Published online Oct 16, 2023. doi: 10.4253/wjge.v15.i10.614
Peer-review started: June 19, 2023
First decision: August 11, 2023
Revised: August 26, 2023
Accepted: September 14, 2023
Article in press: September 14, 2023
Published online: October 16, 2023
Asparaginase (ASP) is an important drug in combined chemotherapy regimens for pediatric acute lymphoblastic leukemia (ALL); ASP-associated pancreatitis (AAP) is the main adverse reaction of ASP. Recurrent pancreatitis is a compli
To evaluate the efficacy and safety of endoscopic retrograde cholangiopancreatography (ERCP) in treating recurrent pancreatitis due to AAP.
From May 2018 to August 2021, ten children (five males and five females; age range: 4–13 years) with AAP were treated using ERCP due to recurrent pancreatitis. Clinical data of the ten children were collected, including their sex, age, weight, ALL risk grading, clinical symptoms at the onset of pancreatitis, time from the first pancreatitis onset to ERCP, ERCP operation status, and posto
The preoperative symptoms were abdominal pain, vomiting, inability to eat, weight loss of 2–7 kg, and 2–9 pancreatitis onsets. After the operation, nine of ten patients did not develop pancreatitis, had no abdominal pain, could eat normally; the remaining patient developed three pancreatitis onsets due to the continuous administration of ASP, but eating was not affected. The postoperative weight gain was 1.5–8 kg. There was one case of post ERCP pancreatitis and two cases of postoperative infections; all recovered after medication.
ERCP improved clinical symptoms and reduced the incidence of pancreatitis, and was shown to be a safe and effective method for improving the management of recurrent pancreatitis due to AAP.
Core Tip: Recurrent pancreatitis is a complication of asparaginase-associated pancreatitis (AAP), and medications do not prevent recurrence. This study was conducted to evaluate the efficacy and safety of endoscopic retrograde cholangiopancreatography (ERCP) in treating recurrent pancreatitis due to AAP. Our research found that ERCP improved clinical symptoms and reduced the incidence of pancreatitis, and was shown to be a safe and effective method for improving the management of recurrent pancreatitis due to AAP.
- Citation: Yang KH, Zeng JQ, Ding S, Zhang TA, Wang WY, Zhang JY, Wang L, Xiao J, Gong B, Deng ZH. Efficacy and safety of endoscopic retrograde cholangiopancreatography in recurrent pancreatitis of pediatric asparaginase-associated pancreatitis. World J Gastrointest Endosc 2023; 15(10): 614-622
- URL: https://www.wjgnet.com/1948-5190/full/v15/i10/614.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i10.614
Acute lymphoblastic leukemia (ALL) is a malignant tumor with a high incidence in children. One of the key drugs in the combined chemotherapy regimen to treat ALL is asparaginase (ASP). ASP is important for inducing remission and achieving long-term disease-free survival, but it has a high probability of inducing ASP-associated pancreatitis (AAP). The overall incidence of AAP is 2%–18%, with 7%–66% of cases classified as severe; the mortality rate due to AAP is as high as 2%[1-5]. Asparaginase-induced pancreatitis is often seen as acute toxicity with lasting issues such as recurrent pancreatitis, causing patients to be at risk for progressing to chronic pancreatitis over time. This may affect children's eating, weight, and quality of life[6-11]. Recurrent pancreatitis is the main complication of AAP, wherein children are unable to eat normally and lose weight, thus seriously affecting their quality of life[6-11]. If pancreatitis repeatedly recurs in AAP, the effects of medications such as somatostatin and its analog octreotide are not efficacious for preventing recurrent pancreatitis, which is a difficulty in clinical treatment.
Endoscopic retrograde cholangiopancreatography (ERCP) is a minimally invasive method for diagnosing and treating recurrent pancreatitis, and its efficacy and safety have been confirmed[12-17]. As patients with ALL are special, research has seldom focused on procedures for the complication of ASP. This study aimed to evaluate the efficacy and safety of ERCP in treating recurrent pancreatitis due to AAP.
This study was approved by the Institutional Review Board of Shanghai Children’s Medical Center (SCMCIRB-K2019005). All the participants’ legal guardians provided written informed consent. From May 2018 to August 2021, ten children with AAP underwent ERCP due to recurrent pancreatitis that persisted for 3 mo and for which medication was ineffective. Their sex, age, ALL risk grading, clinical symptoms at the onset of pancreatitis, time from the first pancreatitis onset to ERCP, weight, ERCP operation status, and postoperative complications of ERCP were summarized. The status of symptomatic relief, number of pancreatitis onsets, and weight change before and after ERCP operation were compared. For all ten children, smoking, alcohol consumption, biliary pancreatitis, hyperlipidemia, hypercalcemia, tumor invasion, trauma, and autoimmune diseases were excluded.
AAP was defined as acute pancreatitis after using ASP, and its diagnosis was based on a combination of clinical, biochemical (amylase, lipase), and imaging evidence. According to the diagnostic criteria put forward by the Toxicity Working Group established by the Ponte di Legno consortium in 2016, cases that meet two or more of the following criteria can be diagnosed as having AAP[18,19]: (1) Acute pancreatitis-related abdominal pain; (2) blood amylase or blood lipase exceeding three times the upper limit of normal; and (3) imaging examination findings (ultrasonography, computed tomography, or magnetic resonance imaging) consistent with pancreatitis.
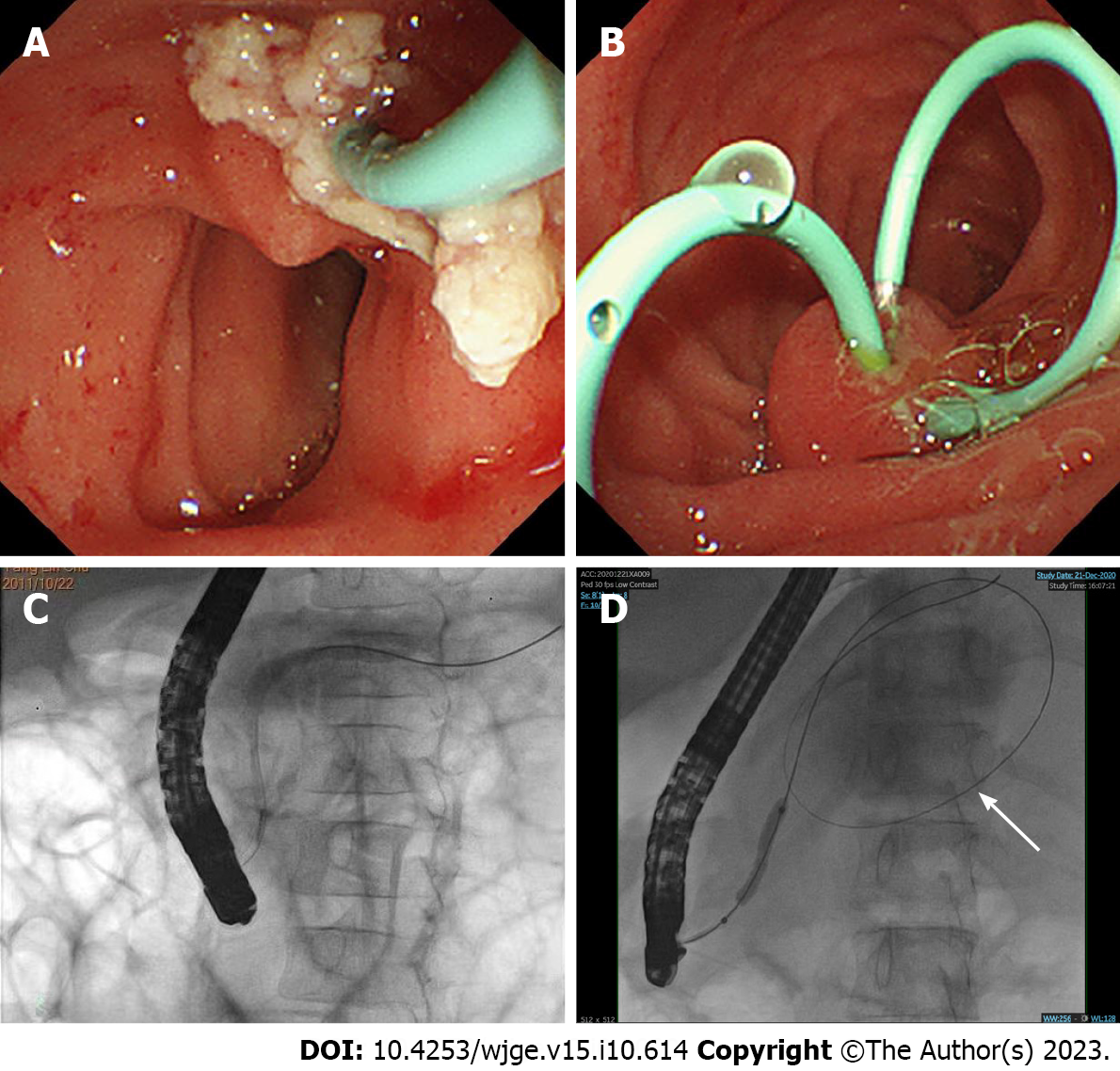
Prior to ERCP, each child’s guardian signed a written informed consent for the procedure. All procedures were performed by the same experienced endoscopist, who had, incidentally, performed more than 30000 ERCPs. The children underwent ERCP in the prone position under general anesthesia using a standard pediatric duodenoscope (JF-240, Olympus, Tokyo, Japan), and vital signs were continuously monitored. Therapeutic maneuvers were selected during the operation according to the pancreatic imaging results, including endoscopic pancreatography sphincterotomy, placement of pancreatic duct stent, balloon dilation, and stone extraction. Figure 1A and B show the most common ERCP procedures of stone extraction and placement of pancreatic duct stent. The fluoroscopic view of the pancreatic duct is shown in Figure 1C. A fluoroscopic view of the pancreatic duct stricture dilation performed by balloon manipulation and huge pseudocyst is shown in Figure 1D. Post-ERCP complications were assessed by monitoring the child’s blood amylase and lipase levels, using pancreatic ultrasound images, and assessing postoperative abdominal pain, fever, and bleeding 24 h after ERCP. Post-ERCP complications were treated using the conventional treatment in internal medicine, and further evaluation and treatment were required for children with severe disease. The discharge criteria were absence of fever and abdominal pain along with a return of blood amylase levels to normal. Patients who did not meet the discharge criteria were evaluated for treatment.
Follow-up time: All patients were followed up for an average of 1.2 years, the shortest follow-up time was 1 year and the longest was 1.5 years.
Observation indicators: The primary observation indicators, including the number of acute pancreatitis onsets, symptoms related to acute pancreatitis such as abdominal pain, vomiting, normal eating habits, and body weight were evaluated every 3 mo. Those with pancreatic pseudocyst underwent reexaminations by B-ultrasonography.
The secondary observations were post-ERCP complications[20]. Post-ERCP pancreatitis (PEP), bleeding, infection, and perforation are the most common complications. PEP was defined as new or worsening abdominal pain with elevated blood amylase three times above the normal value and lasting longer than 24 h. Bleeding was defined as bleeding foci or persistent oozing of blood that was visible to the naked eye during the operation and vomiting of blood, blood in stool, or black stool with a progressive decrease in hemoglobin after the operation. Infection was defined as a postoperative temperature greater than 38°C and lasting for more than 24 h. Perforation was characterized by sudden abdominal pain and signs related to peritonitis during or after the operation. This included signs such as subdiaphragmatic free gas and retroperitoneal gas on imaging.
Among the ten children, there were five males and five females (age range: 4–13 years). There were nine cases at moderate risk and one case at low risk. All ten children showed recurrent abdominal pain and vomiting and were unable to eat. Among them, seven were fed through nasal jejunal tubes. The number of pancreatitis onsets before the operation was equal to or greater than two times. The weight loss before the operation was 2–7 kg. The time from the first AAP onset to ERCP was 3–8 mo (Table 1).
| Case | Sex | Age (yr) | Risk grading | Interval between first AAP onset and ERCP (mo) | Clinical features | Nasal jejunal tube | Number of AAP onsets (times) | Weight loss (kg) |
| 1 | Female | 4 | Medium | 4 | Abdominal pain, vomiting, inability to eat | Yes | 6 | 2 |
| 2 | Male | 10 | Medium | 3 | Abdominal pain, vomiting, inability to eat | Yes | 4 | 6 |
| 3 | Male | 12 | Medium | 3 | Abdominal pain, vomiting, inability to eat | Yes | 7 | 7 |
| 4 | Male | 6 | Low | 6 | Abdominal pain, vomiting, inability to eat | Yes | 9 | 4 |
| 5 | Female | 13 | Medium | 3 | Abdominal pain, vomiting, inability to eat | Yes | 5 | 2 |
| 6 | Male | 4 | Medium | 3 | Abdominal pain, vomiting, inability to eat | No | 5 | 3 |
| 7 | Female | 4 | Medium | 6 | Abdominal pain, vomiting, inability to eat | Yes | 9 | 3 |
| 8 | Male | 7 | Medium | 3 | Abdominal pain, inability to eat | Yes | 2 | 7 |
| 9 | Female | 7 | Medium | 8 | Abdominal pain, vomiting, inability to eat | No | 9 | 3 |
| 10 | Female | 4 | Medium | 4 | Abdominal pain, vomiting, inability to eat | No | 5 | 3 |
There were five cases of pseudocyst (5/10, 50%), six cases of pancreatic duct stones (6/10, 60%), and four cases of pancreatic duct stenosis (4/10, 40%). A total of seven cases (7/10, 70%) underwent pancreatic duct stent implantation; eight cases (8/10, 80%) underwent sphincterotomy; and five cases (5/10, 50%) had balloon manipulation. In total, two patients underwent ERCP operations twice for stent removal, while the remaining eight patients underwent only one operation (Table 2).
| Case | Presence of pseudocyst and its size (mm) | Pancreatic duct stenosis | Pancreatic duct stones | ERCP operation mode | ERCP operation times |
| 1 | 40 × 15 | No | Yes | EPS+ERPD | 2 |
| 2 | / | No | Yes | EPS+balloon manipulation | 1 |
| 3 | 81 × 41 | Yes | No | Balloon manipulation +ERPD | 1 |
| 4 | / | Yes | Yes | EPS+ERPD | 2 |
| 5 | 68 × 40 | Yes | No | Balloon manipulation+EPS+ERPD | 1 |
| 6 | 16 × 12 | No | No | EST | 1 |
| 7 | / | No | Yes | Balloon manipulation+ERPD | 1 |
| 8 | 48 × 40 | Yes | No | Balloon manipulation+EPS+ERPD | 1 |
| 9 | / | No | Yes | EPS | 1 |
| 10 | / | No | Yes | EPS+ERPD | 1 |
Among the five cases of pseudocyst, the cyst disappeared in four cases; no changes were observed in the cyst in one case even following the operation, but no pancreatitis occurred. After ERCP was performed, one patient developed three pancreatitis onsets due to continuous administration of ASP, but eating was not affected. The remaining nine patients did not develop pancreatitis after the operation and could eat normally. Weights increased by 1.5–8 kg across all patients.
After ERCP, one case developed mild pancreatitis, which recovered after medication. Moreover, two children developed postoperative infections but recovered after receiving anti-infective treatment (Table 3).
| Case | Ultrasonography for pseudocyst | After ERCP acute pancreatitis onsets (times) | Clinical symptoms | Postoperative complications | Weight gain/yr (kg) |
| 1 | At 6 mo after the operation, no pseudocyst was found by ultrasonography | 0 | Abdominal pain disappeared, able to eat | No | 5 |
| 2 | / | 0 | Abdominal pain disappeared, able to eat | Postoperative pancreatitis | 5 |
| 3 | At 3 mo after the operation, no pseudocyst was found by ultrasonography | 0 | Abdominal pain disappeared, able to eat | Infection | 8 |
| 4 | / | 0 | Abdominal pain disappeared, able to eat | Infection | 7 |
| 5 | At 4 mo after the operation, ultrasonography revealed that the pseudocyst had disappeared | 0 | Abdominal pain disappeared, able to eat | No | 3 |
| 6 | There was still a 16 mm × 12 mm pseudocyst | 0 | Abdominal pain disappeared, able to eat | No | 7 |
| 7 | / | 0 | Abdominal pain disappeared, able to eat | No | 1.5 |
| 8 | At 2 mo after the operation, no pseudocyst was found by ultrasonography | 0 | Abdominal pain disappeared, able to eat | No | 2 |
| 9 | / | 3 | Abdominal pain, but did not affect eating | No | 2 |
| 10 | / | 0 | Abdominal pain disappeared, able to eat | No | 3 |
AAP is a serious adverse reaction to ASP and is the most common reason for interruption of ASP treatment[21-23]. AAP is defined as an acute inflammatory process within the pancreatic parenchyma following ASP treatment. The diagnosis is based on the presence of at least two of the following three criteria: Abdominal pain suggestive of pancreatitis, serum amylase or lipase three or more times the upper-normal level, and characteristic imaging findings suggestive of pancreatitis. The underlying pathophysiology is not fully understood, but is thought to involve the reduction of protein synthesis, especially in organs with high protein turnover, such as the liver and pancreas, and results from systemic depletion of asparagine[19,24]. The Ponte di Legno consortium classified AAP into three grades according to the degree of pancreatic injury[19]. On the basis of severe pancreatitis, repeated pancreatitis is a high-risk factor for chronic pancreatitis. In addition, recurrent onsets of pancreatitis lead to children’s inability to eat normally during chemotherapy, causing inadequate nutrition. Combined with the impact of chemotherapy drugs, this results in weight loss and insufficient immunity, seriously impacting children’s quality of life.
In our study, ten children with AAP experienced recurrent pancreatitis presenting as abdominal pain, vomiting, inability to eat normally, and weight loss. Although seven patients had been placed on nasal jejunal tube feeding, their weight was still below the ideal due to recurrent pancreatitis. Therefore, once recurrent pancreatitis appears in AAP, intervention is necessary as early as possible to improve the quality of life of children and prevent chronic pancreatitis in the long term. However, most human clinical studies have focused on how to treat acute pancreatitis rather than relapsing pancreatitis.
ERCP is an endoscopic technique that combines gastrointestinal endoscopy and fluoroscopy for the diagnosis and treatment of pancreatic and biliary diseases. The application of ERCP in children has been increasing in recent years and its efficacy and safety have been demonstrated in operations such as sphincterotomy, papillary dilation, and pancreatic duct stenting. ERCP can safely be performed in children with a pooled complication rate of approximately 1.2-10.9%, paralleling that observed with adults[25,26].
Recurrent pancreatitis is a therapeutic indication for ERCP. Our study was comprised of ten children with recurrent pancreatitis related to ASP. Five had pancreatic pseudocyst, four had pancreatic duct stenosis, and six had pancreatic duct stones, suggesting that pseudocyst and pancreatic duct lesions were the root causes of recurrent pancreatitis and might even be the pathological basis of chronic pancreatitis. For pseudocyst and pancreatic duct lesions, medication is ineffective. ERCP is a minimally invasive treatment for biliary and pancreatic diseases in children, and symptomatic pancreatic pseudocyst and pancreatic duct diseases (stenosis and stones) are indications for ERCP[27-29]. Through pancreatic duct stent implantation, dilatation, and sphincterotomy, four cases of pseudocyst were resolved. Pseudocyst remained in one case, but no pancreatitis occurred; therefore, an observation strategy was maintained for natural absorption. A total of nine cases did not develop pancreatitis after the operation and the patients could, therefore, eat normally. Pancreatitis was observed in one case three times due to continuous use of ASP; however, the abdominal pain was mild and did not affect eating. The patient recovered after medication. All ten children gained weight after the operation. Two of the children developed an infection but recovered after anti-infective treatment. This supports ERCP as an effective and safe intervention for AAP with recurrent pancreatitis.
Pancreatic duct stones (PDS) are stones formed in the main pancreatic duct and pancreatic duct branches. They are a manifestation of protein embolism or mineralization (caused by calcium carbonate or protein in the pancreas precipitating in the pancreatic ducts) and are characteristic pathological changes of chronic pancreatitis. PDS and pancreatitis are mutually causal. Recurrent pancreatitis leads to increased secretion of pancreatic juice and the activation and concentration of a large amount of trypsinogen in the pancreatic ducts, leading to the formation of pancreatic stones. PDS block the pancreatic ducts, causing stenosis or dilation of the pancreatic ducts and subsequent recurrent pancreatitis[30]. In this study, six children were found to have PDS during the ERCP operation, and the cause of their formation might be related to the recurrence of pancreatitis after using ASP.
Approximately 23% of AAP episodes resulted in pancreatic pseudocyst, a markedly higher proportion than reported in a case series of pancreatitis due to all etiologies, which ranged from 2% to 16%[31]. Pancreatic pseudocyst is a common complication of pancreatitis that can manifest with abdominal symptoms of pain, nausea, and vomiting; it can also present without any clinical symptoms. Approximately 20%–60% of pancreatic pseudocysts naturally resolve within 6–12 wk[32,33]; therefore, ERCP is not the first treatment choice. When pancreatic pseudocysts are symptomatic, endoscopic intervention should be the therapy of first choice. In this study, the five cases of pseudocysts presented with recurrent pancreatitis. We observed these for at least 3 mo during which time the pseudocysts were still not absorbed. Furthermore, the children lost weight while waiting for the pseudocysts to be naturally absorbed. Therefore, once pancreatic pseudocysts with recurrent pancreatitis are present, we recommend ERCP treatment as early as possible.
In one case (case 9), where AAP recurred even after the ASP dosage was reduced by half, ERCP operation was performed to enable subsequent ASP treatment. After the operation, the child continued to receive three injections of ASP. Although there were still onsets of pancreatitis, compared with that before the operation, the number of onsets was reduced, degree of abdominal pain was alleviated, and eating was not affected. We believe that the implantation of a pancreatic duct stent is the key to ensuring successful chemotherapy with ASP. Meta-analysis data of many clinical reports show that stent placement could effectively relieve the symptoms of abdominal pain, with immediate relief rates of 65%–95% and sustained relief rates of 32%–68%[34]. Moreover, pancreatic duct stents could prevent post-ERCP pancreatitis in high-risk patients. Therefore, in the majority of patients (seven out of ten) in the study, a pancreatic duct stent was implanted, effectively relieving abdominal pain and, in the ninth case, protecting the pancreas from the damage caused by repeated ASP use. Repeated use of ASP in severe AAP is not typically recommended because up to 63% of children had a second recurrence of pancreatitis[5]. Based on our experience of using ERCP treatment in the ninth case, we believe that ERCP can be attempted in children with AAP prior to resuming the use of ASP to ensure that patients can complete their ASP treatment course, thereby improving the event-free survival rate in cases of ALL where children’s ASP therapy is interrupted. To our knowledge, the efficacy and safety of ERCP in recurrent pancreatitis of ASP in children has not been reported to date. This is the largest number reported in China, with further detail than has previously been reported.
There are several limitations in the present study. First, this was a single-center and retrospective study with potential biases in inclusion criteria. Second, the sample size is notably limited due to the technically demanding and less commonly available nature of the ERCP procedure in most hospitals. The prevalent approach for cases of recurrent pancreatitis post-AAP prioritizes fluid replacement therapy, nutrition, and pain management. Additionally, it is important to note that ERCP is still in the process of development and refinement, especially concerning its application for children with leukemia. Third, there was no control group in our study. Fourth, due to the underdeveloped nature of the rural area from which the patients originated, access to hospital resources is limited. This limitation could potentially contribute to the onset of pancreatitis, abdominal pain, vomiting, and weight gain among the patients. Finally, our study has a relatively short follow-up duration, averaging 1.2 years. This shorter duration may also impact the results of ERCP in AAP.
In summary, for AAP complicated by recurrent pancreatitis with pancreatic pseudocyst and pancreatic duct lesions (stones, pancreatic duct stenosis, or dilatation), ERCP appears to be an effective and safe intervention. Furthermore, ERCP seems to have a protective effect against pancreatic injury caused by repeated use of ASP. As a consequence, these patients can rapidly resume chemotherapy, which improves their outcome with regard to the underlying malignant disease.
Asparaginase (ASP) is an important drug in combined chemotherapy regimens for pediatric acute lymphoblastic leukemia (ALL); ASP-associated pancreatitis (AAP) is the main adverse reaction of ASP. Recurrent pancreatitis is a complication of AAP, for which medication is ineffective.
As repeated occurrence of AAP limits the application of chemotherapy regimens for ALL, an effective, less invasive, and safe treatment strategy for AAP is desirable.
To evaluate the efficacy and safety of endoscopic retrograde cholangiopancreatography (ERCP) in treating recurrent pancreatitis due to AAP.
From May 2018 to August 2021, ten children (five males and five females; age range: 4–13 years) with AAP were treated using ERCP due to recurrent pancreatitis. Clinical data of the ten children were collected, including their sex, age, weight, ALL risk grading, clinical symptoms at the onset of pancreatitis, time from the first pancreatitis onset to ERCP, ERCP operation status, and postoperative complications. The symptomatic relief, weight change, and number of pancreatitis onsets before and after ERCP were compared.
The preoperative symptoms were abdominal pain, vomiting, inability to eat, weight loss of 2–7 kg, and 2–9 pancreatitis onsets. After the operation, nine of ten patients did not develop pancreatitis, had no abdominal pain, could eat normally; the remaining patient developed three pancreatitis onsets due to continuous administration of ASP, but eating was not affected. The postoperative weight gain was 1.5–8 kg. There was one case of postoperative pancreatitis and two cases of postoperative infections; all recovered after medication.
ERCP could improve clinical symptoms and reduce the incidence of pancreatitis, and was shown to be a safe and effective method for improving the management of recurrent pancreatitis of AAP.
Based on our experience of using ERCP in treating recurrent pancreatitis due to AAP, we believe that ERCP can be attempted in children with AAP prior to resuming the use of ASP to ensure that patients can complete their ASP treatment course, thereby improving the event-free survival rate in cases of ALL where children’s ASP therapy is interrupted.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Gupta R, India S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Abu-El-Haija M, Hornung L, Lin TK, Nathan JD, Thompson T, Vitale DS, Nasr A, Husain SZ, Denson L. Drug induced pancreatitis is the leading known cause of first attack acute pancreatitis in children. Pancreatology. 2020;20:1103-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Meczker Á, Hanák L, Párniczky A, Szentesi A, Erőss B, Hegyi P; Hungarian Pancreatic Study Group. Analysis of 1060 Cases of Drug-Induced Acute Pancreatitis. Gastroenterology. 2020;159:1958-1961.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Oparaji JA, Rose F, Okafor D, Howard A, Turner RL, Orabi AI, Byersdorfer C, Mi Q, Ritchey K, Lowe ME, Husain SZ. Risk Factors for Asparaginase-associated Pancreatitis: A Systematic Review. J Clin Gastroenterol. 2017;51:907-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Rank CU, Wolthers BO, Grell K, Albertsen BK, Frandsen TL, Overgaard UM, Toft N, Nielsen OJ, Wehner PS, Harila-Saari A, Heyman MM, Malmros J, Abrahamsson J, Norén-Nyström U, Tomaszewska-Toporska B, Lund B, Jarvis KB, Quist-Paulsen P, Vaitkevičienė GE, Griškevičius L, Taskinen M, Wartiovaara-Kautto U, Lepik K, Punab M, Jónsson ÓG, Schmiegelow K. Asparaginase-Associated Pancreatitis in Acute Lymphoblastic Leukemia: Results From the NOPHO ALL2008 Treatment of Patients 1-45 Years of Age. J Clin Oncol. 2020;38:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Stefanović M, Jazbec J, Lindgren F, Bulajić M, Löhr M. Acute pancreatitis as a complication of childhood cancer treatment. Cancer Med. 2016;5:827-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | de Fine Licht S, Winther JF, Gudmundsdottir T, Holmqvist AS, Bonnesen TG, Asdahl PH, Tryggvadottir L, Anderson H, Wesenberg F, Malila N, Holm K, Hasle H, Olsen JH; ALiCCS study group. Hospital contacts for endocrine disorders in Adult Life after Childhood Cancer in Scandinavia (ALiCCS): a population-based cohort study. Lancet. 2014;383:1981-1989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Kichler A, Jang S. Chronic Pancreatitis: Epidemiology, Diagnosis, and Management Updates. Drugs. 2020;80:1155-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Kumar S, Ooi CY, Werlin S, Abu-El-Haija M, Barth B, Bellin MD, Durie PR, Fishman DS, Freedman SD, Gariepy C, Giefer MJ, Gonska T, Heyman MB, Himes R, Husain SZ, Lin TK, Lowe ME, Morinville V, Palermo JJ, Pohl JF, Schwarzenberg SJ, Troendle D, Wilschanski M, Zimmerman MB, Uc A. Risk Factors Associated With Pediatric Acute Recurrent and Chronic Pancreatitis: Lessons From INSPPIRE. JAMA Pediatr. 2016;170:562-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 9. | Liu QY, Abu-El-Haija M, Husain SZ, Barth B, Bellin M, Fishman DS, Freedman SD, Gariepy CE, Giefer MJ, Gonska T, Heyman MB, Himes R, Lin TK, Maqbool A, Mascarenhas M, McFerron BA, Morinville VD, Nathan JD, Ooi CY, Perito ER, Pohl JF, Rhee S, Schwarzenberg SJ, Shah U, Troendle D, Werlin SL, Wilschanski M, Zimmerman MB, Lowe ME, Uc A. Risk Factors for Rapid Progression From Acute Recurrent to Chronic Pancreatitis in Children: Report From INSPPIRE. J Pediatr Gastroenterol Nutr. 2019;69:206-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149:1490-1500.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557-1573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 399] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 12. | Felux J, Sturm E, Busch A, Zerabruck E, Graepler F, Stüker D, Manger A, Kirschner HJ, Blumenstock G, Malek NP, Goetz M. ERCP in infants, children and adolescents is feasible and safe: results from a tertiary care center. United European Gastroenterol J. 2017;5:1024-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Kohoutova D, Tringali A, Papparella G, Perri V, Boškoski I, Hamanaka J, Costamagna G. Endoscopic treatment of chronic pancreatitis in pediatric population: Long-term efficacy and safety. United European Gastroenterol J. 2019;7:270-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Pan G, Yang K, Gong B, Deng Z. Analysis of the Efficacy and Safety of Endoscopic Retrograde Cholangiopancreatography in Children With Symptomatic Pancreas Divisum. Front Pediatr. 2021;9:761331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Poddar U, Yachha SK, Borkar V, Srivastava A, Saraswat VA. Clinical profile and treatment outcome of chronic pancreatitis in children: a long-term follow-up study of 156 cases. Scand J Gastroenterol. 2017;52:773-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Troendle DM, Fishman DS, Barth BA, Giefer MJ, Lin TK, Liu QY, Abu-El-Haija M, Bellin MD, Durie PR, Freedman SD, Gariepy C, Gonska T, Heyman MB, Himes R, Husain SZ, Kumar S, Lowe ME, Morinville VD, Ooi CY, Palermo J, Pohl JF, Schwarzenberg SJ, Werlin S, Wilschanski M, Zimmerman MB, Uc A. Therapeutic Endoscopic Retrograde Cholangiopancreatography in Pediatric Patients With Acute Recurrent and Chronic Pancreatitis: Data From the INSPPIRE (INternational Study group of Pediatric Pancreatitis: In search for a cuRE) Study. Pancreas. 2017;46:764-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Xue N, Lei XF, Xu JJ, Wei XX. [Progression of endoscopic retrograde cholangiopancreatography in children with pancreaticobiliary diseases]. Zhonghua Er Ke Za Zhi. 2021;59:145-149. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 18. | Párniczky A, Abu-El-Haija M, Husain S, Lowe M, Oracz G, Sahin-Tóth M, Szabó FK, Uc A, Wilschanski M, Witt H, Czakó L, Grammatikopoulos T, Rasmussen IC, Sutton R, Hegyi P. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology. 2018;18:146-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Schmiegelow K, Attarbaschi A, Barzilai S, Escherich G, Frandsen TL, Halsey C, Hough R, Jeha S, Kato M, Liang DC, Mikkelsen TS, Möricke A, Niinimäki R, Piette C, Putti MC, Raetz E, Silverman LB, Skinner R, Tuckuviene R, van der Sluis I, Zapotocka E; Ponte di Legno toxicity working group. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17:e231-e239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 20. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1890] [Cited by in F6Publishing: 1934] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 21. | Baruchel A, Brown P, Rizzari C, Silverman L, van der Sluis I, Wolthers BO, Schmiegelow K. Increasing completion of asparaginase treatment in childhood acute lymphoblastic leukaemia (ALL): summary of an expert panel discussion. ESMO Open. 2020;5:e000977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Kearney SL, Dahlberg SE, Levy DE, Voss SD, Sallan SE, Silverman LB. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2009;53:162-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Wolthers BO, Frandsen TL, Baruchel A, Attarbaschi A, Barzilai S, Colombini A, Escherich G, Grell K, Inaba H, Kovacs G, Liang DC, Mateos M, Mondelaers V, Möricke A, Ociepa T, Samarasinghe S, Silverman LB, van der Sluis IM, Stanulla M, Vrooman LM, Yano M, Zapotocka E, Schmiegelow K; Ponte di Legno Toxicity Working Group. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno Toxicity Working Group study. Lancet Oncol. 2017;18:1238-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Troendle DM, Abraham O, Huang R, Barth BA. Factors associated with post-ERCP pancreatitis and the effect of pancreatic duct stenting in a pediatric population. Gastrointest Endosc. 2015;81:1408-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Keil R, Drábek J, Lochmannová J, Šťovíček J, Koptová P, Wasserbauer M, Frýbová B, Šnajdauf J, Matouš J, Kotalová R, Rygl M, Hlava Š. ERCP in infants, children, and adolescents-Different roles of the methods in different age groups. PLoS One. 2019;14:e0210805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 28. | Freeman AJ, Maqbool A, Bellin MD, Goldschneider KR, Grover AS, Hartzell C, Piester TL, Szabo F, Kiernan BD, Khalaf R, Kumar R, Rios M, Husain SZ, Morinville VD, Abu-El-Haija M. Medical Management of Chronic Pancreatitis in Children: A Position Paper by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr. 2021;72:324-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Hart PA, Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020;115:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Skipper MT, Albertsen BK, Schmiegelow K, Andrés-Jensen L. Long-term effects of asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2023;e30528. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 31. | Dua MM, Visser BC. Surgical Approaches to Chronic Pancreatitis: Indications and Techniques. Dig Dis Sci. 2017;62:1738-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Lu X, Uchida E, Yokomuro S, Nakamura Y, Aimoto T, Tajiri T. Features and choice of treatment of acute and chronic pancreatic pseudocysts--with special reference to invasive intervention. Pancreatology. 2008;8:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Tan JH, Chin W, Shaikh AL, Zheng S. Pancreatic pseudocyst: Dilemma of its recent management (Review). Exp Ther Med. 2021;21:159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Adler JM, Gardner TB. Endoscopic Therapies for Chronic Pancreatitis. Dig Dis Sci. 2017;62:1729-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |