Published online Apr 16, 2024. doi: 10.4253/wjge.v16.i4.214
Peer-review started: December 25, 2023
First decision: January 23, 2024
Revised: February 4, 2024
Accepted: April 1, 2024
Article in press: April 1, 2024
Published online: April 16, 2024
Second-look endoscopy (SLE) to prevent recurrent bleeding in patients with peptic ulcer disease (PUD) and those undergoing endoscopic submucosal dissection (ESD) is routinely being performed. Conflicting evidence exists regarding efficacy, risk, benefit, and cost-effectiveness.
To identify the role and effectiveness of SLE in ESD and PUD, associated rebleeding and PUD-related outcomes like mortality, hospital length of stay, need for endoscopic or surgical intervention and blood transfusions.
A systematic review of literature databases PubMed, Cochrane, and Embase was conducted from inception to January 5, 2023. Randomized controlled trials that compared patients with SLE to those who did not have SLE or evaluated the role of prophylactic hemostasis during SLE compared to other conservative interventions were included. The study was conducted per PRISMA guidelines, and the protocol was registered in PROSPERO (ID CRD42023427555:). RevMan was used to perform meta-analysis, and Mantel-Haenszel Odds ratio (OR) were generated using random effect models.
A total of twelve studies with 2687 patients were included in our systematic review and meta-analysis, of which 1074 patients underwent SLE after ESD and 1613 patients underwent SLE after PUD-related bleeding. In ESD, the rates of rebleeding were 7% in the SLE group compared to 4.4% in the non-SLE group with OR 1.65, 95% confidence intervals (CI) of 0.96 to 2.85; P = 0.07, whereas it was 11% in the SLE group compared to 13% in the non-SLE group with OR 0.8 95%CI: 0.50 to 1.29; P = 0.36. The mean difference in the blood transfusion rates in the SLE and no SLE group in PUD was OR 0.01, 95%CI: -0.22 to 0.25; P = 0.91. In SLE vs non-SLE groups with PUD, the OR for Endoscopic intervention was 0.29, 95%CI: 0.08 to 1.00; P = 0.05 while it was OR 2.03, 95%CI: 0.95 to 4.33; P = 0.07, for surgical intervention. The mean difference in the hospital length of stay was -3.57 d between the SLE and no SLE groups in PUD with 95%CI: -7.84 to 0.69; P = 0.10, denoting an average of approximately 3 fewer days of hospital stay among patients with PUD who underwent SLE. For mortality between SLE and non-SLE groups in PUD, the OR was 0.88, 95%CI: 0.45 to 1.72; P = 0.70.
SLE does not confer any benefit in preventing ESD and PUD-associated rebleeding. SLE also does not provide any significant improvement in mortality, need for interventions, or blood transfusions in PUD patients. SLE decreases the hospital length of stay on average by 3.5 d in PUD patients.
Core Tip: Second-look endoscopy (SLE) has been a common practice to prevent recurrent bleeding in patients with peptic ulcer disease (PUD) and those undergoing endoscopic submucosal dissection (ESD). Current guidelines by American college of gastroenterology and American society of gastrointestinal endoscopy do not advocate routine SLE for nonvariceal upper gastrointestinal (GI) bleeding but recommend its consideration in cases of recurrent bleeding or higher recurrence risk. Conflicting evidence exists regarding the cost-effectiveness, efficacy, and potential risks of SLE in non-variceal upper GI bleeds. Second look endoscopy does not have any benefit in preventing ESD and PUD-associated rebleeding. SLE also does not have any significant improvement in mortality, need for interventions, or blood transfusions in PUD patients. SLE reduced the hospital length of stay on average by 3.5 d in PUD patients.
- Citation: Kogilathota Jagirdhar GS, Perez JA, Banga A, Qasba RK, Qasba RK, Pattnaik H, Hussain M, Bains Y, Surani S. Role of second look endoscopy in endoscopic submucosal dissection and peptic ulcer bleeding: Meta-analysis of randomized controlled trials. World J Gastrointest Endosc 2024; 16(4): 214-226
- URL: https://www.wjgnet.com/1948-5190/full/v16/i4/214.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i4.214
Peptic ulcer disease (PUD) and endoscopic submucosal dissection (ESD) are distinct clinical entities, yet they share a common concern-the management of gastrointestinal bleeding. PUD is a prevailing cause of acute upper gastrointestinal (GI) bleeding, entailing significant morbidity and mortality[1], while ESD is a well-established technique for the resection of gastric neoplasms[2]. Despite their differences, both clinical scenarios require careful consideration of the role of second-look endoscopy (SLE).
ESD, while effective in providing high en-bloc resection rates for gastric neoplasms, is associated with the concern of post-procedural bleeding, which can be life-threatening[3]. Efforts have been made to prevent such bleeding, including prophylactic coagulation during ESD[4]. SLE, often performed with or without prophylactic hemostasis, has been a common practice in many institutions. However, recent evidence, including a meta-analysis of randomized controlled trials (RCTs), has cast doubt on the efficacy of SLE in reducing the incidence of post-ESD bleeding[5]. The unpredictability of post-ESD bleeding sites and the limited applicability of prophylactic measures during SLE have further complicated its role.
On the other hand, for PUD, endoscopic treatment is effective in achieving initial hemostasis, but recurrent bleeding poses a substantial risk with potentially severe consequences[1,6,7]. The utility of planned SLE has been a topic of discussion, as it has shown promise in reducing the risk of recurrent bleeding in certain RCTs. However, conflicting results have also emerged, raising questions about the cost-effectiveness and potential risks associated with routine SLE[8].
Therefore, we conducted a systematic review and meta-analysis to assess the role of SLE in ESD and peptic ulcer bleeding to provide a comprehensive evaluation of the role of SLE in both settings by synthesizing evidence from RCTs and addressing the need for high-quality evidence to guide the further decision-making process.
We conducted this review following the PRISMA statement as indicated in the PRISMA checklist and registered our protocol with PROSPERO (CRD42023427555; www.crd.york.ac.uk/prospero).
A comprehensive literature search was performed in three databases, PubMed, Embase, and Cochrane, from inception until January 5, 2023. The search included keywords and subject-specific medical headings for SLE combined with gastrointestinal bleeding. We used vocabulary related to ('second look endoscopy' OR 'repeat endoscopy' OR 'prophylactic hemostasis') AND ('bleed' OR 'endoscopic submucosal dissection'/exp OR 'endoscopic submucosal dissection' OR 'ESD') AND (randomized OR randomized). Five authors were involved in the study selection process (Kogilathota Jagirdhar GS, Perez JA, Banga A, Qasba RK, Qasba RK). After removing duplicates using Endnote reference manager software, four authors independently performed title and abstract screening using the Rayyan software (https://rayyan.ai/)[9]. Studies that satisfied the inclusion criteria were retrieved and screened for full-text eligibility. Conflicts between authors on study selection were resolved through mutual discussion by an additional third arbiter if a consensus could not be reached. We have included studies that were: (1) Only RCTs; (2) patients who had initial endoscopy (EGD) for various reasons (peptic ulcer bleeding, submucosal dissection of polyps, dissection of tumor.), and (3) patients who had intervention such as SLE or prophylactic hemostasis during SLE. These studies compared patients who had SLE to those who did not have SLE, prophylactic hemostasis during SLE, or other conservative interventions.
We excluded the following studies: (1) Case reports; (2) case series; (3) literature reviews; (4) systematic reviews; (5) meta-analyses; (6) single arm studies; (7) non-randomized studies such as retrospective or prospective studies; (8) studies without SLE intervention groups; (9) animal studies; (10) unpublished studies; and (11) publications in a language other than English.
Three authors independently (Perez JA, Banga A, Qasba RK) extracted data including general information (Authors, DOI, Title, Journal, year of publication), Characteristics of studies and participants (site/ country, period of study, number of centers, study design, SLE/no SLE related numbers) and outcomes (SLE/no SLE Rebleeding number, types of treatment, Mean number of units blood transfused, type of intervention, need for surgery, all-cause mortality and hospital length of stay). All this data was transferred into a pre-piloted extraction form in Google Sheets. A Fourth author (GJ) checked the extracted data independently for validity.
Our outcomes were: (1) Recurrent bleeding; (2) all-cause mortality, (3) need for surgery; (4) mean number of units of blood transfused; and (5) mean number of hospital days.
We used RevMan 5.4.1 version, the Cochrane Collaboration, 2020, to assess all results[10], and Microsoft Excel to interpret and assess all results. After extracting raw data for events and non-events from each RCT, we calculated crude odds ratio (OR) using the Mantel-Haenszel method for each study with corresponding 95% confidence intervals (CI) using the random-effects model[11]. Differences were considered statistically significant at a P-value < 0.05. For continuous outcomes, a previously proven technique was used to convert the median to mean[12], and then estimates for mean differences were produced using the random effects model[11]. Further forest plots were generated to present the results of a meta-analysis. Cochrane Q and I2 statistics were used to measure heterogeneity and a low-level heterogeneity was defined as I2 of 20%[11]. The stability of the results was assessed using sensitivity analysis. Funnel plots were used to determine the likelihood of publication bias (Supplementary Figures 1-7)[13].
We used the Cochrane Risk of Bias Assessment Tool (ROB1) to assess the bias in included studies[14]. Two authors (Rakhtan KQ and Ruman KQ) conducted separate evaluations of the risk of bias for each included study. Any discrepancies were deliberated among all authors, and a unanimous decision was reached. The assessment was conducted in the following domains: Sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data risk of bias, selective reporting, and other sources of risk of bias. Each domain was categorized under high risk, low risk, and unclear risk of bias.
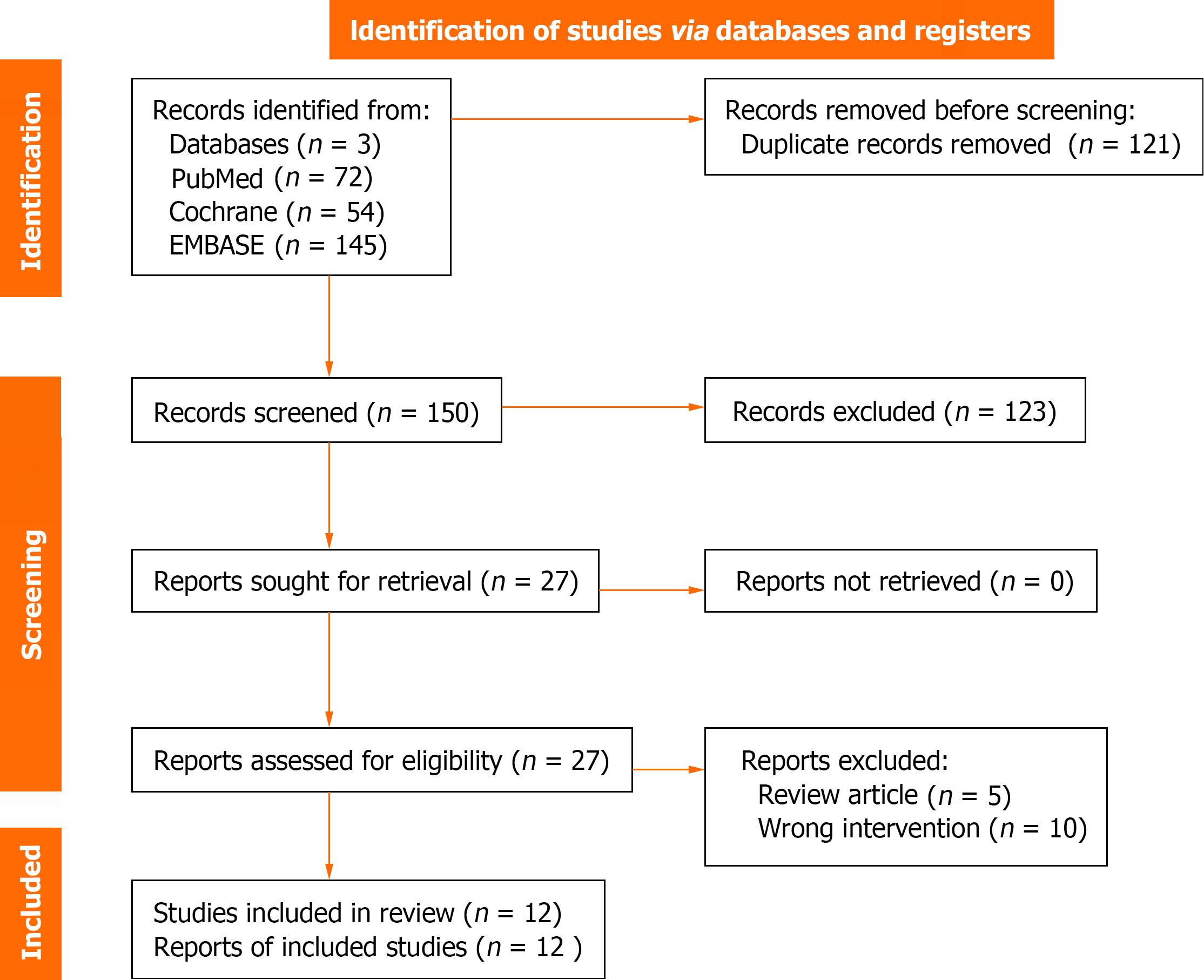
A total of 271 records were identified from the initial search; 121 were excluded as duplicates, and 150 articles were selected for the screening of title and abstract. Twenty-seven were chosen for full-text screening, and a total of 12 studies met the inclusion criteria and were included. These papers were eligible for qualitative and quantitative synthesis. Figure 1 shows the PRISMA diagram for the study selection process. We included studies that included patients who had initial endoscopy (EGD) for various reasons (PUD, submucosal dissection of polyps, dissection of tumors) followed by bleeding or complications post EGD and patients who had intervention such as SLE or prophylactic hemostasis during SLE.
A total of 2687 patients from twelve studies were included in the meta-analysis, of which 1074 patients from four studies belonged to the group of patients who underwent SLE[15-18] after ESD and 1361 patients from eight studies belonged to the group of patients who underwent SLE after PUD[19-27]. The studies observed outcomes of gastrointestinal bleeding in those with and without a SLE. The outcomes recorded were the number of events of gastrointestinal bleeding in the SLE and no-SLE groups, the timing of SLE, and risk factors for the occurrence of bleeding. The main characteristics of the included studies are summarized in Table 1.
| No. | Ref. | Country | Year of publication | Study design | Number of participants in SLE | Number of participants in No SLE | GIB symptoms in SLE total | GIB symptoms In No SLE total | Timing of SLE | Risk factors for the occurrence of post-procedural bleeding | |
| Endoscopic submucosal dissection for gastric neoplasm | |||||||||||
| 1 | Ryu et al[15] | Korea | 2013 | Prospective, randomized, controlled trial study | 74 | 81 | 15 | 11 | > 24 h | Longer procedure: (41.4 ± 28.2 min vs 32.1 ± 25.8 min; P < 0.048) | |
| 2 | Kim et al[16] | Korea | 2014 | Prospective, randomized, single-blind, controlled trial | 220 | 217 | 8 | 6 | > 48 h | Large tumor size > 20 mm | |
| 3 | Mochizuki et al[17] | Japan | 2015 | Multicenter prospective randomized controlled non-inferiority trial | 130 | 132 | 7 | 5 | > 24 h | Large tumor size > 40 mm | |
| 4 | Jee et al[18] | Korea | 2016 | Multicenter prospective randomized-controlled study | 110 | 110 | 7 | 2 | > 24 h | Ulcerative lesions finding | |
| Peptic ulcer bleeding | |||||||||||
| 1 | Chiu et al[19] | China | 2003 | Single center, prospective, randomized, controlled trial | 100 | 94 | 5 | 13 | 16-24 h | N/A | |
| 2 | Chiu et al[20] | China | 2016 | Single center, prospective, randomized, controlled trial | 152 | 153 | 12 | 10 | 16-24 h | Baylor bleeding score | |
| 3 | Park et al[21] | Japan | 2018 | Multicenter, prospective, randomized, controlled trial | 158 | 161 | 16 | 9 | 24 to 36 h | N/A | |
| 4 | Pittayanon et al[22] | Hong Kong | 2022 | Multicenter, prospective, randomized, controlled trial | 75 | 76 | 9 | 14 | 24 h | N/A | |
| 5 | Villanueva et al[23] | Spain | 1994 | Prospective, randomized, controlled trial | 52 | 52 | 11 | 15 | 24 h | N/A | |
| 6 | Messmann et al[24] | Germany | 1998 | Multicenter, prospective, randomized, controlled trial | 52 | 53 | 14 | 11 | 16-24 h | N/A | |
| 7 | Saeed et al[25] | United States | 1996 | Single-center, prospective, randomized, controlled trial | 19 | 21 | 0 | 5 | 24 h | Active bleeding, visible vessel, fresh adherent clot | |
| 8 | Lee[26] | - | 2005 | Randomized, controlled trial | 70 | 73 | 7 | 12 | - | NA | |
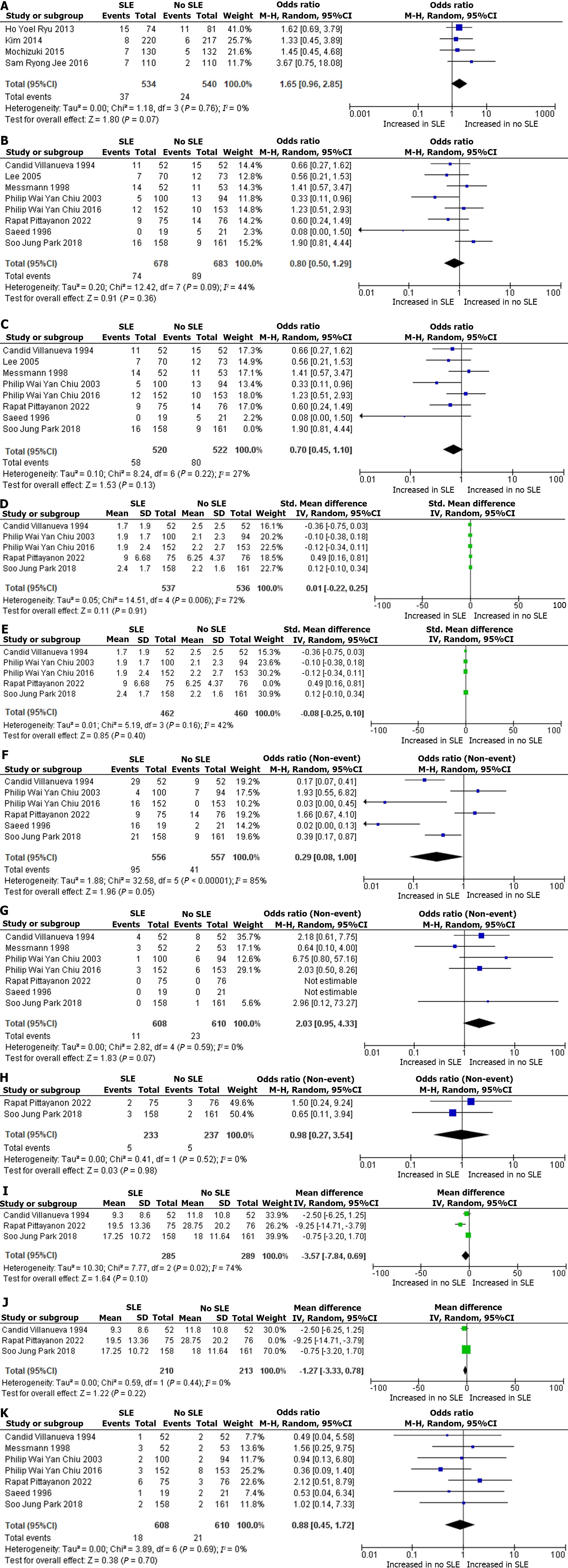
A total of 1074 patients from four RCTs were included in the qualitative analysis. The rates of rebleeding were 7% (SLE) 37/534 and 4.4% (no SLE) 24/540. The OR was 1.65 for ESD rebleeding with a 95%CI: 0.96 to 2.85; P = 0.07, I2 = 0%. Figure 2A shows the Forest plot and meta-analysis for ESD rebleeding. Risk factors for delayed post-ESD bleeding were Lesions with a large size > 20 mm, ulcerative lesions, and a longer procedure time.
A total of 1074 patients from four RCTs were included in the qualitative analysis, of which 534 patients belonged to the SLE group and 540 belonged to no SLE group. The number of patients who underwent interventions in the SLE group was 12% (69/534). Commonly performed interventions in ESD were prophylactic hemostasis using hemostatic clips, hemostatic forceps, Argon plasma coagulation, and endoscopic injection therapy. The number of patients who underwent interventions in the no SLE group was 0.3% (2/540). The intervention method was Hemostatic forceps and hemostatic clips, Argon plasma coagulation, and endoscopic injection with epinephrine.
A total of 1361 patients from eight RCTs were included in the qualitative analysis. The rates of rebleeding were 11% (SLE) 74/678 and 13% (no SLE) 89/683. The OR was 0.8 for PUD rebleeding with a 95%CI: 0.50 to 1.29; P = 0.36, I2 = 44%. Figure 2B shows the forest plot and meta-analysis for PUD rebleeding. Figure 2C shows the sensitivity analysis for PUD rebleeding. Risk factors for delayed post-PUD rebleeding were higher Baylor bleeding score, active bleeding before initial endoscopy, larger amounts of transfused blood, unsatisfactory initial endoscopic hemostasis, and use of nonsteroidal anti-inflammatory drugs (NSAIDs).
A total of 1073 patients from five RCTs were included in the qualitative analysis. 537 patients were in the SLE group and 536 patients in the no SLE group. A qualitative synthesis showed that the mean difference in blood transfusion rates in PUD was 0.01 between the SLE and no SLE group and a 95%CI: -0.22 to 0.25; P = 0.91, I2 = 72%. Figure 2D shows the forest Plot and meta-analysis for blood transfusion in PUD. Figure 2E shows sensitivity analysis for blood transfusion in PUD.
A total of 1113 patients from six RCTs were included in the qualitative analysis. A total of 556 patients were in the SLE group and 557 in the no SLE group. The number of patients who underwent SLE and required intervention in PUD was 17% (SLE) 95/556. The intervention number in patients with no SLE was 7% 41/557. The OR was 0.29 for Endoscopic intervention in PUD with a 95%CI: 0.08 to 1.00; P = 0.05, I2 = 85%. Figure 2F shows the first plot and meta-analysis for endoscopic intervention in PUD. Commonly performed interventions were hemoclip application or thermal (heat probe) coagulation, endo-clips ± 1:10000 epinephrine, fibrin glue injection therapy, hemospray, second emergency adrenaline injection, sequential injection of epinephrine (1:10000v/v) and up to 2 mL of fibrin/ thrombin.
A total of 1218 patients from seven RCTs were included in the qualitative analysis. A total of 608 patients were in the SLE group, and 610 patients were in the no SLE group. The number of patients that required surgical intervention after SLE was 2% (SLE) 11/608, and the number of patients who required surgical intervention without undergoing prior SLE was 4% (no SLE) 23/610. The OR was 2.03 for surgical intervention in PUD with a 95%CI: 0.95 to 4.33; P = 0.07, I2 = 0%. Figure 2G shows the forest plot and meta-analysis for surgical intervention in PUD.
In patients who underwent SLE and no SLE, the rates of angiographic embolization were similar, with 5 patients in each group. Figure 2H shows the forest Plot and meta-analysis for angiographic embolization in PUD.
A total of 574 patients from three RCTs were included in the qualitative analysis. A total of 285 patients were in the SLE group, and 289 patients were in the no SLE group. A qualitative synthesis showed that the mean difference in the hospital length of stay was -3.57 d between the SLE and no SLE groups and a 95%CI: -7.84 to 0.69; P = 0.10, I2 = 74%. Figure 2I shows the forest plot and meta-analysis for hospital length of stay in PUD. Figure 2J shows the sensitivity analysis for Hospital length of stay. This denotes an average of approximately 3 fewer d of hospital stay among patients with PUD (no-SLE).
A total of 1218 patients from seven RCTs were included in the qualitative analysis. A total of 608 were from the SLE group and 610 patients from the no SLE group. The number of patients that underwent mortality in SLE was 3% (SLE) 18/608, and the number of patients that underwent mortality without SLE was 3% (no SLE) 21/610. The OR was 0.88 for mortality in PUD with a 95%CI: 0.45 to 1.72; P = 0.70, I2 = 0%. Figure 2K shows the forest plot for mortality in PUD.
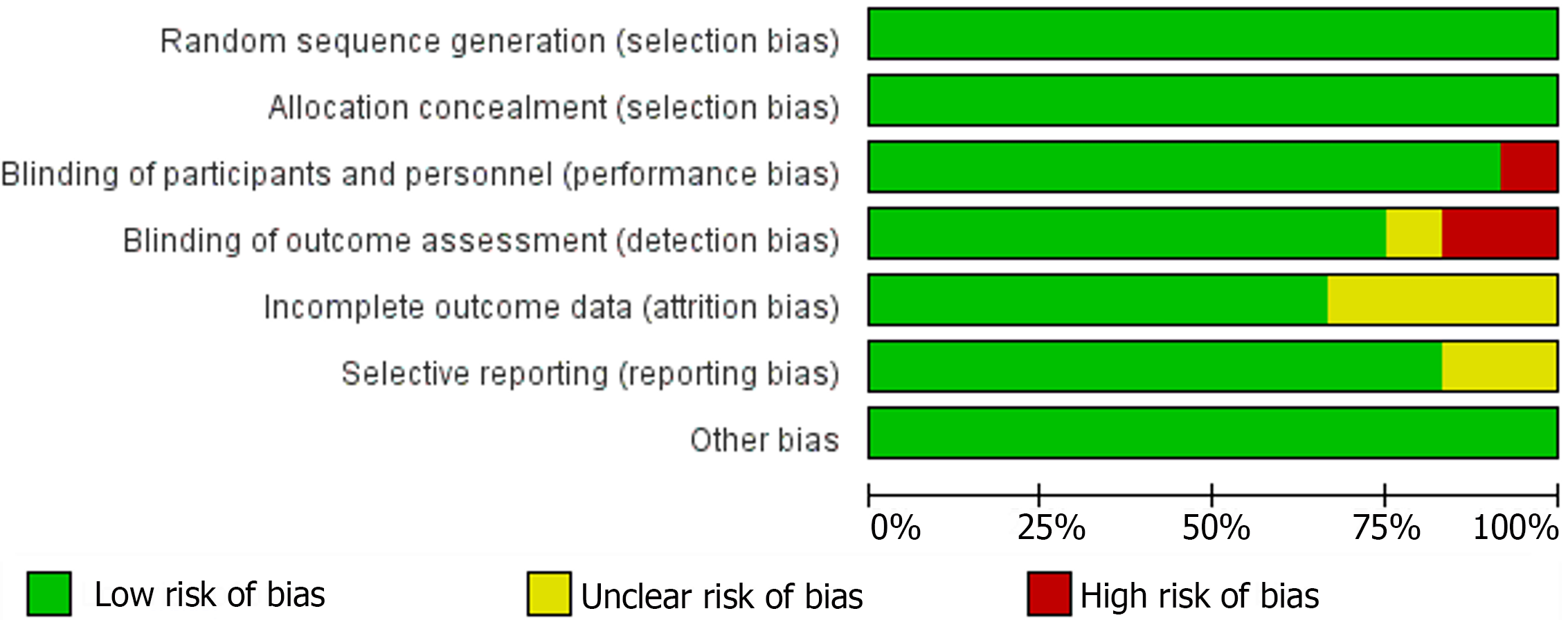
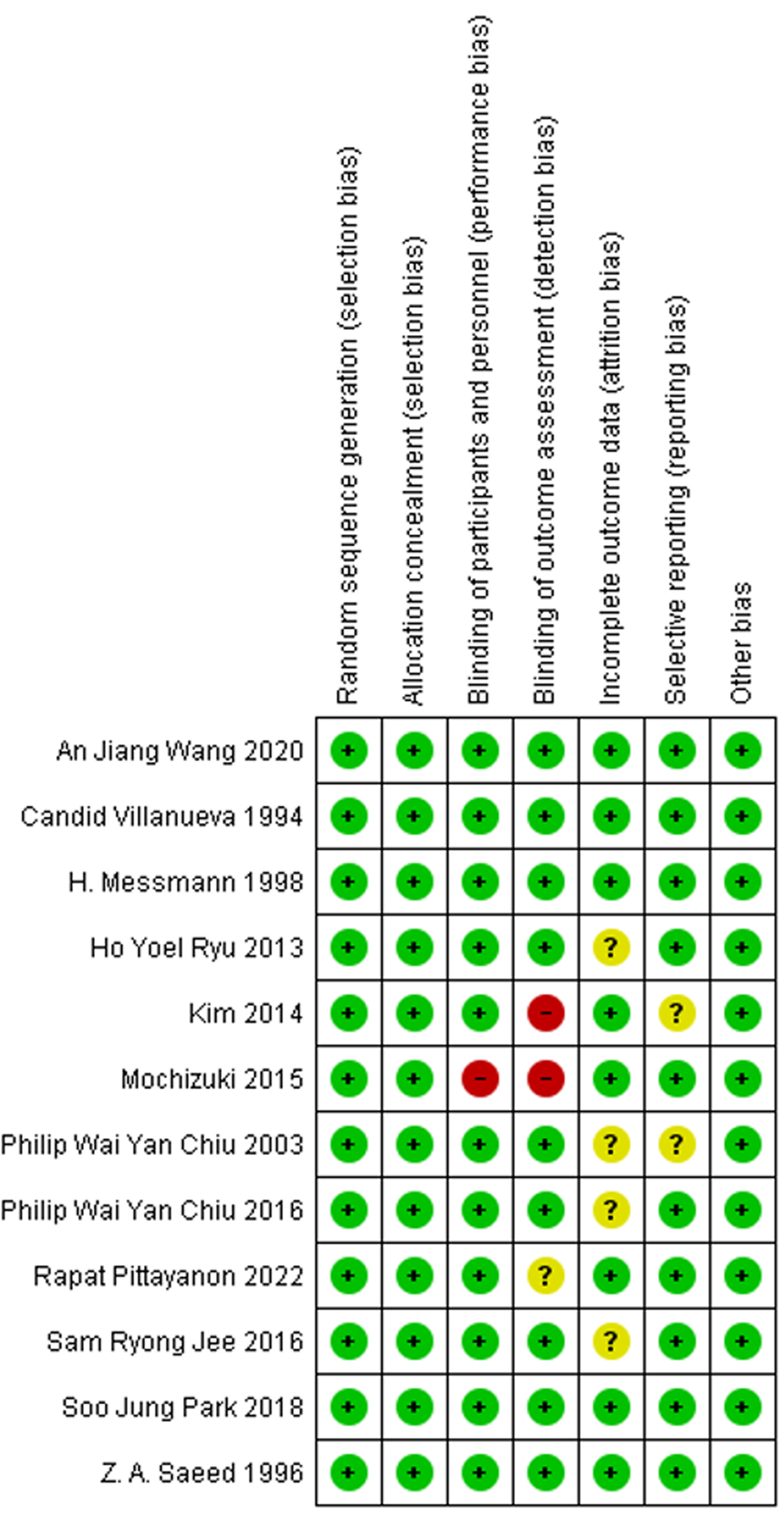
The Cochrane Collaboration tool was used to assess the methodological quality of all included studies, with the summarized outcomes detailed in Figures 3 and 4. All studies were randomized. All of the thirteen studies reported adequate sequence generation and concealment. Only Mochizuki et al[17] did not report blinding of participants and personnel. Additionally, Kim et al[16] and Mochizuki et al[17] did not report blinding of the outcome assessments. In eight of the studies, intent-to-treat analyses were done. Out of thirteen, only seven studies met all criteria for low risk of bias.
Our systematic review and meta-analysis of 12 RCTs, which included 1074 and 1361 patients with ESD and PUD, respectively, aimed to evaluate the role of SLE in preventing gastrointestinal rebleeding and improving outcomes such as mortality, hospital length of stay, need for surgical interventions and blood transfusions in patients who had undergone initial endoscopy.
Our findings suggest that SLE does not affect the rebleeding rate in upper GI bleeding due to ESD or PUD. Interestingly, there was an observed rise in rebleeding incidents in the SLE group compared to the non-SLE group among patients with PUD. However, the trend was the opposite in patients undergoing ESD although neither reached statistical significance. However, PUD patients who underwent SLE had a significantly higher likelihood of undergoing endoscopic interventions. Notably, PUD patients in the SLE group had lower rates of surgical intervention, but this did not reach statistical significance. Furthermore, in PUD patients, SLE also lacks a statistically significant impact on mortality, the requirement for blood transfusions, and angiographic embolization when compared to the non-SLE group. Nevertheless, individuals with PUD who underwent SLE experienced, on average, a reduction in hospital stay by three and a half days.
A 2017 meta-analysis by Kim et al[16] reported that SLE after ESD did not reduce the risk of post-ESD bleeding (pooled OR =1.27, 95%CI: 0.80 to 2.00). Patients who were found to be at high risk for post-ESD bleeding during SLE underwent prophylactic hemostasis. These patients ended up with high rates of delayed post-ESD bleeding compared to those who were not prophylactically treated [pooled OR = 3.40, 95%CI: 1.87 to 6.18]. This is an interesting observation, wherein being aggressive with early/prophylactic intervention led to higher rebleeding rates and, hence, worse outcomes. SLE encourages higher rates of interventions without improved outcomes which may not be in the best interest of patients. In our research, patients treated with SLE showed notably increased rates of endoscopic interventions, but these did not lead to improved outcomes such as mortality or decreased blood transfusion units.
In our study, SLE and non-SLE groups had no difference in the rebleeding rates after ESD. This is corroborated by a meta-analysis of risk factors for bleeding after gastric ESD by Libânio et al[28], which suggested that SLE was not associated with decreased post-procedural bleeding. Similarly, for PUD, SLE did not affect rebleeding, mortality, or the need for surgical intervention in our analysis, which is supported by previous studies[8,29]. However, SLE has been shown to reduce rebleeding if the risk of rebleeding is greater than or equal to 31%[8]. However, from a cost-effectiveness point of view, SLE in PUD patients who are not at an exceedingly high risk of bleeding is discouraged, especially in the current era of high-dose PPI[8,30,31].
More than half of bleeding episodes occur before SLE, and even prophylactic hemostasis on SLE was not capable of reducing bleeding[27]. Risk factors that contribute to delayed rebleeding like large size lesions, ulcerative lesions, and longer procedure time in the setting of ESD; higher bleeding score, active bleeding before initial endoscopy, a large amount of transfused blood, unsatisfactory initial endoscopic hemostasis, and use of NSAID’s in the setting of PUD. This evidence suggests that the creation of risk stratification models to assess post-procedural bleeding based on patient, procedure, and high-risk lesion needs to be researched and practiced. These models can allow a cost-effective strategy by categorizing patients so that SLE can be performed in high-risk categories only[27].
According to a meta-analysis by Kamal et al[29], which included 9 RCTs, there was no significant difference in recurrent bleeding, need for surgery, or mean units of blood transfused. In our study, the bleeding rates were higher in the no-SLE group, although this was not statistically significant. There was no statistical difference in the mean number of transfusions nor the need for surgical intervention. There was no difference in mortality rate in our study. Interestingly, our study showed a statistically decreased length of stay in patients with PUD who had SLE. From a cost-effectiveness perspective, this is interesting as hospital systems continue to improve and address strategies to decrease the cost of care for patients and healthcare entities.
Additional research is required to assess the actual efficacy of SLE in patients with PUD and to investigate the factors contributing to a reduced hospital length of stay without a concurrent decrease in adverse outcomes.
In the study by Kim et al[16], for every 25 patients who stay longer in the hospital after getting preventive treatment for post-ESD bleeding during a SLE, one patient promptly received treatment for delayed bleeding.
Based on the available literature, there are no established guidelines on whether a SLE is beneficial in upper GI bleeding due to non-variceal bleeding. Studies report inconclusive results regarding its benefits. In regard to the recommendations in the setting of non-variceal bleeding by the American College of Gastroenterology (ACG) and the European Society of Gastrointestinal Endoscopy (ESGE), they do not recommend a routine SLE in patients with non-variceal upper GI bleeding unless there is recurrent bleeding[32,33]. The recommendation from the ACG is that patients with recurrent bleeding after endoscopic therapy for a bleeding ulcer undergo repeat endoscopy and endoscopic therapy rather than surgery or transcatheter arterial embolization[33,34].
The current consensus on SLE is reflected by the guidelines laid down by the ACG and the ESGE[32-34], which do not recommend performing routine SLE in patients with nonvariceal-upper-GI-bleeding. However, they recommend using SLE in cases of recurrent bleeding or in those who demonstrate a higher risk of recurrence. ACG guidelines also advise caution in choosing the type of endoscopic therapy, particularly heated probes, during SLE due to the demonstrated higher risk of perforation[33]. These recommendations are further bolstered by the findings of the International Consensus Group[35,36]. The National Institute for Health and Care Excellence, United Kingdom guidelines recommend considering SLE in all patients with a high risk of re-bleeding with emphasis on those patients whose initial endoscopic therapy was found to be inadequate to achieve hemostasis[37]. This is supported by an Asia-Pacific working group that recommends SLE in patients at high risk for recurrent bleeding[38]. In summary, the general care practice is to avoid a repeat endoscopy, to avoid iatrogenic injury in patients as non-invasive modality such as high-dose proton pump therapy is considered first line.
Our meta-analysis followed PRISMA guidelines, and our study was duly registered in PROSPERO. All the studies included in our meta-analysis were prospective RCTs, thus offering the highest grade of evidence and lending high confidence and low risk of bias to their results and, by extension, to our findings.
No previous study has conducted such an extensive meta-analysis of twelve studies, which were all prospective RCTs evaluating both ESD and PUD. We also discussed in detail the risk factors for delayed post-ESD and PUD bleeding and provided a comprehensive view of associated clinical outcomes through forest plots.
The studies included in our analysis were majorly from Asia with two from Europe and one from North America. However, given that Asia has the highest age-standardized prevalence rate of PUD[39] more studies are expected from this region. Due to a limited number of studies from the initial pool, it might be underpowered to assess their summary statistics. We consider the results of our study to be generalizable globally as they reflect the global burden of the disease.
For individuals with ESD and PUD, considering patient factors such as comorbidities, prior use of anticoagulants and antiplatelets, clinical status, hemoglobin levels, and units of blood transfused can guide decision-making for SLE. This personalized and individualized approach to decision-making can enhance cost-effectiveness, prevent unnecessary procedures, and reduce procedural complications.
Future studies should focus on types of high-risk lesions predisposing to rebleeding and patient factors that influence worse outcomes. Larger and more robust RCTs are necessary to find the true relationship between SLE and patient outcomes. Our study suggests the importance of developing risk stratification models to evaluate the risk of post-procedural bleeding, considering patient characteristics, procedural factors, and high-risk lesions. Implementing such models could facilitate a cost-effective strategy by classifying patients and ensuring that SLE is conducted specifically in high-risk categories.
Second look, endoscopy seems to offer no advantage in the prevention of ESD and PUD-associated rebleeding. The decision to perform a SLE must be personalized and individualized, despite SLE decreasing the hospital length of stay on average by 3.5 d in PUD patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Critical Care Medicine.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bernardes A, Portugal S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Park CH, Shin S, Park JC, Shin SK, Lee SK, Lee YC, Lee H. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis. 2013;45:651-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Park CH, Lee SK. Preventing and controlling bleeding in gastric endoscopic submucosal dissection. Clin Endosc. 2013;46:456-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Guo Z, Miao L, Chen L, Hao H, Xin Y. Efficacy of second-look endoscopy in preventing delayed bleeding after endoscopic submucosal dissection of early gastric cancer. Exp Ther Med. 2018;16:3855-3862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Han YJ, Cha JM, Park JH, Jeon JW, Shin HP, Joo KR, Lee JI. Successful Endoscopic Hemostasis Is a Protective Factor for Rebleeding and Mortality in Patients with Nonvariceal Upper Gastrointestinal Bleeding. Dig Dis Sci. 2016;61:2011-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Hong MJ, Lee SY, Kim JH, Sung IK, Park HS, Shim CS, Jin CJ. Rebleeding after initial endoscopic hemostasis in peptic ulcer disease. J Korean Med Sci. 2014;29:1411-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Imperiale TF, Kong N. Second-look endoscopy for bleeding peptic ulcer disease: a decision-effectiveness and cost-effectiveness analysis. J Clin Gastroenterol. 2012;46:e71-e75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5711] [Cited by in F6Publishing: 7770] [Article Influence: 971.3] [Reference Citation Analysis (0)] |
| 10. | Shah A, Usman O, Zahra T, Chaudhari SS, Mulaka GSR, Masood R, Batool S, Saleem F. Efficacy and Safety of Potassium-Competitive Acid Blockers Versus Proton Pump Inhibitors as Helicobacter pylori Eradication Therapy: A Meta-Analysis of Randomized Clinical Trials. Cureus. 2023;15:e48465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 11. | Deeks JJ, Higgins JPT, Altman DG; Group on behalf of the CSM. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed. 2022. [DOI] [Cited in This Article: ] |
| 12. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3433] [Cited by in F6Publishing: 5211] [Article Influence: 521.1] [Reference Citation Analysis (0)] |
| 13. | Boutron I PM, Higgins JP, Altman DG, Lundh A, Hróbjartsson A; Group on behalf of the CBM. Considering bias and conflicts of interest among the included studies. Cochrane Handbook for Systematic Reviews of Interventions version 63 Cochrane. 2022. [DOI] [Cited in This Article: ] |
| 14. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18487] [Cited by in F6Publishing: 21361] [Article Influence: 1643.2] [Reference Citation Analysis (2)] |
| 15. | Ryu HY, Kim JW, Kim HS, Park HJ, Jeon HK, Park SY, Kim BR, Lang CC, Won SH. Second-look endoscopy is not associated with better clinical outcomes after gastric endoscopic submucosal dissection: a prospective, randomized, clinical trial analyzed on an as-treated basis. Gastrointest Endosc. 2013;78:285-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Kim JS, Chung MW, Chung CY, Park HC, Ryang DY, Myung DS, Cho SB, Lee WS, Joo YE. The need for second-look endoscopy to prevent delayed bleeding after endoscopic submucosal dissection for gastric neoplasms: a prospective randomized trial. Gut Liver. 2014;8:480-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Mochizuki S, Uedo N, Oda I, Kaneko K, Yamamoto Y, Yamashina T, Suzuki H, Kodashima S, Yano T, Yamamichi N, Goto O, Shimamoto T, Fujishiro M, Koike K; SAFE Trial Study Group. Scheduled second-look endoscopy is not recommended after endoscopic submucosal dissection for gastric neoplasms (the SAFE trial): a multicentre prospective randomised controlled non-inferiority trial. Gut. 2015;64:397-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Jee SR, Park MI, Lim SK, Kim SE, Ku KH, Hwang JW, Lee SH, Kim JH, Seol SY, Um SH. Clinical impact of second-look endoscopy after endoscopic submucosal dissection of gastric neoplasm: a multicenter prospective randomized-controlled trial. Eur J Gastroenterol Hepatol. 2016;28:546-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Chiu PW, Lam CY, Lee SW, Kwong KH, Lam SH, Lee DT, Kwok SP. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003;52:1403-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Chiu PW, Joeng HK, Choi CL, Tsoi KK, Kwong KH, Lam SH, Sung JJ. High-dose omeprazole infusion compared with scheduled second-look endoscopy for prevention of peptic ulcer rebleeding: a randomized controlled trial. Endoscopy. 2016;48:717-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Park SJ, Park H, Lee YC, Choi CH, Jeon TJ, Park JC, Kim JH, Youn YH, Kim YJ, Lee KJ, Lim SG, Kim H, Bang BW. Effect of scheduled second-look endoscopy on peptic ulcer bleeding: a prospective randomized multicenter trial. Gastrointest Endosc. 2018;87:457-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Pittayanon R, Suen BY, Kongtub N, Tse YK, Rerknimitr R, Lau JYW. Scheduled second look endoscopy after endoscopic hemostasis to patients with high risk bleeding peptic ulcers: a Randomized Controlled Trial. Surg Endosc. 2022;36:6497-6506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Villanueva C, Balanzó J, Torras X, Soriano G, Sáinz S, Vilardell F. Value of second-look endoscopy after injection therapy for bleeding peptic ulcer: a prospective and randomized trial. Gastrointest Endosc. 1994;40:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Messmann H, Schaller P, Andus T, Lock G, Vogt W, Gross V, Zirngibl H, Wiedmann KH, Lingenfelser T, Bauch K, Leser HG, Schölmerich J, Holstege A. Effect of programmed endoscopic follow-up examinations on the rebleeding rate of gastric or duodenal peptic ulcers treated by injection therapy: a prospective, randomized controlled trial. Endoscopy. 1998;30:583-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Saeed ZA, Cole RA, Ramirez FC, Schneider FE, Hepps KS, Graham DY. Endoscopic retreatment after successful initial hemostasis prevents ulcer rebleeding: a prospective randomized trial. Endoscopy. 1996;28:288-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Lee S. The effect of second look endoscopy in patients with bleeding peptic ulcers. Gastroenterology. 2005;128:A639. [DOI] [Cited in This Article: ] |
| 27. | Kim EH, Park SW, Nam E, Eun CS, Han DS, Park CH. Role of second-look endoscopy and prophylactic hemostasis after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:756-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc. 2016;84:572-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Kamal F, Khan MA, Lee-Smith W, Sharma S, Imam Z, Henry C, Jowhar D, Khan Z, Petryna E, Iqbal U, Tombazzi C, Ismail MK, Howden CW. Role of routine second-look endoscopy in patients with acute peptic ulcer bleeding: meta-analysis of randomized controlled trials. Gastrointest Endosc. 2021;93:1228-1237.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | El Ouali S, Barkun AN, Wyse J, Romagnuolo J, Sung JJ, Gralnek IM, Bardou M, Martel M. Is routine second-look endoscopy effective after endoscopic hemostasis in acute peptic ulcer bleeding? A meta-analysis. Gastrointest Endosc. 2012;76:283-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Spiegel BM, Ofman JJ, Woods K, Vakil NB. Minimizing recurrent peptic ulcer hemorrhage after endoscopic hemostasis: the cost-effectiveness of competing strategies. Am J Gastroenterol. 2003;98:86-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 445] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 33. | Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am J Gastroenterol. 2021;116:899-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 150] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 34. | Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345-60; quiz 361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 449] [Article Influence: 37.4] [Reference Citation Analysis (1)] |
| 35. | Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F, Leontiadis GI, Abraham NS, Calvet X, Chan FKL, Douketis J, Enns R, Gralnek IM, Jairath V, Jensen D, Lau J, Lip GYH, Loffroy R, Maluf-Filho F, Meltzer AC, Reddy N, Saltzman JR, Marshall JK, Bardou M. Management of Nonvariceal Upper Gastrointestinal Bleeding: Guideline Recommendations From the International Consensus Group. Ann Intern Med. 2019;171:805-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 36. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P; International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 682] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 37. | Dworzynski K, Pollit V, Kelsey A, Higgins B, Palmer K; Guideline Development Group. Management of acute upper gastrointestinal bleeding: summary of NICE guidance. BMJ. 2012;344:e3412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Sung JJ, Chiu PW, Chan FKL, Lau JY, Goh KL, Ho LH, Jung HY, Sollano JD, Gotoda T, Reddy N, Singh R, Sugano K, Wu KC, Wu CY, Bjorkman DJ, Jensen DM, Kuipers EJ, Lanas A. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67:1757-1768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 39. | Xie X, Ren K, Zhou Z, Dang C, Zhang H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol. 2022;22:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |