Published online Oct 16, 2023. doi: 10.4253/wjge.v15.i10.602
Peer-review started: July 31, 2023
First decision: August 24, 2023
Revised: August 28, 2023
Accepted: September 11, 2023
Article in press: September 11, 2023
Published online: October 16, 2023
Transoral outlet reduction (TORe) is a minimally invasive endoscopic revision of Roux-en-Y gastric bypass (RYGB) for weight recurrence; however, little has been published on its clinical implementation in the community setting.
To characterize the safety and efficacy of TORe in the community setting for adults with weight recurrence after RYGB.
This is a retrospective cohort study of argon plasma coagulation and purse-string suturing for gastric outlet reduction in consecutive adults with weight recurrence after RYGB at a single community center from September 2020 to September 2022. Patients were provided longitudinal nutritional support via virtual visits. The primary outcome was total body weight loss (TBWL) at twelve months from TORe. Secondary outcomes included TBWL at three months and six months; excess weight loss (EWL) at three, six, and twelve months; twelve-month TBWL by obesity class; predictors of twelve-month TBWL; rates of post-TORe stenosis; and serious adverse events (SAE). Outcomes were reported with descriptive statistics.
Two hundred eighty-four adults (91.9% female, age 51.3 years, body mass index 39.3 kg/m2) underwent TORe an average of 13.3 years after RYGB. Median pre- and post-TORe outlet diameter was 35 mm and 8 mm, respectively. TBWL was 11.7% ± 4.6% at three months, 14.3% ± 6.3% at six months, and 17.3% ± 7.9% at twelve months. EWL was 38.4% ± 28.2% at three months, 46.5% ± 35.4% at six months, and 53.5% ± 39.2% at twelve months. The number of follow-up visits attended was the strongest predictor of TBWL at twelve months (R2 = 0.0139, P = 0.0005). Outlet stenosis occurred in 11 patients (3.9%) and was successfully managed with endoscopic dilation. There was one instance of post-procedural nausea requiring overnight observation (SAE rate 0.4%).
When performed by an experienced endoscopist and combined with longitudinal nutritional support, purse-string TORe is safe and effective in the community setting for adults with weight recurrence after RYGB.
Core Tip: Given the chronic, progressive nature of obesity, weight recurrence after Roux-en-Y gastric bypass (RYGB) is common. Transoral outlet reduction (TORe) is a minimally invasive, same-day, Food and Drug Administration-authorized endoscopic procedure that restricts the gastrojejunal anastomosis to facilitate safe and clinically meaningful weight loss in patients experiencing post-RYGB weight recurrence. To date, nearly all TORe literature has originated in the academic setting. Here, we show that TORe is safe, effective, and technically feasible in the community setting when performed by an experienced bariatric endoscopist and coupled with longitudinal aftercare.
- Citation: Maselli DB, Chittajallu V, Wooley C, Waseem A, Lee D, Secic M, Donnangelo LL, Coan B, McGowan CE. Transoral outlet reduction: Outcomes of endoscopic Roux-en-Y gastric bypass revision in 284 patients at a community practice. World J Gastrointest Endosc 2023; 15(10): 602-613
- URL: https://www.wjgnet.com/1948-5190/full/v15/i10/602.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i10.602
The Roux-en-Y gastric bypass (RYGB), characterized by its restrictive and hypoabsorptive properties, is one of the most effective therapeutic interventions for obesity. Following RYGB, patients typically reach their weight nadir between 18-24 mo, corresponding to an approximate 35% total body weight loss (TBWL). However, over the five subsequent years, there is a linear recurrence of 20%-30% of the maximal weight previously lost, thereby increasing risk for the exacerbation or recurrence of weight-associated medical conditions[1-3]. The incidence of post-RYGB weight recurrence ranges from 24%-79%, depending on patient characteristics and assessment methodology[4-6], and greater than one-third of patients will have a clinically significant weight recurrence surpassing 25% of their initial weight lost[7]. One of the strongest predictors of weight recurrence is time from RYGB, underscoring obesity’s chronic, progressive nature, even after one of the most effective weight loss options currently available[8].
This natural history has led to a substantial increase in the number of revisional procedures after metabolic and bariatric surgeries—from 9480 cases in 2011 (6.0% of bariatric procedures) to 42881 cases in 2019 (16.7% of bariatric procedures)[9]. Weight recurrence after RYGB is multifactorial, and one component is dilation of the gastrojejunal anastomosis (GJA), which has been linked to a reduction in satiation from the RYGB and increased caloric intake[10]. While revisional surgery can include restriction of the gastric pouch and outlet, as well as other techniques, these interventions are associated with increased operative risk and are not widely offered after RYGB, leading to a management gap for individuals with weight recurrence[11,12].
The emergence of endobariatric therapies has provided a minimally invasive alternative for weight recurrence after metabolic and bariatric surgeries. Transoral outlet reduction (TORe) using the Apollo ReviseTM System (Apollo Endosurgery, Inc., Austin, TX, United States) is now the first and only United States Food and Drug Administration (FDA)-authorized device for reduction of the GJA to induce weight loss in adults with weight recurrence after RYGB with body mass index (BMI) between 30-50 kg/m2. TBWL after TORe ranges from 3.5%-8.6% at one year[13,14], with published durability of weight loss both after three years (TBWL 6.9% ± 10.1%) and five years (TBWL 8.8% ± 12.5%)[15].
While the principle behind TORe remains consistent—namely, reduction of the GJA—the technical approach is heterogeneous. Endoscopic suturing patterns for GJA reduction include interrupted, sequential, or purse-string suture closure, with greater efficacy at one year observed from the purse-string technique[14,15]. The GJA reduction may also take the form of argon plasma coagulation (APC) with or without full-thickness endoscopic suturing[16]. Furthermore, existing publications of TORe nearly exclusively arise from tertiary hospital-affiliated centers, further limiting our understanding of how to successfully implement TORe in the community setting.
To address knowledge gaps surrounding the safety, efficacy, and feasibility of TORe for weight recurrence after RYGB in an ambulatory, community setting, we performed a retrospective review of prospectively collected data from 284 consecutive adults who underwent purse-string TORe with longitudinal nutritional support at a single community practice with expertise in endoscopic bariatric therapies.
The study was approved by an Institutional Review Board (WCG IRB, Puyallup, WA) and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. This was a retrospective study of prospectively collected data from a single community practice with expertise in endoscopic weight loss procedures. Consecutive patients aged 21 years and older undergoing TORe by a single endoscopist (Christopher E McGowan) from September 2020 to September 2022 for weight recurrence after RYGB were included. Patients were excluded from this study for the following: anti-obesity medication use within the study duration following TORe; excess weight loss (EWL) from initial RYGB < 25%; time from RYGB < 2 years; GJA diameter < 20 mm at time of TORe attempt; presence of a gastro-gastric fistula; and presence of a silastic ring at the GJA. In addition, all patients needed to describe diminished satiety from meals during their consultation to be a candidate for TORe. All subjects were self-pay. All patients underwent TORe as a same-day procedure with comprehensive virtual follow-up offered by licensed registered dieticians and scheduled visits with a medical team of physicians and nurse practitioners.
All procedures were performed under general anesthesia. The steps of the TORe procedure are shown in Figure 1. First, an endoscopic evaluation with a single-channel gastroscope was performed to identify anatomy, including the gastric pouch, GJA, and blind and efferent limbs of the jejunum. TORe diameter was estimated by standard foreign body forceps, as implemented in guidelines and studies of TORe[17,18]. If present, visible surgical material was removed from the GJA with forceps and/or endoscopic scissors. If no contraindications to TORe were identified, gastric tissue surrounding the GJA was circumferentially ablated using APC (80 W, 1.2 L/min2) for a golden-brown effect approximately 5-10 mm in width. A dual-channel therapeutic gastroscope equipped with a full thickness endoscopic suturing system (Apollo Endosurgery, Inc., Austin, TX, United States) was then used to perform a purse-string outlet reduction as described previously[14]. Outlet reduction was performed with suture tightening over a through-the-scope fluid-filled balloon inserted through the GJA and into the efferent limb for a consistent final outlet diameter. For gastric pouches > 2 cm in length, full-thickness suturing of the gastric pouch from the anterior to posterior direction was performed to reinforce the outlet and reduce the size of the pouch. All reinforcement sutures were placed within the pouch distal to the level of gastroesophageal junction.
The primary outcome was TBWL at twelve months from TORe. Post-TORe TBWL was expressed as a percentage and was defined as follows: (weight at time point after TORe – weight at TORe)/ (weight at TORe) × 100. Thus, weight loss from original RYGB is not incorporated into post-TORe TBWL. Secondary outcomes included technical success; TBWL at three months and six months from TORe; TBWL at 12 mo from TORe by obesity classification; clinical response rates at 12 mo by TBWL category; EWL and body mass index (BMI) at three months, six months, and twelve months from TORe; and predictive factors of 12-mo TBWL after TORe that included age, sex, BMI, time from RYGB to TORe, percent weight recurrence from RYGB at time of TORe, procedure duration, number of sutures, post-TORe GJA diameter, and number of follow-up visits attended. Technical success was defined as the ability to perform circumferential ablation and purse-string suture pattern (i.e. not resorting to interrupted or running suture patterns). Weight recurrence was expressed as a percentage of weight lost from RYGB and was calculated as (weight at time of TORe – lowest weight after RYGB)/(weight at time of RYGB – lowest weight after RYGB) × 100. At 12 mo from TORe, TBWL < 5% was considered non-response; < 10% was considered suboptimal response; ≥ 10% was considered clinically meaningful; and ≥ 15% was considered optimal response. Follow-up visits included those with either a registered dietician or medical team provider (physician or nurse practitioner). Serious adverse events were reported throughout the study and graded according to the lexicon[19]. Rates and resolution of post-TORe outlet stenosis were also reported.
For patients who experienced post-TORe outlet stenosis, endoscopic dilations of the GJA were performed using a single-channel gastroscope and a through-the-scope fluid-filled balloon to dilate the GJA, with the goal of dilation to 2 mm beyond the initial post-TORe outlet diameter.
A biomedical statistician performed the statistical analysis of this study. One-way ANOVA was used to evaluate differences in TBWL at 12 mo between obesity classes. The remaining study data were summarized with descriptive statistics. Continuous variables were summarized with means, standard deviations, medians, and ranges. Categorical variables were summarized with counts and percentages. Multiple linear regression was performed at the 12-mo visit to evaluate predictors of TBWL and included dependent variables of age, sex, BMI, time from original RYGB, percent weight recurrence, GJA diameter, or number of sutures used.
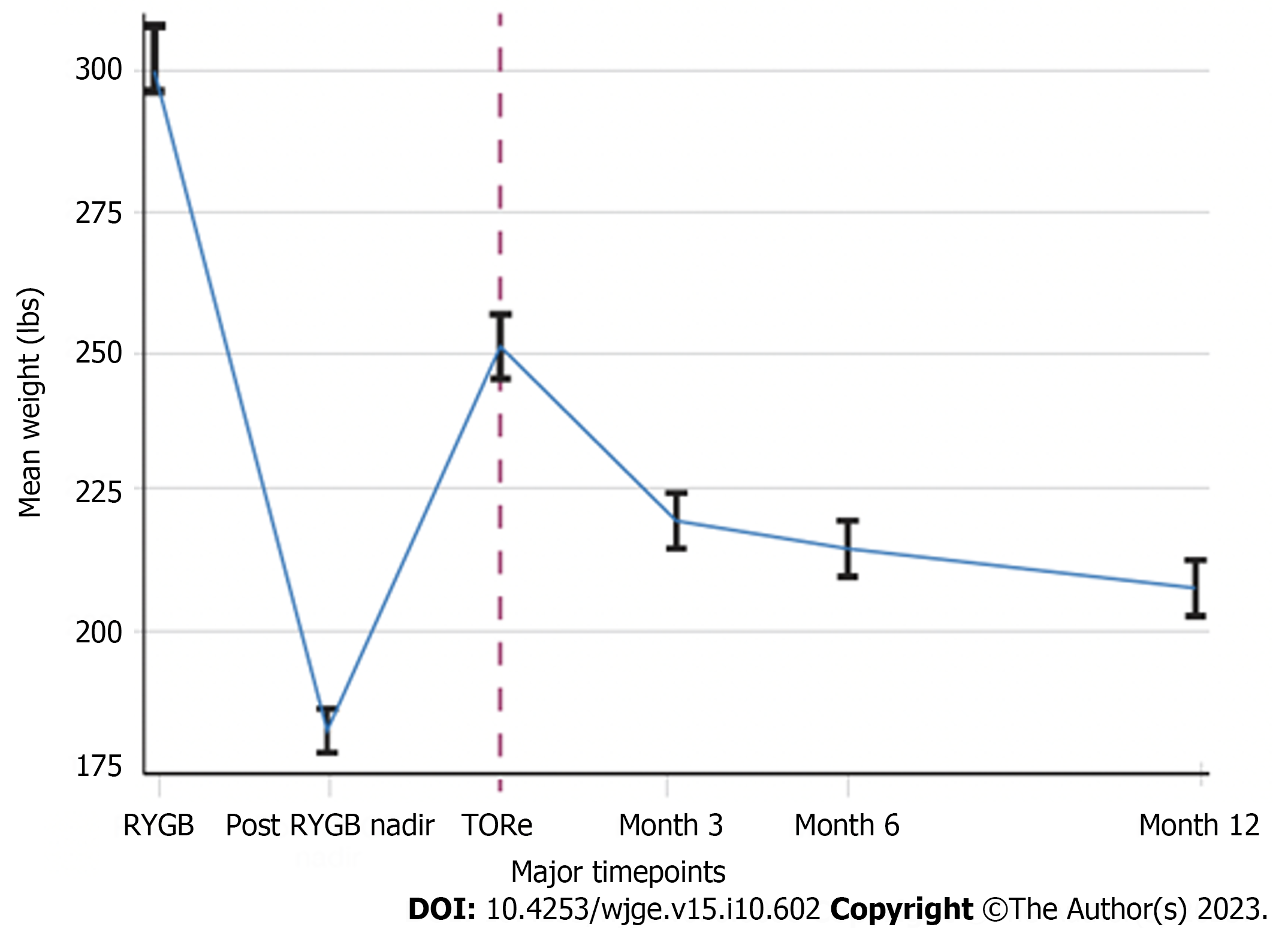
Patient characteristics are shown in Table 1. This study included 284 consecutive adult patients (91.9% female, mean age 51.3 years, mean BMI 39.3 kg/m2) who underwent TORe from September 2020 to September 2022 for weight recurrence after RYGB, without use of anti-obesity medications in the 12 mo following TORe. At the time of TORe, eight subjects (2.8%) had pre-obesity (BMI 25.0-29.9 kg/m2), 74 (26.1%) had class I obesity (BMI 30.0-34.9 kg/m2), 90 (31.7%) had class II obesity (BMI 35.0-39.9 kg/m2), and 112 (39.4%) had class III obesity (BMI ≥ 40.0 kg/m2). 159 (56.0%) patients had at least one of the following comorbidities: hypertension, hyperlipidemia, type II diabetes, or obstructive sleep apnea. From time of RYGB to post-RYGB weight nadir, the cohort had experienced TBWL of 40.5% ± 10.0%. From post-RYGB weight nadir to time of TORe, the cohort had experienced weight recurrence of 35.4% (range 5.3%-128.0%). TORe was performed an average of 13.3 years from their RYGB, took a mean of 27.5 ± 5.8 min to perform, and involved outlet reinforcement/pouch reduction sutures in 67.2% of the cases. Technical success for purse-string outlet reduction was 100%. The median pre-TORe GJA diameter was 35 mm (range 20-50 mm), and the median post-TORe GJA diameter was 8 mm (range 5-10 mm). Procedural characteristics are summarized in Table 2.
| Patient Characteristics | Value |
| Weight at time of RYGB (kg) | |
| mean ± SD | 134.2 ± 26.0 |
| Median, range | 130.0, 72.7-231.8 |
| Post-RYGB weight nadir (kg) | |
| mean ± SD | 79.0 ± 16.3 |
| Median, range | 75.0, 51.8-147.7 |
| Weight recurrence from post-RYGB nadir to TORe (%) | |
| mean ± SD | 39.5 ± 19.8 |
| Median, range | 35.4, 5.3-128 |
| Duration from RYGB to TORe (yr) | 13.3 ± 5.8 |
| Weight at time of TORe (kg) | |
| mean ± SD | 109.1 ± 20.7 |
| Median, range | 106.8, 72.7-171.4 |
| BMI at time of TORe (kg/m2) | |
| mean ± SD | 39.3 ± 6.7 |
| Median, range | 38.3, 26.5-60.0 |
| Class of obesity at time of TORe, n (%) | |
| Pre-obesity (BMI 25.1-29.9 kg/m2) | 8 (2.8) |
| Class I (BMI 30.0-34.9 kg/m2) | 74 (26.1) |
| Class II (BMI 35.0-39.9 kg/m2) | 90 (31.7) |
| Class III (BMI ≥ 40.0 kg/m2) | 112 (39.4%) |
| No. of female subjects (%) | 260 (91.9) |
| Age at time of TORe (yr) | |
| mean ± SD | 51.3 ± 7.9 |
| Median, range | 51, 32-72 |
| Obesity-associated medical problems at time of TORe, n (%) | |
| Hypertension | 90 (31.7%) |
| Dyslipidemia | 74 (26.1%) |
| Diabetes, type II | 31 (10.9%) |
| Obstructive sleep apnea | 18 (6.3%) |
| Procedural characteristics | Value |
| Procedure duration (min) | 27.5 ± 12.9 |
| Procedure Technique | |
| Purse-string of GJA only | 93 (32.7) |
| Purse-string of GJA + 1 reinforcement suture in pouch | 98 (34.5) |
| Purse-string of GJA + 2 reinforcement sutures in pouch | 93 (32.7) |
| Pre-TORe GJA diameter estimation (mm) | |
| mean ± SD | 33.4 ± 6.5 |
| Median, range | 35, 20-50 |
| Post-TORe GJA diameter (mm) | |
| mean ± SD | 7.6 ± 1.0 |
| Median, range | 8, 5-10 |
Of the 284 patients in the patient cohort at the time of data evaluation, 234, 187, and 110 were eligible for follow-up at three, six, and twelve months, respectively. Of patients eligible for follow-up, follow-up rates were 84.2%, 79.7%, and 77.3% at three, six, and twelve months, respectively. For those with 12 mo of follow-up, patients attended a median of 8 follow-up visits after TORe (range 1-17 visits).
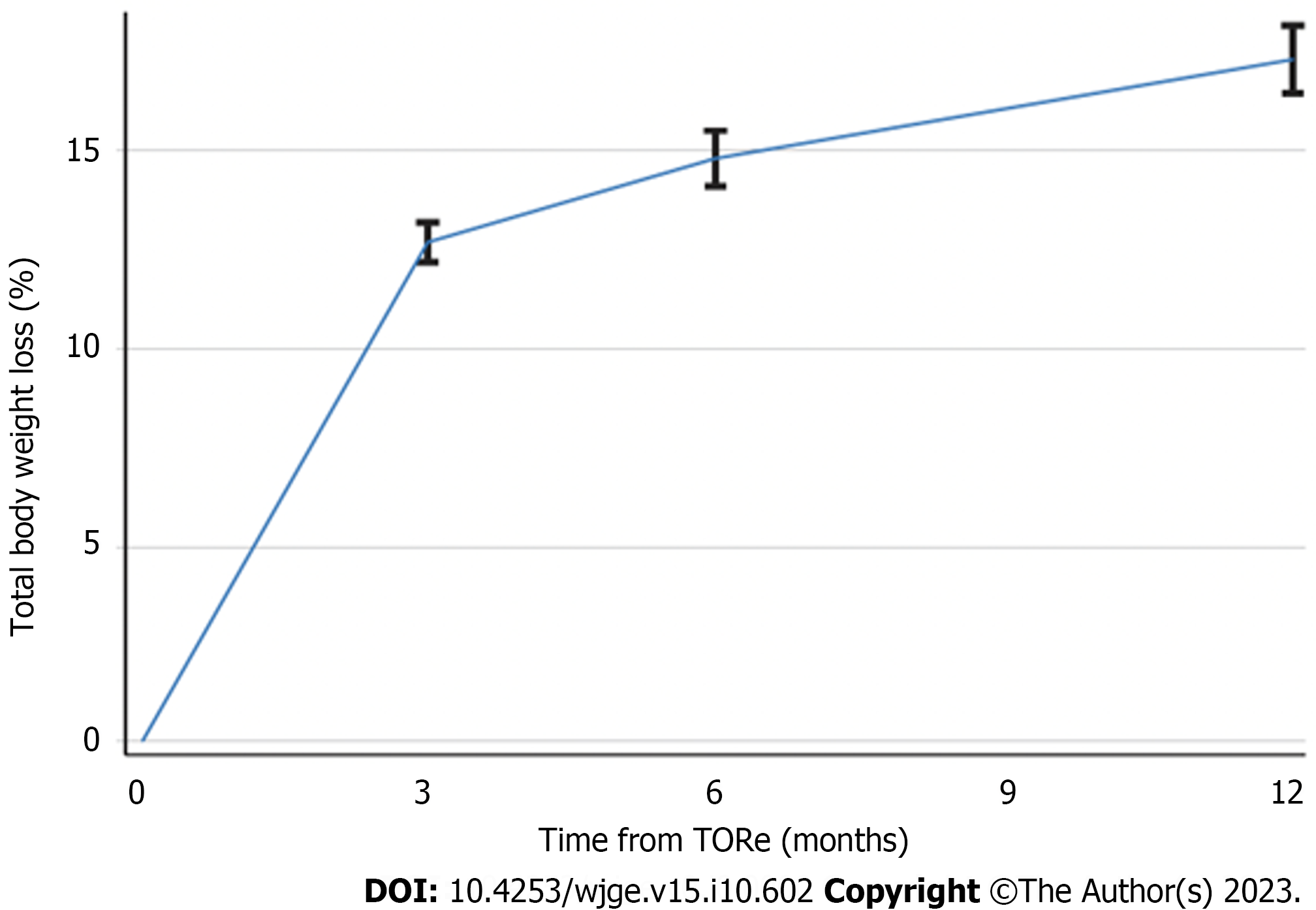
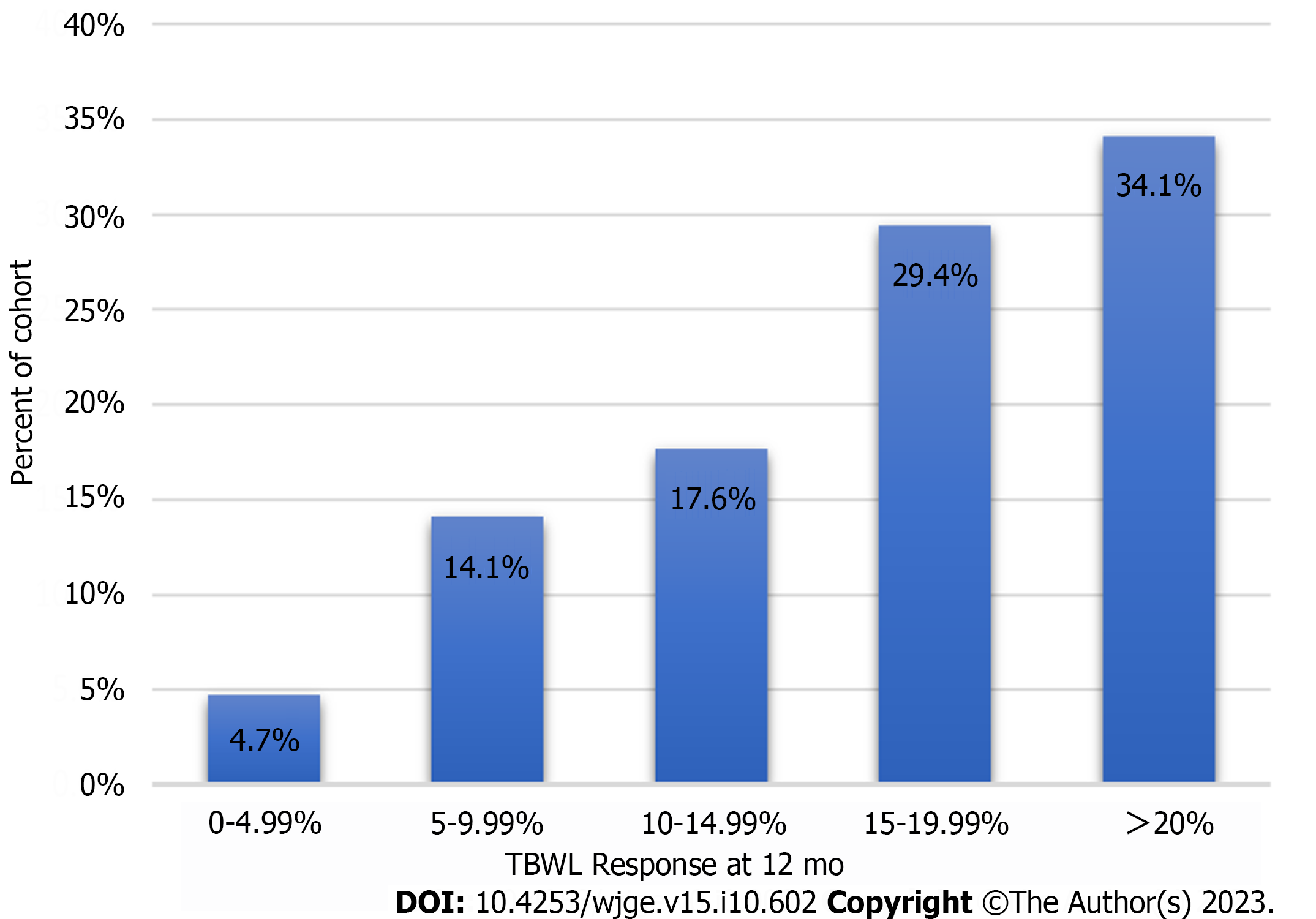
TBWL following TORe was 11.7% ± 4.6% at three months, 14.3% ± 6.3% at six months, and 17.3% ± 7.9% at twelve months (Figure 2). Clinical response rates for the overall cohort are shown in Figure 3. Weight loss was similar between obesity classes at twelve months, with mean TBWL of 12.6% ± 4.3% for pre-obesity; 17.4% ± 7.8% for class I obesity; 16.4% ± 8.4% for class II obesity; and 17.3% ± 8.0% for class III obesity (P = 0.36). TBWL was 18.2% ± 7.2% for those with BMI exceeding 50 kg/m2 (n = 11). For the overall cohort, EWL was 38.4% ± 28.2% at three months, 46.5% ± 35.4% at six months, and 53.5% ± 39.2% at twelve months. For the overall cohort, BMI was 39.3 ± 6.6 kg/m2 at baseline, 35.0 ± 6.4 kg/m2 at 3 mo, 34.6 ± 7.0 kg/m2 at 6 mo, and 33.9 ± 6.9 kg/m2 at 12 mo. The number of follow-up visits attended was the strongest predictor of TBWL at 12 mo (R2 = 0.139, P = 0.0005). There was no association between TBWL at 12 mo and patient age, sex, BMI, time from original RYGB, percent weight recurrence, GJA diameter, or number of sutures used. Two patients (0.7%) underwent upper endoscopy to manage insufficient clinical response. One underwent a repeat TORe with APC + endoscopic purse-string suturing for GJA dilation, and one underwent APC alone for mild stomal dilation. The overall weight trajectory of the cohort from RYGB to 12 mo after TORe is illustrated in Figure 4.
There were no instances of death, gastrointestinal bleeding, gastrointestinal tract leak or perforation, infection/sepsis, or pulmonary embolism from TORe. 283 patients (99.6%) were discharged home same-day, and one subject (0.4%) required in-patient observation for persistent vomiting, which self-resolved, representing a serious adverse event rate of 0.4%.
Eleven patients (3.9%) developed post-TORe stenosis requiring GJA dilation. Components of outlet stenosis and management are shown in Table 3. Of the eleven patients with post-TORe stenosis, the majority (55.6%) had their GJA narrowed to 8 mm at the time of TORe. The average time from TORe to symptom onset suggestive of stenosis was 56.5 ± 30.6 d, and average time from TORe to endoscopic dilation of stenosis was 87.7 ± 60.6 d. Nine patients (81.8%) responded to a single dilation, whereas two (18.2%) required two separate endoscopic procedures for resolution. While none of these eleven patients had a history of type II diabetes, non-steroidal anti-inflammatory drug use, or tobacco use, five (45.5%) had a history of GJA stenosis following RYGB.
| No. of patients with stenosis | 11 (3.9) |
| Post-TORe GJA diameter (mm) | |
| 5 | 1 (0.35) |
| 6 | 57 (20) |
| 7 | 39 (13.7) |
| 8 | 158 (55.6) |
| 9-10 | 25 (7.4) |
| Not reported | 4 (1.4) |
| Post-Stenosis dilation diameter for resolution (mm) | |
| 10 | 6 (54.5) |
| 11 | 1 (9.1) |
| 12 | 2 (18.2) |
| 13.5 | 1 (9.1) |
| 15 | 1 (9.1) |
| Days from TORe to symptoms suggestive of stenosis | 56.5 ± 30.6 |
| Days from TORe to first endoscopic dilation for stenosis | 87.7 ± 60.6 |
| No. of endoscopic dilations to resolve stenosis | |
| 1 | 9 (82) |
| 2 | 2 (18) |
| Medical history of patients who developed stenosis | |
| Post-RYGB Stenosis | 5 (45.5) |
| Diabetes, Type II | 0 |
| NSAID Use | 0 |
| Tobacco use | 0 |
This study demonstrates that purse-string TORe, when performed by an experienced bariatric endoscopist and supported in conjunction with longitudinal nutritional follow-up, is safe and effective in the community setting for the management of weight recurrence after RYGB. The weight loss and safety outcomes in this cohort satisfy the expert-level thresholds for clinical adoption of a novel endoscopic bariatric therapy[20]. Over 80% of the cohort achieved > 10% TBWL, a threshold observed to have a meaningful impact on weight related medical conditions[21].
Notably, TORe appears safe and effective for a wide range of BMIs, including those above or below the FDA authorized ranges of 30-50 kg/m2. While further study is needed, we believe this shows TORe is a reasonable, minimally-invasive option for patients with BMI exceeding 50 kg/m2, particularly given the risks of revisional surgery[12]. Additionally, obesity is a chronic, progressive disease, and increased weight recurrence attenuates the success of a revisional surgery and heightens the risk of weight-related medical conditions[22,23]. Therefore, our practice is to implement TORe for patients with weight recurrence after RYGB early, even for those with pre-obesity on a case-by-case basis, as was done here for eight subjects.
While our cohort’s rates and severity of weight recurrence comport with the existing literature[2,7], a 12-mo TBWL of 17% from TORe is discordant with the published TORe experience[14,15,24]. In a systematic review and meta-analysis of thirteen studies and 850 patients, Dhindsa et al[25] noted a mean TBWL of 8.55% at 12 mo from TORe. While these studies also employed the same full-thickness suturing device and were predominantly female participants, major differences included the inclusion of only studies conducted at academic centers in both the inpatient and outpatient setting, inconsistent use of purse-string technique and ablation, and inclusion of patients on concomitant anti-obesity medications.
We suspect that this discrepancy is driven by two important factors within our cohort: first, consistent use of the purse-string technique, which leads to superior weight loss outcomes compared to other suture patterns for TORe[14]; second, longitudinal and frequent follow-up with medical and nutritional support, which is a critical factor in weight loss and weight loss maintenance in endobariatric therapies[26,27]. In this cohort, attendance of follow-up visits was the strongest predictor of weight loss from TORe, a feature largely afforded by a dedicated team of registered dieticians and the transition to a telemedicine care model amid the coronavirus-19 pandemic. Regular outpatient follow-up with reinforcement of comprehensive lifestyle programming likely maximizes the therapeutic effect of the TORe procedure. Finally, this may also be a result of a self-pay model in our practice, which may confer a higher degree of patient investment in weight loss efforts.
As such, we recommend that gastroenterologists or surgeons looking to incorporate TORe into their practice perform the purse-string technique and provide longitudinal aftercare. The purse-string technique is more challenging to master than alternative techniques[28]. While published data on the learning curve for TORe are sparse, our experience mirrors that of the endoscopic sleeve gastroplasty, for which 25-50 cases are needed, at minimum[29]. However, a novel endoscopic suturing simulator has shown promise in markedly reducing the number of cases needed for independence in TORe, including among novices[30].
Intriguingly, pouch reduction did not contribute to a greater weight loss at one year. This is suggestive of a few possibilities: first, narrowing of the GJA disproportionately governs satiation signals compared to pouch restriction, and second, mucosal ablation and purse-string suturing of the GJA may lead to a durable construct that is not enhanced within the first year by additional reinforcement sutures. Mucosal ablation of the pouch in combination with endoscopic suturing was not performed in this study, and the effect of this is not known at this time. Other factors previously shown to be associated with greater weight loss after TORe—including degree of weight recurrence following RYGB, smaller GJA post-TORe, greater change in pre- to post-TORe diameter, and number of pouch sutures— were not noted in our study[16,31,32].
For those wishing to provide or undergo this procedure, it is important to be cognizant of post-TORe stenosis. This presents as a consistent inability to pass ingested meals with subsequent regurgitation and is not responsive to prolonging a liquid diet. From our cohort, post-TORe stenosis followed a predictable timeline, predominantly occurring within a few weeks of starting a regular diet (day 50 in our program). The major risk factor appears to be GJA stenosis after RYGB; thus, it is routine to ask about this during consultation for TORe at our center. In these patients, our approach is modified by narrowing the GJA to 10 mm rather than 8 mm, our current standard practice. The rate of post-TORe stenosis observed in this cohort is consistent with those published by Jaruvongvanich et al[16] but nearly ten times higher than those observed in Jirapinyo et al[31] and Dhindsa et al[25]. We suspect that this is driven by the high wattage (80 W) mucosal ablation—which is associated with a higher absolute rate of stenosis—plus the purse-string suture technique[33]. The combination of these approaches may be more likely to induce an overly robust healing response beyond the original post-TORe diameter in a predisposed patient. Nevertheless, given that this combination technique offers superior weight loss outcomes, we contend this is an acceptable risk, provided patients are sufficiently counseled and aware that it typically resolves with one to two endoscopic dilations.
Adverse event rates in our cohort were otherwise lower than other published studies, with only one patient admitted for overnight observation of persistent nausea and vomiting with self-resolution[25]. Still, as with other endoscopic suturing procedures, TORe can be associated with a low but serious risk of gastrointestinal bleeding, intraabdominal infection, perigastric leak, and perforation[25,34]. It is therefore critical to inform patients about warning signs and symptoms and to ensure that they have direct access to an on-call physician for assessment and risk stratification should concerns arise. Though this study showed that TORe can be successfully and safely performed in a community ambulatory surgical center, these rare complications may require inpatient management. As such, community physicians performing TORe should have privileges at or at least a relationship with nearby hospitals.
Strengths of this study include a consistent TORe procedural technique and a high subject accountability rate over twelve months. Study limitations include its single-center, retrospective design, and procedural performance by a single experienced endoscopist with expertise in endobariatric procedures, as these limit the generalizability of our findings. Restricting the study duration to 12 mo was necessary due to loss of follow-up beyond this time point but precludes understanding of TORe durability beyond one year. Other limitations include lack of follow-up on comorbidity resolution and lack of capture of improvement of dumping syndrome, for which TORe is a proposed therapy[34,35]. Finally, the results here are, by definition, from those patients who continued with follow-up, and—given that follow-up support is linked to improved weight loss outcomes in endobariatrics—this cohort may over-represent weight loss response[26,27]. For this reason, we emphasize the importance of both TORe technique and aftercare in the interpretation of these data.
Ultimately, TORe provides a critically needed tool for addressing weight recurrence after metabolic and bariatric surgery for those wishing to avoid the risks of revisional surgery. While there are still challenges, including accessibility due to the lack of widespread insurance coverage in the United States and inconsistency regarding what constitutes sufficient training to obtain competency in TORe[36], the procedure nevertheless fits well within the model of obesity as a chronic, progressive, relapsing disease state, particularly as it has been shown to be safe to use with anti-obesity pharmacotherapy[15,37] and, as seen with two patients in our cohort, can be repeated to enhance weight loss effect.
When performed by a physician with experience in endoscopic bariatric therapies, TORe is a feasible, safe, and effective approach to weight recurrence after RYGB in a community-based practice. Successful TORe implementation should focus on mucosal ablation with purse-string technique and frequent, intensive aftercare. Patients and providers should be aware of the risk of post-TORe stenosis that responds well to non-urgent endoscopic balloon dilation.
Given the chronic, progressive nature of obesity, recurrence of 20%-30% of weight lost is common in the decade following Roux-en-Y gastric bypass (RYGB).
Surgical interventions for weight recurrence after RYGB carry heightened risks. Patients may be more amenable to the minimally-invasive endoscopic revision known as transoral outlet reduction (TORe). Though United States Food and Drug Administration-authorized, very little data exists on the implementation of TORe in the community setting.
To clarify the safety, efficacy, and technically feasibility of purse-string TORe in the community setting.
This was a retrospective evaluation of a prospectively-maintained cohort of adult patients undergoing purse-string TORe in an ambulatory surgical center at a practice with expertise in endoscopic bariatric therapies. The primary outcome was total body weight loss at 12 mo. Secondary outcomes included excess weight loss within the first year, safety, predictors of total body weight loss (TBWL) response at 12 mo, and rates of post-TORe gastrojejunal anastomosis (GJA) stenosis.
In this cohort of 284 adults who underwent TORe in the community setting for weight recurrence following RYGB, 12-mo total body weight loss was 17.4%, and 81.2% achieved ≥ 10% TBWL. The number of follow up visits was the strongest predictor of 12-mo TBWL. Serious adverse events were rare and included one episode of post-operative nausea and vomiting requiring hospitalization (0.4%). Post-TORe stenosis occurred in 3.9% of subjects after an average of 57 d from TORe and was successfully managed with 1-2 endoscopic dilations. In this single largest cohort of patients undergoing TORe with a consistent purse-string technique, the procedure was shown to be safe and effective in the community setting.
When performed by experienced endoscopists and supported by longitudinal nutritional aftercare, purse-string TORe is an effective, safe, and feasible tool in the community setting to address weight recurrence after RYGB.
Further study of TORe should evaluate the impact of the procedure on weight related comorbidities, which are shown to reemerge with weight recurrence after RYGB. Investigation into application of TORe to other metabolic and bariatric surgeries with a GJA (such as the one-anastomosis gastric bypass) and other clinical entities in RYGB (such as dumping syndrome and bile acid reflux) will also be valuable to the field.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai KL, China; Gica N, Romania; Liao Z, Singapore; Wang ZF, China S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Thomas DD, Anderson WA, Apovian CM, Hess DT, Yu L, Velazquez A, Carmine B, Istfan NW. Weight Recidivism After Roux-en-Y Gastric Bypass Surgery: An 11-Year Experience in a Multiethnic Medical Center. Obesity (Silver Spring). 2019;27:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, Hopkins PN, McKinlay R, Simper SC, Hunt SC. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017;377:1143-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 534] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 3. | Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3301] [Cited by in F6Publishing: 2914] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 4. | Monaco-Ferreira DV, Leandro-Merhi VA. Weight Regain 10 Years After Roux-en-Y Gastric Bypass. Obes Surg. 2017;27:1137-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 429] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 6. | Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, Altattan M, Balasubramaniam M, Gibbs DS, Krause KR, Chengelis DL, Franklin BA, McCullough PA. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20:349-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in Weight Regain Following Roux-en-Y Gastric Bypass (RYGB) Bariatric Surgery. Obes Surg. 2015;25:1474-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Shantavasinkul PC, Omotosho P, Corsino L, Portenier D, Torquati A. Predictors of weight regain in patients who underwent Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12:1640-1645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Clapp B, Ponce J, DeMaria E, Ghanem O, Hutter M, Kothari S, LaMasters T, Kurian M, English W. American Society for Metabolic and Bariatric Surgery 2020 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2022;18:1134-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 10. | Maleckas A, Gudaitytė R, Petereit R, Venclauskas L, Veličkienė D. Weight regain after gastric bypass: etiology and treatment options. Gland Surg. 2016;5:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Tran DD, Nwokeabia ID, Purnell S, Zafar SN, Ortega G, Hughes K, Fullum TM. Revision of Roux-En-Y Gastric Bypass for Weight Regain: a Systematic Review of Techniques and Outcomes. Obes Surg. 2016;26:1627-1634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Pokala B, Giannopoulos S, Athanasiadis DI, Motamedi SMK, Stefanidis D. Distal gastric bypass revision for weight recurrence or nonresponse to primary procedure: initial experience and outcomes in an academic practice. Surg Endosc. 2023;37:5538-5546. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Thompson CC, Chand B, Chen YK, DeMarco DC, Miller L, Schweitzer M, Rothstein RI, Lautz DB, Slattery J, Ryan MB, Brethauer S, Schauer P, Mitchell MC, Starpoli A, Haber GB, Catalano MF, Edmundowicz S, Fagnant AM, Kaplan LM, Roslin MS. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology. 2013;145:129-137.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Schulman AR, Kumar N, Thompson CC. Transoral outlet reduction: a comparison of purse-string with interrupted stitch technique. Gastrointest Endosc. 2018;87:1222-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Jirapinyo P, Kumar N, AlSamman MA, Thompson CC. Five-year outcomes of transoral outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest Endosc. 2020;91:1067-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Jaruvongvanich V, Vantanasiri K, Laoveeravat P, Matar RH, Vargas EJ, Maselli DB, Alkhatry M, Fayad L, Kumbhari V, Fittipaldi-Fernandez RJ, Hollenbach M, Watson RR, Gustavo de Quadros L, Galvao Neto M, Aepli P, Staudenmann D, Brunaldi VO, Storm AC, Martin JA, Gomez V, Abu Dayyeh BK. Endoscopic full-thickness suturing plus argon plasma mucosal coagulation versus argon plasma mucosal coagulation alone for weight regain after gastric bypass: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:1164-1175.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Brunaldi VO, Peixoto de Oliveira GH, Kerbage A, Ribas PH, Nunes F, Faria G, de Moura D, Riccioppo D, Santo M, de Moura E. Long-term follow-up after transoral outlet reduction following Roux-en-Y gastric bypass: Back to stage 0? Endosc Int Open. 2023;11:E538-E545. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 18. | Galvao Neto M, Brunaldi VO, Grecco E, Silva LB, de Quadros LG, de Souza TF, Teixeira A, de Morais HWP, de Lima JHF, Concon Filho A, Amorim A, de Santana MF, Teixeira N, Marchesini JC; Brazilian Bariatric Endoscopy Collaborative working group. Good Clinical Practices on Argon Plasma Coagulation Treatment for Weight Regain Associated with Dilated Gastrojejunostomy Following Roux-en-Y Gastric Bypass: a Brazilian-Modified Delphi Consensus. Obes Surg. 2022;32:273-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1553] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 20. | Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, Thompson CC, Banerjee S; ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425-38.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 281] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 21. | Ryan DH, Yockey SR. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr Obes Rep. 2017;6:187-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 316] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 22. | Łabul M, Wysocki M, Bartosiak K, Orłowski M, Katkowski B, Jaworski P, Małczak P, Major P; PROSS–Collaborative Study Group. Analysis of the Factors Contributing to Bariatric Success After Laparoscopic Redo Bariatric Procedures: Results from Multicenter Polish Revision Obesity Surgery Study (PROSS). Obes Surg. 2022;32:3879-3890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 23. | Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 671] [Article Influence: 95.9] [Reference Citation Analysis (1)] |
| 24. | Vargas EJ, Bazerbachi F, Rizk M, Rustagi T, Acosta A, Wilson EB, Wilson T, Neto MG, Zundel N, Mundi MS, Collazo-Clavell ML, Meera S, Abu-Lebdeh HS, Lorentz PA, Grothe KB, Clark MM, Kellogg TA, McKenzie TJ, Kendrick ML, Topazian MD, Gostout CJ, Abu Dayyeh BK. Transoral outlet reduction with full thickness endoscopic suturing for weight regain after gastric bypass: a large multicenter international experience and meta-analysis. Surg Endosc. 2018;32:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Dhindsa BS, Saghir SM, Naga Y, Dhaliwal A, Ramai D, Cross C, Singh S, Bhat I, Adler DG. Efficacy of transoral outlet reduction in Roux-en-Y gastric bypass patients to promote weight loss: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E1332-E1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Lopez-Nava G, Galvao M, Bautista-Castaño I, Fernandez-Corbelle JP, Trell M. Endoscopic sleeve gastroplasty with 1-year follow-up: factors predictive of success. Endosc Int Open. 2016;4:E222-E227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Lopez-Nava G, Asokkumar R, Rull A, Corbelle F, Beltran L, Bautista I. Bariatric endoscopy procedure type or follow-up: What predicted success at 1 year in 962 obese patients? Endosc Int Open. 2019;7:E1691-E1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Jirapinyo P, Thompson CC. Training in Bariatric and Metabolic Endoscopic Therapies. Clin Endosc. 2018;51:430-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Saumoy M, Schneider Y, Zhou XK, Shukla A, Kahaleh M, Aronne L, Sharaiha RZ. A single-operator learning curve analysis for the endoscopic sleeve gastroplasty. Gastrointest Endosc. 2018;87:442-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Jirapinyo P, Thompson CC. Development of a Novel Endoscopic Suturing Simulator: Validation and Impact on Clinical Learning Curve. Gastrointest Endosc. 2023;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 31. | Jirapinyo P, Kröner PT, Thompson CC. Purse-string transoral outlet reduction (TORe) is effective at inducing weight loss and improvement in metabolic comorbidities after Roux-en-Y gastric bypass. Endoscopy. 2018;50:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Meyers MH, Swei EC, Tarter W, Schoen J, Rothchild K, Pratap A, Sullivan SA. Factors Associated with Weight Loss After Endoscopic Transoral Outlet Reduction (TORe). J Gastrointest Surg. 2023;27:1587-1593. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 33. | Jirapinyo P, de Moura DTH, Dong WY, Farias G, Thompson CC. Dose response for argon plasma coagulation in the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest Endosc. 2020;91:1078-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Pontecorvi V, Matteo MV, Bove V, De Siena M, Giannetti G, Carlino G, Polidori G, Vinti L, Angelini G, Iaconelli A, Familiari P, Raffaelli M, Costamagna G, Boškoski I. Long-term Outcomes of Transoral Outlet Reduction (TORe) for Dumping Syndrome and Weight Regain After Roux-en-Y Gastric Bypass. Obes Surg. 2023;33:1032-1039. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 35. | Vargas EJ, Abu Dayyeh BK, Storm AC, Bazerbachi F, Matar R, Vella A, Kellogg T, Stier C. Endoscopic management of dumping syndrome after Roux-en-Y gastric bypass: a large international series and proposed management strategy. Gastrointest Endosc. 2020;92:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Spota A, Laracca GG, Perretta S. Training in bariatric and metabolic endoscopy. Ther Adv Gastrointest Endosc. 2020;13:2631774520931978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Jirapinyo P, Thompson CC. Combining transoral outlet reduction with pharmacotherapy yields similar 1-year efficacy with improved safety compared with surgical revision for weight regain after Roux-en-Y gastric bypass (with videos). Gastrointest Endosc. 2023;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |