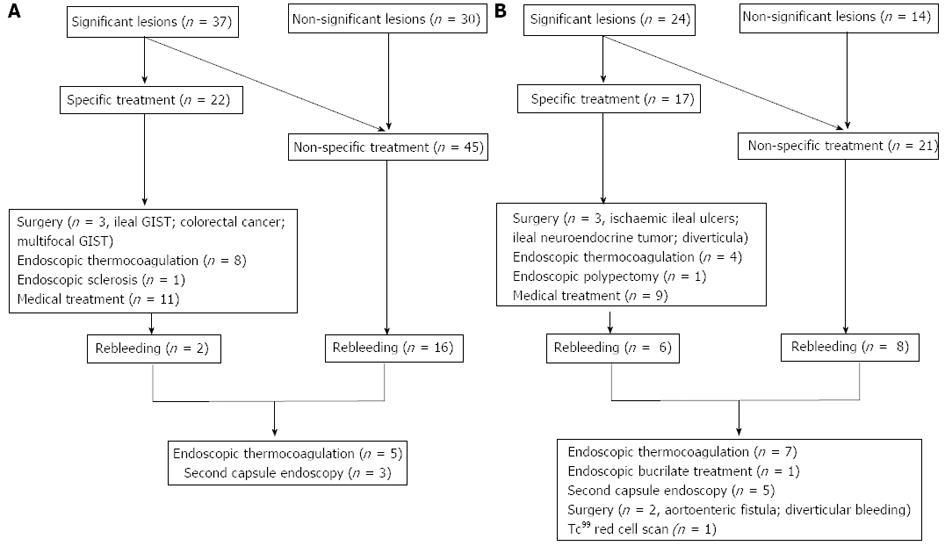
Therapeutic strategy after CE and outcome according to OGIB presentation and CE findings are detailed in Figure 3. Specific treatment was performed in 39 patients (37.5%): surgery (n = 6), endoscopic therapy with thermocoagulation or sclerosis (n = 13) and specific medical treatment (n = 21). Up to 87% of patients with significant lesions in CE received specific treatment. NSAIDs were withdrawn in 22 patients (21%): 9 of them with small bowel ulcers and 13 with normal capsule or non-significant lesions.
Overall follow-up time was 321 d (IQR 115-626). In the cohort, 73 patients (69.5%) had a favorable outcome according to the definition and hemoglobin levels improved significantly [∆Hb 4.4 (95%CI: 5.0-3.9) g/dL, P < 0.001]. Surgery was curative in 100% of patients (n = 6). Endoscopic therapies (n = 13) such as thermocoagulation, sclerosis or polypectomy had a resolution rate of 61.5%.
There were 32 patients (30.8%) who had a recurrence of OGIB, in a median time of 157 d (IQR 81-326) after the index episode. Rebleeding rates were 20.5% and 36.4%, depending on specific and non-specific treatment (P = 0.8).
In 8 of the 32 patients, a second-look capsule endoscopy was performed, which enabled the diagnosis of angiodysplasias in two patients with a previously normal CE. After rebleeding, specific treatment was performed on 15 of the 32 patients (46.8%): 12 endoscopic thermocoagulation, one bucrylate injection and two surgeries.
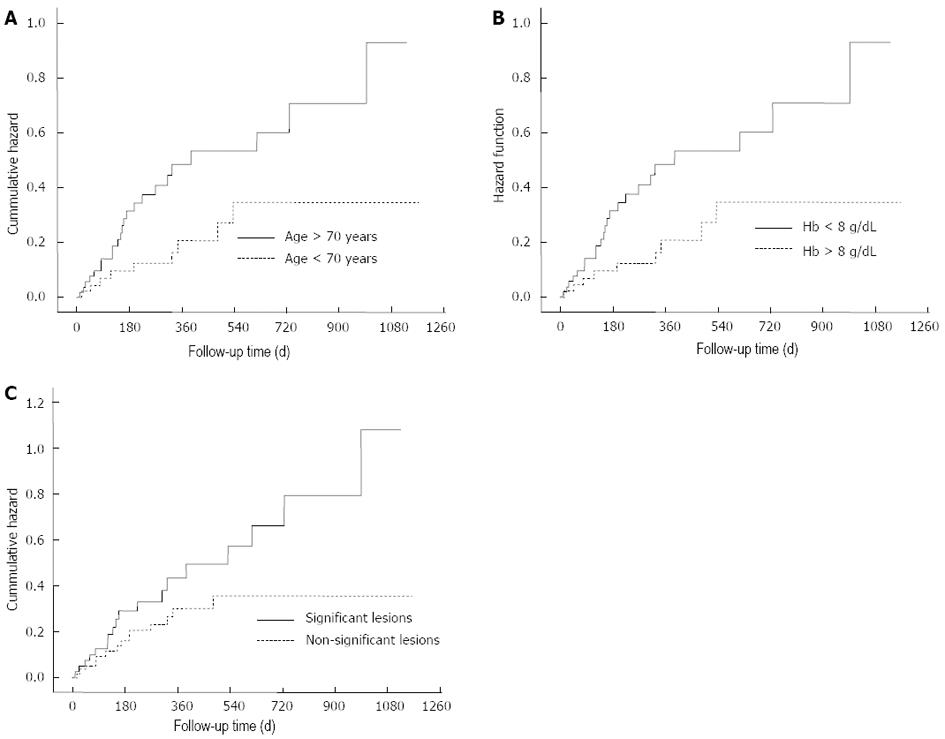
In order to elucidate factors associated with a higher risk of rebleeding, univariate and multivariate analysis were performed. Association analysis is detailed in Table 2 and hazard plots are represented in Figure 4. In this study, Hb levels lower than 8 g/dL at diagnosis (HR = 2.7, 95%CI: 1.9-6.3), patients older than 70 years of age (HR = 2.1, 95%CI: 1.2-6.1) and significant findings in CE (HR = 2.4, 95%CI: 1.1-5.8) were independent predictors of rebleeding. The analysis depending on the type of lesion did not show differences. However, angiodysplasia was the lesion that rebled more frequently (8/32 patients, 25%). Moreover, angiodysplasia was diagnosed after rebleeding in another eight of the 32 patients (25%), whose cause of OGIB was different or unknown after the CE in the index episode.
| Risk factors | Univariate Log-rank test | Multivariate | ||
| P | HR | 95%CI | P | |
| Age > 70 yr | 0.037 | 2.1 | 1.2-6.1 | 0.05 |
| Gender | 0.800 | - | - | - |
| Overt OGIB presentation | 0.130 | - | - | - |
| Hb < 8.0 g/dL | 0.027 | 2.7 | 1.9-6.3 | 0.03 |
| Blood units transfused | 0.180 | - | - | - |
| Transfusions requirement | 0.020 | - | - | - |
| T ≥ 2 blood units | 0.023 | - | - | - |
| NSAID intake | 0.900 | - | - | - |
| Significant lesions in CE | 0.036 | 2.4 | 1.1-5.8 | 0.01 |
| Specific treatment carried out | 0.500 | - | - | - |
Diagnostic work-up and treatment of OGIB is an important challenge for gastroenterologists. Obscure gastrointestinal bleeding is the first accepted indication for capsule endoscopy and there are several diagnostic algorithms proposed by different medical societies[1,6,7]. In the present study of 105 patients with OGIB, a high diagnostic yield of CE in patients with occult-OGIB and overt-OGIB is described.
Optimal bowel preparation is essential to improve CE diagnostic yield[8] and a two liter, polyethylene glycol-based solution ingested the day before CE permitted adequate bowel visualization in almost all patients. Several studies have already shown the superiority of CE compared to other techniques[5,9]. In this cohort, a diagnostic yield of 58.1% was observed, which is concordant with published data[10-16]. However, differences between obscure-overt bleeding and obscure-occult bleeding[17,18] were not observed. The time between the acute bleeding episodes and CE has been analyzed in previous studies, showing that the diagnostic yield would be higher in ongoing bleeding cases if CE were performed within the first 48 h[12,19,20], although this factor does not seem to influence the diagnostic yield in this study.
As has been previously described, the most frequent findings in capsule studies were angiodysplasia and intestinal ulcers[12,21,22]. In seven patients (6.6%), CE diagnosed lesions in the upper-GI tract that were not seen in previous upper-GIE. These results suggest that the repetition of upper- or lower-GIE prior to a CE could be useful in diagnosing accessible lesions by conventional endoscopy[20].
A favorable outcome after CE, reaching bleeding resolution, was observed in two-thirds of patients in this study. Capsule retention occurred in 1.9% of patients, which is consistent with previously published data[23].
The diagnostic and therapeutic work-up were based on CE results in our series: patients with significant lesions received specific treatment more frequently compared with those who had no lesions or non-significant lesions. Nevertheless, this treatment was not associated with a higher resolution rate (20.5% vs 36.4% of rebleeding after specific or non specific treatment, P = 0.8, respectively). These results may have several explanations. Firstly, despite not finding differences according to the kind of lesions and risk of rebleeding, the lesion that most frequently rebled was angiodysplasia. Secondly, angiodysplasia often has a multifocal nature and it has a high rate of rebleeding, even when patients are treated by an endoscopy[14,24].
Moreover, special attention is required in patients treated with NSAIDs and antiplatelet drugs. It is well established that a high percentage of NSAID consumers may present small bowel erosions or ulcers in CE[25]. In this study’s cohort, the use of NSAIDs was stopped in 22 patients after CE findings and 14 of them (14/22, 63.6%) achieved a resolution of the bleeding. However, in eight of these patients, CE findings were non-significant. It is important to remark that an accurate anamnesis regarding NSAID intake is important and its withdrawal should be individually evaluated.
In this study’s cohort, one-third of patients presented with rebleeding episodes during follow-up. Hemoglobin levels lower than 8 g/dL at diagnosis, patients older than 70 years of age, and significant findings in CE were independent predictors of rebleeding, as has been described in previous papers[20,26]. So far, the role of CE findings as a rebleeding risk factor remains controversial. Macdonald et al[27] described a higher rebleeding rate in patients with significant lesions, although no regression analysis was performed in that study[27]. On the contrary, Park et al[28] did not find significant differences in the cumulative rebleeding rate between significant and non-significant findings in the CE. Type of lesion was not a predictor of rebleeding, probably due to stratification and the low number of relapses. However, nearly 50% of patients that presented with rebleeding had a final diagnosis of angiodysplasia in CE. Interestingly, second-look CE or conventional endoscopy after rebleeding revealed angiodysplasia not found in previous procedures, underlining the usefulness of a second-look CE in selected patients, as has been published before[29,30].
The main limitation of this study is that it is a retrospective design. However, data were collected from electronic charts that permitted accuracy in terms of in-hospital and outpatient data, reducing data collection bias. Strengths of the study were the long follow-up that allowed the outcome to be evaluated and the regression analysis of rebleeding risk factors.
In conclusion, this study offers a long follow-up of a large, clinical based, cohort from a single tertiary hospital. Diagnostic yield of CE was high in both OGIB presentations. One-third of the patients presented with rebleeding after CE; risk factors of rebleeding were Hb < 8 g/dL, age ≥ 70 years or the presence of significant lesions in CE.
Capsule endoscopy (CE) is a device that allows visualization of the entire small bowel mucosa. It has become essential in the diagnosis work-up of gut pathologies, especially evaluation of obscure gastrointestinal bleeding (OGIB). Although published studies are focused on diagnostic yield of CE, outcome of patients that undergo a CE has not been analyzed extensively.
CE is widely used in OGIB diagnosis. However, reports about patient outcomes presenting OGIB that received a CE are rare in the medical literature.
This study analyzed a large group of OGIB patients during a long follow-up time. The authors concluded that CE is useful in different types of OGIB and further treatments permitted the resolution of OGIB in a high proportion of patients. Ulcers and angiodysplasias were the most frequently diagnosed lesions by CE. The authors identified several risk factors of rebleeding: old age, a low hemoglobin level at diagnosis and the presence of significant lesions in the CE.
CE is safe, well tolerated and useful in the diagnosis of several gastrointestinal disorders. The risk factors described in this study should help physicians in OGIB management.
CE is a device a little bit bigger than a pill that can be easily swallowed by patients. It is able to take photos as it passes through the gut that are saved in an external memory disk via wireless technology. Photos are studied later by a gastroenterologist at a workstation.
In this retrospective study, the authors investigated the outcome of obscure gastrointestinal bleeding after capsule endoscopy. They concluded that hemoglobin levels < 8 g/dL at diagnosis, patients > 70 years and significant findings in CE were independent factors of a high rebleeding rate.
P- Reviewers: Ladas SD, Riccioni ME, Triantafyllou K S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1694-1696. [Cited in This Article: ] |
| 2. | Saurin JC, Delvaux M, Gaudin JL, Fassler I, Villarejo J, Vahedi K, Bitoun A, Canard JM, Souquet JC, Ponchon T. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003;35:576-584. [Cited in This Article: ] |
| 3. | Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685-689. [Cited in This Article: ] |
| 4. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [Cited in This Article: ] |
| 5. | Saperas E, Dot J, Videla S, Alvarez-Castells A, Perez-Lafuente M, Armengol JR, Malagelada JR. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:731-737. [Cited in This Article: ] |
| 6. | Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-227. [Cited in This Article: ] |
| 7. | Teshima CW. Small bowel endoscopy for obscure GI bleeding. Best Pract Res Clin Gastroenterol. 2012;26:247-261. [Cited in This Article: ] |
| 8. | Rokkas T, Papaxoinis K, Triantafyllou K, Pistiolas D, Ladas SD. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol. 2009;104:219-227. [Cited in This Article: ] |
| 9. | Zhang BL, Jiang LL, Chen CX, Zhong BS, Li YM. Diagnosis of obscure gastrointestinal hemorrhage with capsule endoscopy in combination with multiple-detector computed tomography. J Gastroenterol Hepatol. 2010;25:75-79. [Cited in This Article: ] |
| 10. | van Turenhout ST, Jacobs MA, van Weyenberg SJ, Herdes E, Stam F, Mulder CJ, Bouma G. Diagnostic yield of capsule endoscopy in a tertiary hospital in patients with obscure gastrointestinal bleeding. J Gastrointestin Liver Dis. 2010;19:141-145. [Cited in This Article: ] |
| 11. | Rondonotti E, Soncini M, Girelli C, Ballardini G, Bianchi G, Brunati S, Centenara L, Cesari P, Cortelezzi C, Curioni S. Small bowel capsule endoscopy in clinical practice: a multicenter 7-year survey. Eur J Gastroenterol Hepatol. 2010;22:1380-1386. [Cited in This Article: ] |
| 12. | Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643-653. [Cited in This Article: ] |
| 13. | Liao Z, Gao R, Li F, Xu C, Zhou Y, Wang JS, Li ZS. Fields of applications, diagnostic yields and findings of OMOM capsule endoscopy in 2400 Chinese patients. World J Gastroenterol. 2010;16:2669-2676. [Cited in This Article: ] |
| 14. | Hindryckx P, Botelberge T, De Vos M, De Looze D. Clinical impact of capsule endoscopy on further strategy and long-term clinical outcome in patients with obscure bleeding. Gastrointest Endosc. 2008;68:98-104. [Cited in This Article: ] |
| 15. | Esaki M, Matsumoto T, Yada S, Yanaru-Fujisawa R, Kudo T, Yanai S, Nakamura S, Iida M. Factors associated with the clinical impact of capsule endoscopy in patients with overt obscure gastrointestinal bleeding. Dig Dis Sci. 2010;55:2294-2301. [Cited in This Article: ] |
| 16. | Apostolopoulos P, Liatsos C, Gralnek IM, Kalantzis C, Giannakoulopoulou E, Alexandrakis G, Tsibouris P, Kalafatis E, Kalantzis N. Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest Endosc. 2007;66:1174-1181. [Cited in This Article: ] |
| 17. | Apostolopoulos P, Liatsos C, Gralnek IM, Giannakoulopoulou E, Alexandrakis G, Kalantzis C, Gabriel P, Kalantzis N. The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy. 2006;38:1127-1132. [Cited in This Article: ] |
| 18. | Selby W. Can clinical features predict the likelihood of finding abnormalities when using capsule endoscopy in patients with GI bleeding of obscure origin? Gastrointest Endosc. 2004;59:782-787. [Cited in This Article: ] |
| 19. | Almeida N, Figueiredo P, Lopes S, Freire P, Lérias C, Gouveia H, Leitão MC. Urgent capsule endoscopy is useful in severe obscure-overt gastrointestinal bleeding. Dig Endosc. 2009;21:87-92. [Cited in This Article: ] |
| 20. | Lepileur L, Dray X, Antonietti M, Iwanicki-Caron I, Grigioni S, Chaput U, Di-Fiore A, Alhameedi R, Marteau P, Ducrotté P. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol. 2012;10:1376-1380. [Cited in This Article: ] |
| 21. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [Cited in This Article: ] |
| 22. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [Cited in This Article: ] |
| 23. | Li F, Gurudu SR, De Petris G, Sharma VK, Shiff AD, Heigh RI, Fleischer DE, Post J, Erickson P, Leighton JA. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc. 2008;68:174-180. [Cited in This Article: ] |
| 24. | Saurin JC, Delvaux M, Vahedi K, Gaudin JL, Villarejo J, Florent C, Gay G, Ponchon T. Clinical impact of capsule endoscopy compared to push enteroscopy: 1-year follow-up study. Endoscopy. 2005;37:318-323. [Cited in This Article: ] |
| 25. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [Cited in This Article: ] |
| 26. | Shahidi NC, Ou G, Svarta S, Law JK, Kwok R, Tong J, Lam EC, Enns R. Factors associated with positive findings from capsule endoscopy in patients with obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2012;10:1381-1385. [Cited in This Article: ] |
| 27. | Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008;68:1122-1127. [Cited in This Article: ] |
| 28. | Park JJ, Cheon JH, Kim HM, Park HS, Moon CM, Lee JH, Hong SP, Kim TI, Kim WH. Negative capsule endoscopy without subsequent enteroscopy does not predict lower long-term rebleeding rates in patients with obscure GI bleeding. Gastrointest Endosc. 2010;71:990-997. [Cited in This Article: ] |
| 29. | Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69:850-856. [Cited in This Article: ] |
| 30. | Bar-Meir S, Eliakim R, Nadler M, Barkay O, Fireman Z, Scapa E, Chowers Y, Bardan E. Second capsule endoscopy for patients with severe iron deficiency anemia. Gastrointest Endosc. 2004;60:711-713. [Cited in This Article: ] |